Abstract
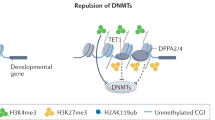
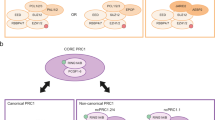
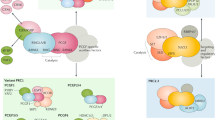
Polycomb group (PcG) proteins are crucial chromatin regulators that maintain repression of lineage-inappropriate genes and are therefore required for stable cell fate. Recent advances show that PcG proteins form distinct multi-protein complexes in various cellular environments, such as in early development, adult tissue maintenance and cancer. This surprising compositional diversity provides the basis for mechanistic diversity. Understanding this complexity deepens and refines the principles of PcG complex recruitment, target-gene repression and inheritance of memory. We review how the core molecular mechanism of Polycomb complexes operates in diverse developmental settings and propose that context-dependent changes in composition and mechanism are essential for proper epigenetic regulation in development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lewis, P. H. Pc: polycomb. Drosoph. Inf. Serv. 21, 69 (1947).
Slifer, E. H. A mutant stock of Drosophila with extra sex-combs. J. Exp. Zool. 90, 31–40 (1942).
Struhl, G. & Akam, M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. EMBO J. 4, 3259–3264 (1985).
Wedeen, C., Harding, K. & Levine, M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell 44, 739–748 (1986).
Zink, B. & Paro, R. In vivo binding pattern of a trans-regulator of homoeotic genes in Drosophila melanogaster. Nature 337, 468–471 (1989).
Whitcomb, S. J., Basu, A., Allis, C. D. & Bernstein, E. Polycomb group proteins: an evolutionary perspective. Trends Genet. 23, 494–502 (2007).
Parreno, V., Martinez, A. M. & Cavalli, G. Mechanisms of polycomb group protein function in cancer. Cell Res. 32, 231–253 (2022).
de Napoles, M. et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7, 663–676 (2004).
Wang, H. et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873–878 (2004).
Francis, N. J., Kingston, R. E. & Woodcock, C. L. Chromatin compaction by a polycomb group protein complex. Science 306, 1574–1577 (2004).
Grau, D. J. et al. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev. 25, 2210–2221 (2011).
Cao, R. et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science 298, 1039–1043 (2002).
Czermin, B. et al. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal polycomb sites. Cell 111, 185–196 (2002).
Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the enhancer of zeste protein. Genes Dev. 16, 2893–2905 (2002).
Muller, J. et al. Histone methyltransferase activity of a Drosophila polycomb group repressor complex. Cell 111, 197–208 (2002).
Boyer, L. A. et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349–353 (2006).
Bracken, A. P., Dietrich, N., Pasini, D., Hansen, K. H. & Helin, K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123–1136 (2006).
Schwartz, Y. B. et al. Genome-wide analysis of polycomb targets in Drosophila melanogaster. Nat. Genet. 38, 700–705 (2006).
Tolhuis, B. et al. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat. Genet. 38, 694–699 (2006).
Margueron, R. et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767 (2009).
Yuan, W. et al. Dense chromatin activates polycomb repressive complex 2 to regulate H3 lysine 27 methylation. Science 337, 971–975 (2012).
Zhao, J. et al. RYBP/YAF2-PRC1 complexes and histone H1-dependent chromatin compaction mediate propagation of H2AK119ub1 during cell division. Nat. Cell Biol. 22, 439–452 (2020). This study demonstrates that the interaction between RYBP and H2AK119ub1 is crucial for spreading of H2AK119ub1 by ncPRC1.
Fischle, W. et al. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 (2003).
Kalb, R. et al. Histone H2A monoubiquitination promotes histone H3 methylation in polycomb repression. Nat. Struct. Mol. Biol. 21, 569–571 (2014).
Min, J., Zhang, Y. & Xu, R. M. Structural basis for specific binding of polycomb chromodomain to histone H3 methylated at Lys27. Genes Dev. 17, 1823–1828 (2003).
Gao, Z. et al. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 45, 344–356 (2012). Through systematic pull-downs and follow-up experiments, this study shows that the composition of distinct PRC1 complexes are based on combinations of PCGF, CBX and RYBP.
Lagarou, A. et al. dKDM2 couples histone H2A ubiquitylation to histone H3 demethylation during polycomb group silencing. Genes Dev. 22, 2799–2810 (2008).
Nekrasov, M. et al. Pcl-PRC2 is needed to generate high levels of H3-K27 trimethylation at polycomb target genes. EMBO J. 26, 4078–4088 (2007).
Zheng, H. et al. Resetting epigenetic memory by reprogramming of histone modifications in mammals. Mol. Cell 63, 1066–1079 (2016). Using low-input ChIP–seq experiments, the authors identify atypical H3K27me3 distribution in mouse oocytes and preimplantation embryos.
Jadhav, U. et al. Acquired tissue-specific promoter bivalency is a basis for PRC2 necessity in adult cells. Cell 165, 1389–1400 (2016).
Cohen, I. et al. PRC1 fine-tunes gene repression and activation to safeguard skin development and stem cell specification. Cell Stem Cell 22, 726–739 e727 (2018).
Blackledge, N. P. & Klose, R. J. The molecular principles of gene regulation by polycomb repressive complexes. Nat. Rev. Mol. Cell Biol. 22, 815–833 (2021).
Piunti, A. & Shilatifard, A. The roles of Polycomb repressive complexes in mammalian development and cancer. Nat. Rev. Mol. Cell Biol. 22, 326–345 (2021).
Ferrari, K. J. et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol. Cell 53, 49–62 (2014).
Lee, H. G., Kahn, T. G., Simcox, A., Schwartz, Y. B. & Pirrotta, V. Genome-wide activities of Polycomb complexes control pervasive transcription. Genome Res. 25, 1170–1181 (2015).
Ku, M. et al. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 4, e1000242 (2008).
Lee, T. I. et al. Control of developmental regulators by polycomb in human embryonic stem cells. Cell 125, 301–313 (2006).
Mendenhall, E. M. et al. GC-rich sequence elements recruit PRC2 in mammalian ES cells. PLoS Genet. 6, e1001244 (2010).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Bernstein, B. E. et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315–326 (2006).
Dobrinic, P., Szczurek, A. T. & Klose, R. J. PRC1 drives Polycomb-mediated gene repression by controlling transcription initiation and burst frequency. Nat. Struct. Mol. Biol. 28, 811–824 (2021).
Henikoff, S. & Shilatifard, A. Histone modification: cause or cog? Trends Genet. 27, 389–396 (2011).
O’Geen, H. et al. dCas9-based epigenome editing suggests acquisition of histone methylation is not sufficient for target gene repression. Nucleic Acids Res. 45, 9901–9916 (2017).
Shao, Z. et al. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37–46 (1999).
Tavares, L. et al. RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell 148, 664–678 (2012).
Farcas, A. M. et al. KDM2B links the polycomb repressive complex 1 (PRC1) to recognition of CpG islands. eLife 1, e00205 (2012).
Taherbhoy, A. M., Huang, O. W. & Cochran, A. G. BMI1-RING1B is an autoinhibited RING E3 ubiquitin ligase. Nat. Commun. 6, 7621 (2015).
Endoh, M. et al. Histone H2A mono-ubiquitination is a crucial step to mediate PRC1-dependent repression of developmental genes to maintain ES cell identity. PLoS Genet. 8, e1002774 (2012).
Kallin, E. M. et al. Genome-wide uH2A localization analysis highlights Bmi1-dependent deposition of the mark at repressed genes. PLoS Genet. 5, e1000506 (2009).
Fursova, N. A. et al. Synergy between variant PRC1 complexes defines polycomb-mediated gene repression. Mol. Cell 74, 1020–1036.e1028 (2019). Through systematic deletion of PCGF proteins in mES cells, the authors of this study and that of Scelfo et al. (2019) characterize the synergistic roles of the PCGF proteins in H2A ubiquitylation and gene repression.
Rose, N. R. et al. RYBP stimulates PRC1 to shape chromatin-based communication between Polycomb repressive complexes. eLife 5, e18591 (2016).
Morey, L., Aloia, L., Cozzuto, L., Benitah, S. A. & Di Croce, L. RYBP and Cbx7 define specific biological functions of polycomb complexes in mouse embryonic stem cells. Cell Rep. 3, 60–69 (2013).
Wu, X., Johansen, J. V. & Helin, K. Fbxl10/Kdm2b recruits polycomb repressive complex 1 to CpG islands and regulates H2A ubiquitylation. Mol. Cell 49, 1134–1146 (2013).
Tamburri, S. et al. Histone H2AK119 mono-ubiquitination is essential for polycomb-mediated transcriptional repression. Mol. Cell 77, 840–856 e845 (2020).
Blackledge, N. P. et al. PRC1 catalytic activity is central to polycomb system function. Mol. Cell 77, 857–874 e859 (2020).
Illingworth, R. S. et al. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 29, 1897–1902 (2015). This study, along with that of Pengelly et al. (2015), shows that the bulk of ubiquitylation is dispensable for correct patterning of animals by generating ubiquitylation-deficient flies and mice.
Pengelly, A. R., Kalb, R., Finkl, K. & Muller, J. Transcriptional repression by PRC1 in the absence of H2A monoubiquitylation. Genes Dev. 29, 1487–1492 (2015). This study, along with that of Illingworth et al. (2015), shows that the bulk of ubiquitylation is dispensable for correct patterning of animals by generating ubiquitylation-deficient flies and mice.
Cohen, I., Bar, C. & Ezhkova, E. Activity of PRC1 and histone H2AK119 monoubiquitination: revising popular misconceptions. Bioessays 42, e1900192 (2020).
Wang, R. et al. Polycomb group targeting through different binding partners of RING1B C-terminal domain. Structure 18, 966–975 (2010).
Isono, K. et al. SAM domain polymerization links subnuclear clustering of PRC1 to gene silencing. Dev. Cell 26, 565–577 (2013). This study demonstrates that the SAM domain-mediated clustering of the PHC protein is crucial for Polycomb body formation and proper mouse development.
Kim, C. A., Gingery, M., Pilpa, R. M. & Bowie, J. U. The SAM domain of polyhomeotic forms a helical polymer. Nat. Struct. Biol. 9, 453–457 (2002).
Kundu, S. et al. Polycomb repressive complex 1 generates discrete compacted domains that change during differentiation. Mol. Cell 65, 432–446 e435 (2017).
Wani, A. H. et al. Chromatin topology is coupled to Polycomb group protein subnuclear organization. Nat. Commun. 7, 10291 (2016).
Plys, A. J. et al. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 33, 799–813 (2019). This study, along with that of Tatavosian et al. (2019), shows that the cPRC1 component CBX2 can phase separate, suggesting another potential way of physically regulating Polycomb domains.
Seif, E. et al. Phase separation by the polyhomeotic sterile alpha motif compartmentalizes Polycomb group proteins and enhances their activity. Nat. Commun. 11, 5609 (2020).
Tatavosian, R. et al. Nuclear condensates of the polycomb protein chromobox 2 (CBX2) assemble through phase separation. J. Biol. Chem. 294, 1451–1463 (2019). This study, along with that of Plys et al. (2019), shows that the cPRC1 component CBX2 can phase separate, suggesting another potential way of physically regulating Polycomb domains.
Satijn, D. P. et al. RING1 is associated with the polycomb group protein complex and acts as a transcriptional repressor. Mol. Cell Biol. 17, 4105–4113 (1997).
Saurin, A. J. et al. The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol. 142, 887–898 (1998).
Boettiger, A. N. et al. Super-resolution imaging reveals distinct chromatin folding for different epigenetic states. Nature 529, 418–422 (2016). Using oligopaint FISH and super resolution microscopy, this study is one of the first imaging-based studies to show that Polycomb-bound regions are more densely packed than transcriptionally active regions.
Schoenfelder, S. et al. Polycomb repressive complex PRC1 spatially constrains the mouse embryonic stem cell genome. Nat. Genet. 47, 1179–1186 (2015). Through promoter capture Hi-C, this study demonstrates the role of PRC1 in genome organization.
Vieux-Rochas, M., Fabre, P. J., Leleu, M., Duboule, D. & Noordermeer, D. Clustering of mammalian Hox genes with other H3K27me3 targets within an active nuclear domain. Proc. Natl Acad. Sci. USA 112, 4672–4677 (2015).
Lau, M. S. et al. Mutation of a nucleosome compaction region disrupts Polycomb-mediated axial patterning. Science 355, 1081–1084 (2017).
Morey, L. et al. Nonoverlapping functions of the polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell 10, 47–62 (2012). This study, along with that of O’Loghlen et al. (2012), shows that CBX component switching occurs during mES cell differentiation, suggesting that cPRC1 composition can be dynamically changed depending on the cellular contexts.
O’Loghlen, A. et al. MicroRNA regulation of Cbx7 mediates a switch of polycomb orthologs during ESC differentiation. Cell Stem Cell 10, 33–46 (2012). This study, along with that of Morey et al. (2012), shows that CBX component switching occurs during mES cell differentiation, suggesting that cPRC1 composition can be dynamically changed depending on the cellular contexts.
Isono, K. et al. Mammalian polyhomeotic homologues Phc2 and Phc1 act in synergy to mediate polycomb repression of Hox genes. Mol. Cell Biol. 25, 6694–6706 (2005).
Core, N. et al. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development 124, 721–729 (1997).
Katoh-Fukui, Y. et al. Male-to-female sex reversal in M33 mutant mice. Nature 393, 688–692 (1998).
Blackledge, N. P. et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445–1459 (2014). This study, along with that of Cooper et al. (2014), demonstrates through ectopic recruitment of various PRC components to artificial loci that ncPRC1 is upstream of PRC2 and cPRC1 in the hierarchical pathway in mES cells.
Cooper, S. et al. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep. 7, 1456–1470 (2014). This study, along with that of Blackledge et al. (2014), demonstrates through ectopic recruitment of various PRC components to artificial loci that ncPRC1 is upstream of PRC2 and cPRC1 in the hierarchical pathway in mES cells.
He, J. et al. Kdm2b maintains murine embryonic stem cell status by recruiting PRC1 complex to CpG islands of developmental genes. Nat. Cell Biol. 15, 373–384 (2013).
Cooper, S. et al. Jarid2 binds mono-ubiquitylated H2A lysine 119 to mediate crosstalk between Polycomb complexes PRC1 and PRC2. Nat. Commun. 7, 13661 (2016).
Oksuz, O. et al. Capturing the onset of PRC2-mediated repressive domain formation. Mol. Cell 70, 1149–1162.e1145 (2018).
Arrigoni, R. et al. The Polycomb-associated protein Rybp is a ubiquitin binding protein. FEBS Lett. 580, 6233–6241 (2006).
Kagey, M. H., Melhuish, T. A. & Wotton, D. The polycomb protein Pc2 is a SUMO E3. Cell 113, 127–137 (2003).
Jaensch, E. S. et al. A Polycomb domain found in committed cells impairs differentiation when introduced into PRC1 in pluripotent cells. Mol. Cell 81, 4677–4691 (2021).
Kim, J. & Kingston, R. E. The CBX family of proteins in transcriptional repression and memory. J. Biosci. 45, 16 (2020).
Bernstein, E. et al. Mouse polycomb proteins bind differentially to methylated histone H3 and RNA and are enriched in facultative heterochromatin. Mol. Cell Biol. 26, 2560–2569 (2006).
Zhen, C. Y. et al. Live-cell single-molecule tracking reveals co-recognition of H3K27me3 and DNA targets polycomb Cbx7-PRC1 to chromatin. eLife 5, e17667 (2016).
Zhen, C. Y., Duc, H. N., Kokotovic, M., Phiel, C. J. & Ren, X. Cbx2 stably associates with mitotic chromosomes via a PRC2- or PRC1-independent mechanism and is needed for recruiting PRC1 complex to mitotic chromosomes. Mol. Biol. Cell 25, 3726–3739 (2014).
Tardat, M. et al. Cbx2 targets PRC1 to constitutive heterochromatin in mouse zygotes in a parent-of-origin-dependent manner. Mol. Cell 58, 157–171 (2015).
Junco, S. E. et al. Structure of the polycomb group protein PCGF1 in complex with BCOR reveals basis for binding selectivity of PCGF homologs. Structure 21, 665–671 (2013).
Gray, F. et al. BMI1 regulates PRC1 architecture and activity through homo- and hetero-oligomerization. Nat. Commun. 7, 13343 (2016).
Scelfo, A. et al. Functional landscape of PCGF proteins reveals both RING1A/B-dependent-and RING1A/B-independent-specific activities. Mol. Cell 74, 1037–1052.e1037 (2019). Through systematic deletion of PCGF proteins in mES cells, the authors of this study and that of Fursova et al. (2019) characterize the synergistic roles of the PCGF proteins in H2A ubiquitylation and gene repression.
Almeida, M. et al. PCGF3/5-PRC1 initiates polycomb recruitment in X chromosome inactivation. Science 356, 1081–1084 (2017). Using livecell imaging and genetic mouse models, the authors show the specific roles of PCGF3 and PCGF5 in X chromosome-inactivation.
Endoh, M. et al. PCGF6-PRC1 suppresses premature differentiation of mouse embryonic stem cells by regulating germ cell-related genes. eLife 6, e21064 (2017).
Zdzieblo, D. et al. Pcgf6, a polycomb group protein, regulates mesodermal lineage differentiation in murine ESCs and functions in iPS reprogramming. Stem Cell 32, 3112–3125 (2014).
Yu, J. R., Lee, C. H., Oksuz, O., Stafford, J. M. & Reinberg, D. PRC2 is high maintenance. Genes Dev. 33, 903–935 (2019).
Li, H. et al. Polycomb-like proteins link the PRC2 complex to CpG islands. Nature 549, 287–291 (2017).
Perino, M. et al. MTF2 recruits polycomb repressive complex 2 by helical-shape-selective DNA binding. Nat. Genet. 50, 1002–1010 (2018).
Healy, E. et al. PRC2.1 and PRC2.2 synergize to coordinate H3K27 trimethylation. Mol. Cell 76, 437–452 e436 (2019).
Hojfeldt, J. W. et al. Non-core subunits of the PRC2 complex are collectively required for its target-site specificity. Mol. Cell 76, 423–436.e423 (2019).
Petracovici, A. & Bonasio, R. Distinct PRC2 subunits regulate maintenance and establishment of polycomb repression during differentiation. Mol. Cell 81, 2625–2639.e2625 (2021).
Kloet, S. L. et al. The dynamic interactome and genomic targets of polycomb complexes during stem-cell differentiation. Nat. Struct. Mol. Biol. 23, 682–690 (2016).
Pemberton, H. et al. Genome-wide co-localization of Polycomb orthologs and their effects on gene expression in human fibroblasts. Genome Biol. 15, R23 (2014).
Endoh, M. et al. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development 135, 1513–1524 (2008).
Shen, X. et al. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 (2008).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 (2011).
Akasaka, T. et al. Mice doubly deficient for the polycomb group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development 128, 1587–1597 (2001).
Moussa, H. F. et al. Canonical PRC1 controls sequence-independent propagation of Polycomb-mediated gene silencing. Nat. Commun. 10, 1931 (2019).
Posfai, E. et al. Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev. 26, 920–932 (2012).
Leeb, M. et al. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 24, 265–276 (2010).
Cohen, I. et al. Polycomb complexes redundantly maintain epidermal stem cell identity during development. Genes Dev. 35, 354–366 (2021).
Zepeda-Martinez, J. A. et al. Parallel PRC2/cPRC1 and vPRC1 pathways silence lineage-specific genes and maintain self-renewal in mouse embryonic stem cells. Sci. Adv. 6, eaax5692 (2020).
Takada, Y. et al. Mammalian Polycomb Scmh1 mediates exclusion of Polycomb complexes from the XY body in the pachytene spermatocytes. Development 134, 579–590 (2007).
Garcia-Moreno, S. A. et al. CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet. 15, e1007895 (2019).
Hasegawa, K. et al. SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev. Cell 32, 574–588 (2015).
Luo, M. et al. Polycomb protein SCML2 associates with USP7 and counteracts histone H2A ubiquitination in the XY chromatin during male meiosis. PLoS Genet. 11, e1004954 (2015).
Haupt, Y., Alexander, W. S., Barri, G., Klinken, S. P. & Adams, J. M. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65, 753–763 (1991).
van Lohuizen, M. et al. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 65, 737–752 (1991).
Gil, J., Bernard, D., Martinez, D. & Beach, D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat. Cell Biol. 6, 67–72 (2004).
Klauke, K. et al. Polycomb Cbx family members mediate the balance between haematopoietic stem cell self-renewal and differentiation. Nat. Cell Biol. 15, 353–362 (2013).
Luis, N. M. et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell 9, 233–246 (2011).
Chung, C. Y. et al. Cbx8 acts non-canonically with Wdr5 to promote mammary tumorigenesis. Cell Rep. 16, 472–486 (2016).
Tan, J. et al. CBX8, a polycomb group protein, is essential for MLL-AF9-induced leukemogenesis. Cancer Cell 20, 563–575 (2011).
Morey, L. et al. Polycomb regulates mesoderm cell fate-specification in embryonic stem cells through activation and repression mechanisms. Cell Stem Cell 17, 300–315 (2015).
Xu, J. et al. Developmental control of polycomb subunit composition by GATA factors mediates a switch to non-canonical functions. Mol. Cell 57, 304–316 (2015).
Stojic, L. et al. Chromatin regulated interchange between polycomb repressive complex 2 (PRC2)-Ezh2 and PRC2-Ezh1 complexes controls myogenin activation in skeletal muscle cells. Epigenetics Chromatin 4, 16 (2011).
Margueron, R. et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell 32, 503–518 (2008).
Laible, G. et al. Mammalian homologues of the polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J. 16, 3219–3232 (1997).
O’Carroll, D. et al. The polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell Biol. 21, 4330–4336 (2001).
Voncken, J. W. et al. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc. Natl Acad. Sci. USA 100, 2468–2473 (2003).
del Mar Lorente, M. et al. Loss- and gain-of-function mutations show a polycomb group function for Ring1A in mice. Development 127, 5093–5100 (2000).
Bhattacharya, D., Talwar, S., Mazumder, A. & Shivashankar, G. V. Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys. J. 96, 3832–3839 (2009).
Li, G. et al. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 24, 368–380 (2010).
Oliviero, G. et al. Dynamic protein interactions of the polycomb repressive complex 2 during differentiation of pluripotent cells. Mol. Cell Proteom. 15, 3450–3460 (2016).
Beringer, M. et al. EPOP functionally links elongin and polycomb in pluripotent stem cells. Mol. Cell 64, 645–658 (2016).
Silva, J. et al. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 4, 481–495 (2003).
Frey, F. et al. Molecular basis of PRC1 targeting to polycomb response elements by PhoRC. Genes Dev. 30, 1116–1127 (2016).
Kang, H. et al. Sex comb on midleg (Scm) is a functional link between PcG-repressive complexes in Drosophila. Genes Dev. 29, 1136–1150 (2015).
DeLuca, S. Z., Ghildiyal, M., Pang, L. Y. & Spradling, A. C. Differentiating Drosophila female germ cells initiate Polycomb silencing by regulating PRC2-interacting proteins. eLife 9, e56922 (2020).
Gao, Z. et al. An AUTS2-Polycomb complex activates gene expression in the CNS. Nature 516, 349–354 (2014).
Liu, S. et al. NRF1 association with AUTS2-polycomb mediates specific gene activation in the brain. Mol. Cell 81, 4663–4676 (2021).
Frangini, A. et al. The aurora B kinase and the polycomb protein ring1B combine to regulate active promoters in quiescent lymphocytes. Mol. Cell 51, 647–661 (2013).
Piunti, A. et al. CATACOMB: An endogenous inducible gene that antagonizes H3K27 methylation activity of Polycomb repressive complex 2 via an H3K27M-like mechanism. Sci. Adv. 5, eaax2887 (2019).
Jain, S. U. et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat. Commun. 10, 2146 (2019).
Ragazzini, R. et al. EZHIP constrains polycomb repressive complex 2 activity in germ cells. Nat. Commun. 10, 3858 (2019).
Pajtler, K. W. et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 136, 211–226 (2018).
Tsuboi, M. et al. Ubiquitination-independent repression of PRC1 targets during neuronal fate restriction in the developing mouse neocortex. Dev. Cell 47, 758–772.e755 (2018). This study shows that distinct PRC1 activity (ubiquitylation versus PHC-mediated clustering) is required for gene silencing in different stages of NPCs during cortical development.
Li, X. Y., Harrison, M. M., Villalta, J. E., Kaplan, T. & Eisen, M. B. Establishment of regions of genomic activity during the Drosophila maternal to zygotic transition. eLife 3, e03737 (2014).
Vastenhouw, N. L. et al. Chromatin signature of embryonic pluripotency is established during genome activation. Nature 464, 922–926 (2010).
Xia, W. et al. Resetting histone modifications during human parental-to-zygotic transition. Science 365, 353–360 (2019).
Du, Z. et al. Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol. Cell 77, 825–839.e827 (2020).
Chen, Z., Djekidel, M. N. & Zhang, Y. Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat. Genet. 53, 551–563 (2021).
Mei, H. et al. H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat. Genet. 53, 539–550 (2021).
Puschendorf, M. et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 40, 411–420 (2008).
Koyama-Nasu, R., David, G. & Tanese, N. The F-box protein Fbl10 is a novel transcriptional repressor of c-Jun. Nat. Cell Biol. 9, 1074–1080 (2007).
Lynch, M. D. et al. An interspecies analysis reveals a key role for unmethylated CpG dinucleotides in vertebrate polycomb complex recruitment. EMBO J. 31, 317–329 (2012).
Brinkman, A. B. et al. Sequential ChIP-bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 22, 1128–1138 (2012).
Reddington, J. P. et al. Redistribution of H3K27me3 upon DNA hypomethylation results in de-repression of Polycomb target genes. Genome Biol. 14, R25 (2013).
Harris, C. et al. Conversion of random X-inactivation to imprinted X-inactivation by maternal PRC2. eLife 8, e44258 (2019).
Inoue, A., Chen, Z., Yin, Q. & Zhang, Y. Maternal Eed knockout causes loss of H3K27me3 imprinting and random X inactivation in the extraembryonic cells. Genes Dev. 32, 1525–1536 (2018).
Inoue, A., Jiang, L., Lu, F., Suzuki, T. & Zhang, Y. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature 547, 419–424 (2017). This study identifies a set of genes that require maternally inherited H3K27me3 for their allele-specific repression, demonstrating that the Polycomb system is involved in imprinting in early mouse embryos.
Inoue, A., Jiang, L., Lu, F. & Zhang, Y. Genomic imprinting of Xist by maternal H3K27me3. Genes Dev. 31, 1927–1932 (2017).
Conway, E. et al. BAP1 enhances Polycomb repression by counteracting widespread H2AK119ub1 deposition and chromatin condensation. Mol. Cell 81, 3526–3541.e3528 (2021).
Fursova, N. A. et al. BAP1 constrains pervasive H2AK119ub1 to control the transcriptional potential of the genome. Genes Dev. 35, 749–770 (2021).
Kadoch, C. et al. Dynamics of BAF-polycomb complex opposition on heterochromatin in normal and oncogenic states. Nat. Genet. 49, 213–222 (2017).
Kennison, J. A. & Tamkun, J. W. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc. Natl Acad. Sci. USA 85, 8136–8140 (1988).
Stanton, B. Z. et al. Smarca4 ATPase mutations disrupt direct eviction of PRC1 from chromatin. Nat. Genet. 49, 282–288 (2017).
Weber, C. M. et al. mSWI/SNF promotes Polycomb repression both directly and through genome-wide redistribution. Nat. Struct. Mol. Biol. 28, 501–511 (2021).
Sharp, E. J., Martin, E. C. & Adler, P. N. Directed overexpression of suppressor 2 of zeste and posterior sex combs results in bristle abnormalities in Drosophila melanogaster. Dev. Biol. 161, 379–392 (1994).
Bel, S. et al. Genetic interactions and dosage effects of polycomb group genes in mice. Development 125, 3543–3551 (1998).
Alkema, M. J., van der Lugt, N. M., Bobeldijk, R. C., Berns, A. & van Lohuizen, M. Transformation of axial skeleton due to overexpression of bmi-1 in transgenic mice. Nature 374, 724–727 (1995).
Lau, M. S. Mutations in the Charged Domain of CBX2 Disrupt PRC1 Function in Vivo. Doctoral thesis, Harvard University (2016).
Zhu, L. & Brangwynne, C. P. Nuclear bodies: the emerging biophysics of nucleoplasmic phases. Curr. Opin. Cell Biol. 34, 23–30 (2015).
Hosogane, M., Funayama, R., Shirota, M. & Nakayama, K. Lack of transcription triggers H3K27me3 accumulation in the gene body. Cell Rep. 16, 696–706 (2016).
Riising, E. M. et al. Gene silencing triggers polycomb repressive complex 2 recruitment to CpG islands genome wide. Mol. Cell 55, 347–360 (2014).
Stielow, B., Finkernagel, F., Stiewe, T., Nist, A. & Suske, G. MGA, L3MBTL2 and E2F6 determine genomic binding of the non-canonical Polycomb repressive complex PRC1.6. PLoS Genet. 14, e1007193 (2018).
Miller, S. A., Damle, M., Kim, J. & Kingston, R. E. Full methylation of H3K27 by PRC2 is dispensable for initial embryoid body formation but required to maintain differentiated cell identity. Development 148, dev196329 (2021).
Lu, T. T. et al. The polycomb-dependent epigenome controls beta cell dysfunction, dedifferentiation, and diabetes. Cell Metab. 27, 1294–1308 e1297 (2018).
Hirabayashi, Y. et al. Polycomb limits the neurogenic competence of neural precursor cells to promote astrogenic fate transition. Neuron 63, 600–613 (2009).
Iovino, N., Ciabrelli, F. & Cavalli, G. PRC2 controls Drosophila oocyte cell fate by repressing cell cycle genes. Dev. Cell 26, 431–439 (2013).
Coleman, R. T. & Struhl, G. Causal role for inheritance of H3K27me3 in maintaining the OFF state of a Drosophila HOX gene. Science 356, eaai8236 (2017). This study, together with that of Laprell et al. (2017), demonstrates that existing H3K27me3 is not sufficient for self-maintenance and gene repression by inducing the deletion of DNA elements needed for de novo PcG recruitment.
Laprell, F., Finkl, K. & Muller, J. Propagation of polycomb-repressed chromatin requires sequence-specific recruitment to DNA. Science 356, 85–88 (2017). This study, together with that of Coleman et al. (2017), demonstrates that existing H3K27me3 is not sufficient for self-maintenance and gene repression by inducing the deletion of DNA elements needed for de novo PcG recruitment.
Jadhav, U. et al. Replicational dilution of H3K27me3 in mammalian cells and the role of poised promoters. Mol. Cell 78, 141–151 e145 (2020). This study shows slow replicative dilution and resulting gene derepression in mammalian systems including intestinal stem cells and human lymphoma cells after disrupting PRC2.
Francis, N. J., Follmer, N. E., Simon, M. D., Aghia, G. & Butler, J. D. Polycomb proteins remain bound to chromatin and DNA during DNA replication in vitro. Cell 137, 110–122 (2009).
Yang, H. et al. Distinct phases of polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science 357, 1142–1145 (2017). This study shows that the loss of Polycomb-mediated memory can be variable between cells within an organism.
Bintu, L. et al. Dynamics of epigenetic regulation at the single-cell level. Science 351, 720–724 (2016).
Struhl, G. A gene product required for correct initiation of segmental determination in Drosophila. Nature 293, 36–41 (1981).
Chan, C. S., Rastelli, L. & Pirrotta, V. A polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13, 2553–2564 (1994).
De, S., Mitra, A., Cheng, Y., Pfeifer, K. & Kassis, J. A. Formation of a Polycomb-domain in the absence of strong polycomb response elements. PLoS Genet. 12, e1006200 (2016).
Mukund, A. & Bintu, L. Temporal signaling, population control, and information processing through chromatin-mediated gene regulation. J. Theor. Biol. 535, 110977 (2022).
Sneppen, K. & Ringrose, L. Theoretical analysis of Polycomb-trithorax systems predicts that poised chromatin is bistable and not bivalent. Nat. Commun. 10, 2133 (2019).
Akasaka, T. et al. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122, 1513–1522 (1996).
Capdevila, M. P., Botas, J. & Garcia-Bellido, A. Genetic interactions between the polycomb locus and the antennapedia and bithorax complexes of Drosophila. Rouxs Arch. Dev. Biol. 195, 417–432 (1986).
Dura, J. M., Brock, H. W. & Santamaria, P. Polyhomeotic: a gene of Drosophila melanogaster required for correct expression of segmental identity. Mol. Gen. Genet. 198, 213–220 (1985).
Tokunaga, C. & Stern, C. The developmental autonomy of extra sex combs in Drosophila melanogaster. Dev. Biol. 11, 50–81 (1965).
van der Lugt, N. M. et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757–769 (1994).
Beuchle, D., Struhl, G. & Muller, J. Polycomb group proteins and heritable silencing of Drosophila hox genes. Development 128, 993–1004 (2001).
Beh, L. Y., Colwell, L. J. & Francis, N. J. A core subunit of polycomb repressive complex 1 is broadly conserved in function but not primary sequence. Proc. Natl Acad. Sci. USA 109, E1063–E1071 (2012).
Sugishita, H. et al. Variant PCGF1-PRC1 links PRC2 recruitment with differentiation-associated transcriptional inactivation at target genes. Nat. Commun. 12, 5341 (2021).
Hickey, G. J. et al. Establishment of developmental gene silencing by ordered polycomb complex recruitment in early zebrafish embryos. eLife 11, e67738 (2022).
Zylicz, J. J. et al. The implication of early chromatin changes in X chromosome inactivation. Cell 176, 182–197.e123 (2019).
Cheutin, T. & Cavalli, G. Progressive polycomb assembly on H3K27me3 compartments generates polycomb bodies with developmentally regulated motion. PLoS Genet. 8, e1002465 (2012).
Buchwald, G. et al. Structure and E3-ligase activity of the ring-ring complex of polycomb proteins Bmi1 and Ring1b. EMBO J. 25, 2465–2474 (2006).
Li, Z. et al. Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J. Biol. Chem. 281, 20643–20649 (2006).
Shen, Y. et al. A map of the 32-cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Acknowledgements
The authors thank J. Zhu, M. Mauger, E. Steinson, J. Wucherpfennig, U. Cho and E. Grow for critical reading of the manuscript. This work was supported by NIH grant R35-GM131743 to R.E.K.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks I. Cohen, H. Koseki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Homeotic transformation
-
A class of mutant phenotypes in which a body segment transforms into another body segment, usually as a result of misregulation of Hox genes. A classic example is the Antennapedia mutation in Drosophila melanogaster, which results in legs instead of antennae.
- Paralogous
-
Paralogous genes are derived from an ancestral gene by gene duplication events within the same species. Paralogous proteins can retain similar functions, but they can also acquire distinct functions.
- Preimplantation embryos
-
Placental animal embryos from zygote to before implantation stages. The first lineage specification between inner cell mass (which gives rise to embryo proper) and trophectoderm (which gives rise to the placenta) happens during this stage.
- Orthologue
-
Orthologous genes are derived by speciation events, therefore, orthologues are present in different species. Orthologous proteins can retain similar functions, but they can also acquire distinct functions.
- Nucleosome arrays
-
In vitro reconstituted chromatin templates used to study biochemical properties of chromatin-modifying proteins, which are made from DNA with nucleosome positioning sequences and linker mixed with histone octamers.
- mSWI/SNF
-
A protein complex that can destabilize histone–DNA interactions in an ATP-dependent manner. It can create accessibility to DNA and counteract Polycomb-mediated repression.
- Phase separation
-
A phenomenon whereby proteins transition to another phase with different physicochemical properties, often through multivalent interactions between themselves. It is potentially one of the driving forces in the formation of membraneless organelles and condensates in the cell.
- Pericentromeres
-
Regions of chromosome adjacent to the centromeres, composed of AT-rich satellite DNA tandem repeats, usually DNA-methylated and methylated at lysine 9 of histone H3.
- CpG islands
-
Approximately 1 kb DNA regions in vertebrates with overrepresentation of CpG dinucleotides compared with the genome average. They are often a site of transcription initiation, and more than half of annotated gene promoters are CpG islands.
- Polycomb response element
-
(PRE). Discrete regulatory DNA element that can nucleate recruitment of Polycomb complexes and silencing in Drosophila melanogaster.
- Vernalization
-
A process of prolonged exposure to the cold that induces flowering in plants. Genetic screens to find genes required for vernalization uncovered several genes later identified to be part of plant Polycomb complexes.
- Trithorax group
-
(trxG). A group of chromatin regulators that maintains an active state of gene expression that includes mSWI/SNF complex. Genes encoding trxG proteins were originally discovered by a genetic suppression screen in Drosophila melanogaster to suppress the Polycomb mutant phenotype.
- Zygotic genome activation
-
After fertilization, transcription is absent in the zygotic genome, therefore, embryos develop with maternally provided transcripts. Zygotic genome activation happens as maternal mRNA decays, and genes are transcribed from the zygotic genome through the process called maternal-to-zygotic transition.
Rights and permissions
About this article
Cite this article
Kim, J.J., Kingston, R.E. Context-specific Polycomb mechanisms in development. Nat Rev Genet 23, 680–695 (2022). https://doi.org/10.1038/s41576-022-00499-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-022-00499-0
This article is cited by
-
Cuproptosis-related ceRNA axis triggers cell proliferation and cell cycle through CBX2 in lung adenocarcinoma
BMC Pulmonary Medicine (2024)
-
Nuclear mRNA decay: regulatory networks that control gene expression
Nature Reviews Genetics (2024)
-
Uncoupled evolution of the Polycomb system and deep origin of non-canonical PRC1
Communications Biology (2023)
-
Functional dissection of PRC1 subunits RYBP and YAF2 during neural differentiation of embryonic stem cells
Nature Communications (2023)
-
New genetic and epigenetic insights into the chemokine system: the latest discoveries aiding progression toward precision medicine
Cellular & Molecular Immunology (2023)