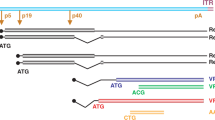
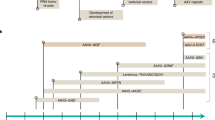
Abstract
Adeno-associated virus (AAV) vector-mediated gene delivery was recently approved for the treatment of inherited blindness and spinal muscular atrophy, and long-term therapeutic effects have been achieved for other rare diseases, including haemophilia and Duchenne muscular dystrophy. However, current research indicates that the genetic modification of AAV vectors may further facilitate the success of AAV gene therapy. Vector engineering can increase AAV transduction efficiency (by optimizing the transgene cassette), vector tropism (using capsid engineering) and the ability of the capsid and transgene to avoid the host immune response (by genetically modifying these components), as well as optimize the large-scale production of AAV.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Moore, N. A., Bracha, P., Hussain, R. M., Morral, N. & Ciulla, T. A. Gene therapy for age-related macular degeneration. Expert. Opin. Biol. Ther. 17, 1235–1244 (2017).
Martinez-Navio, J. M. et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50, 567–575.e5 (2019).
Bass-Stringer, S. et al. Adeno-associated virus gene therapy: translational progress and future prospects in the treatment of heart failure. Heart Lung Circ. 27, 1285–1300 (2018).
Dunbar, C. E. et al. Gene therapy comes of age. Science 359, eaan4672 (2018).
Kotterman, M. A., Chalberg, T. W. & Schaffer, D. V. Viral vectors for gene therapy: translational and clinical outlook. Annu. Rev. Biomed. Eng. 17, 63–89 (2015).
Lundstrom, K. Viral vectors in gene therapy. Diseases 6, 42 (2018).
Kay, M. A. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 12, 316–328 (2011).
Buning, H. Gene therapy enters the pharma market: the short story of a long journey. EMBO Mol. Med. 5, 1–3 (2013).
Yla-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol. Ther. 20, 1831–1832 (2012).
Aiuti, A., Roncarolo, M. G. & Naldini, L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 9, 737–740 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Keeler, A. M. & Flotte, T. R. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu. Rev. Virol. 6, 601–621 (2019).
Wu, Z., Asokan, A. & Samulski, R. J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327 (2006).
Hastie, E. & Samulski, R. J. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success — a personal perspective. Hum. Gene Ther. 26, 257–265 (2015).
Xie, Q. et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl Acad. Sci. USA 99, 10405–10410 (2002).
Govindasamy, L. et al. Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J. Virol. 80, 11556–11570 (2006).
Buning, H., Huber, A., Zhang, L., Meumann, N. & Hacker, U. Engineering the AAV capsid to optimize vector–host-interactions. Curr. Opin. Pharmacol. 24, 94–104 (2015).
Kotterman, M. A. & Schaffer, D. V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 15, 445–451 (2014). This review describes, in detail, the development of AAV mutants using directed evolution.
Pillay, S. & Carette, J. E. Host determinants of adeno-associated viral vector entry. Curr. Opin. Virol. 24, 124–131 (2017).
Bleker, S., Sonntag, F. & Kleinschmidt, J. A. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J. Virol. 79, 2528–2540 (2005).
Kronenberg, S., Bottcher, B., von der Lieth, C. W., Bleker, S. & Kleinschmidt, J. A. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J. Virol. 79, 5296–5303 (2005).
Haberman, R. P., McCown, T. J. & Samulski, R. J. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J. Virol. 74, 8732–8739 (2000).
Wu, Z. et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol. Ther. 16, 280–289 (2008).
Mendell, J. R. et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010).
George, L. A. et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 377, 2215–2227 (2017).
Rodrigues, G. A. et al. Pharmaceutical development of AAV-based gene therapy products for the eye. Pharm. Res. 36, 29 (2018).
Nathwani, A. C. et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 371, 1994–2004 (2014).
Nathwani, A. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV–factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006). This paper is the first report of successful gene therapy using an AAV vector in patients with haemophilia; the paper also shows that transduced target cells are eliminated by capsid-specific CTLs.
Rangarajan, S. et al. AAV5–factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 377, 2519–2530 (2017).
Nathwani, A. C. et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 107, 2653–2661 (2006).
He, Y. et al. Kinetics of adeno-associated virus serotype 2 (AAV2) and AAV8 capsid antigen presentation in vivo are identical. Hum. Gene Ther. 24, 545–553 (2013).
Shao, W. et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight 3, e120474 (2018). This study demonstrates activation of the innate immune response in the later phase of AAV transduction in human cells.
Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M. & Sah, D. W. Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug. Discov. 17, 767 (2018).
McCarty, D. M. et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 10, 2112–2118 (2003). This paper describes the development of a novel approach involving the mutation of AAV ITRs to generate self-complementary AAV vectors to achieve faster and higher transgene expression.
Fu, H. et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol. Ther. 8, 911–917 (2003).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017). This paper presents findings that led to the second AAV-based drug, Zolgensma, being approved by the FDA.
Greig, J. A. et al. Characterization of adeno-associated viral vector-mediated human factor VIII gene therapy in hemophilia A mice. Hum. Gene Ther. 28, 392–402 (2017).
Yan, Z. et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum. Gene Ther. 26, 334–346 (2015).
Chuah, M. K. et al. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol. Ther. 22, 1605–1613 (2014).
Rincon, M. Y. et al. Genome-wide computational analysis reveals cardiomyocyte-specific transcriptional cis-regulatory motifs that enable efficient cardiac gene therapy. Mol. Ther. 23, 43–52 (2015).
Garel, J. Quantitative adaptation of isoacceptor tRNAs to mRNA codons of alanine, glycine and serine. Nature 260, 805–806 (1976).
Dong, H., Nilsson, L. & Kurland, C. G. Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J. Mol. Biol. 260, 649–663 (1996).
Dittmar, K. A., Goodenbour, J. M. & Pan, T. Tissue-specific differences in human transfer RNA expression. PLOS Genet. 2, e221 (2006).
Brown, H. C. et al. Target-cell-directed bioengineering approaches for gene therapy of hemophilia A. Mol. Ther. Methods Clin. Dev. 9, 57–69 (2018).
Bowles, D. E. et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20, 443–455 (2012).
Duan, D., Yue, Y. & Engelhardt, J. F. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol. Ther. 4, 383–391 (2001).
Hirsch, M. L., Wolf, S. J. & Samulski, R. J. Delivering transgenic DNA exceeding the carrying capacity of AAV vectors. Methods Mol. Biol. 1382, 21–39 (2016).
Koo, T., Popplewell, L., Athanasopoulos, T. & Dickson, G. Triple trans-splicing adeno-associated virus vectors capable of transferring the coding sequence for full-length dystrophin protein into dystrophic mice. Hum. Gene Ther. 25, 98–108 (2014).
Hirsch, M. L. et al. Oversized AAV transductifon is mediated via a DNA-PKcs-independent, Rad51C-dependent repair pathway. Mol. Ther. 21, 2205–2216 (2013).
Ghosh, A., Yue, Y., Lai, Y. & Duan, D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther. 16, 124–130 (2008).
Fakhiri, J. et al. Novel chimeric gene therapy vectors based on adeno-associated virus and four different mammalian bocaviruses. Mol. Ther. Methods Clin. Dev. 12, 202–222 (2019).
Srivastava, C. H., Samulski, R. J., Lu, L., Larsen, S. H. & Srivastava, A. Construction of a recombinant human parvovirus B19: adeno-associated virus 2 (AAV) DNA inverted terminal repeats are functional in an AAV–B19 hybrid virus. Proc. Natl Acad. Sci. USA 86, 8078–8082 (1989).
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24, 1519–1525 (2018).
Tornabene, P. et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci. Transl Med. 11, eaav4523 (2019).
Herrmann, A. K. & Grimm, D. High-throughput dissection of AAV–host interactions: the fast and the curious. J. Mol. Biol. 430, 2626–2640 (2018).
Shen, S. et al. Engraftment of a galactose receptor footprint onto adeno-associated viral capsids improves transduction efficiency. J. Biol. Chem. 288, 28814–28823 (2013).
Wang, D. et al. A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol. Ther. Methods Clin. Dev. 9, 234–246 (2018).
Zhong, L. et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl Acad. Sci. USA 105, 7827–7832 (2008).
Petrs-Silva, H. et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 17, 463–471 (2009).
Markusic, D. M. et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol. Ther. 18, 2048–2056 (2010).
Ling, C., Li, B., Ma, W. & Srivastava, A. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum. Gene Ther. Methods 27, 143–149 (2016).
White, S. J. et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 109, 513–519 (2004).
Grifman, M. et al. Incorporation of tumor-targeting peptides into recombinant adeno-associated virus capsids. Mol. Ther. 3, 964–975 (2001).
Adachi, K. & Nakai, H. A new recombinant adeno-associated virus (AAV)-based random peptide display library system: infection-defective AAV1.9-3 as a novel detargeted platform for vector evolution. Gene Ther. Regul. 5, 31–55 (2010).
Muller, O. J. et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 21, 1040–1046 (2003).
Korbelin, J. et al. Pulmonary targeting of adeno-associated viral vectors by next-generation sequencing-guided screening of random capsid displayed peptide libraries. Mol. Ther. 24, 1050–1061 (2016).
Varadi, K. et al. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene Ther. 19, 800–809 (2012).
Korbelin, J. et al. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med. 8, 609–625 (2016).
Maheshri, N., Koerber, J. T., Kaspar, B. K. & Schaffer, D. V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198–204 (2006). This study is the first to apply directed evolution to the selection of AAV vectors with the ability to enhance transgene expression and evade NAbs.
Pulicherla, N. et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 19, 1070–1078 (2011).
Voigt, C. A., Martinez, C., Wang, Z. G., Mayo, S. L. & Arnold, F. H. Protein building blocks preserved by recombination. Nat. Struct. Biol. 9, 553–558 (2002).
Ojala, D. S. et al. In vivo selection of a computationally designed SCHEMA AAV library yields a novel variant for infection of adult neural stem cells in the SVZ. Mol. Ther. 26, 304–319 (2018).
Marsic, D. et al. Vector design tour de force: integrating combinatorial and rational approaches to derive novel adeno-associated virus variants. Mol. Ther. 22, 1900–1909 (2014).
Deverman, B. E. et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 (2016).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Dalkara, D. et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl Med. 5, 189ra176 (2013).
Santiago-Ortiz, J. et al. AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene Ther. 22, 934–946 (2015).
Zinn, E. et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 12, 1056–1068 (2015).
Landegger, L. D. et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 35, 280–284 (2017).
Pan, B. et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 35, 264–272 (2017).
Rabinowitz, J. E. et al. Cross-dressing the virion: the transcapsidation of adeno-associated virus serotypes functionally defines subgroups. J. Virol. 78, 4421–4432 (2004).
Chai, Z. et al. Application of polyploid adeno-associated virus vectors for transduction enhancement and neutralizing antibody evasion. J. Control Rel. 262, 348–356 (2017).
Monahan, P. E. et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol. Ther. 18, 1907–1916 (2010).
Mitchell, A. M. & Samulski, R. J. Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors. J. Virol. 87, 13035–13041 (2013).
Mitchell, A. M., Li, C. & Samulski, R. J. Arsenic trioxide stabilizes accumulations of adeno-associated virus virions at the perinuclear region, increasing transduction in vitro and in vivo. J. Virol. 87, 4571–4583 (2013).
Nicolson, S. C., Li, C., Hirsch, M. L., Setola, V. & Samulski, R. J. Identification and validation of small molecules that enhance recombinant adeno-associated virus transduction following high-throughput screens. J. Virol. 90, 7019–7031 (2016).
Berry, G. E. & Asokan, A. Chemical modulation of endocytic sorting augments adeno-associated viral transduction. J. Biol. Chem. 291, 939–947 (2016).
Maddalena, A. et al. High-throughput screening identifies kinase inhibitors that increase dual adeno-associated viral vector transduction in vitro and in mouse retina. Hum. Gene Ther. 29, 886–901 (2018).
Chandler, L. C. et al. Enhancement of adeno-associated virus-mediated gene therapy using hydroxychloroquine in murine and human tissues. Mol. Ther. Methods Clin. Dev. 14, 77–89 (2019).
Denard, J. et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J. Virol. 86, 6620–6631 (2012).
Denard, J. et al. C-reactive protein (CRP) is essential for efficient systemic transduction of recombinant adeno-associated virus vector 1 (rAAV-1) and rAAV-6 in mice. J. Virol. 87, 10784–10791 (2013).
Wang, M. et al. Direct interaction of human serum proteins with AAV virions to enhance AAV transduction: immediate impact on clinical applications. Gene Ther. 24, 49–59 (2017).
Pei, X. et al. AAV8 virions hijack serum proteins to increase hepatocyte binding for transduction enhancement. Virology 518, 95–102 (2018).
Chai, Z. et al. Cryoprecipitate augments the global transduction of the adeno-associated virus serotype 9 after a systemic administration. J. Control Rel. 286, 415–424 (2018).
Denard, J. et al. AAV-8 and AAV-9 vectors cooperate with serum proteins differently than AAV-1 and AAV-6. Mol. Ther. Methods Clin. Dev. 10, 291–302 (2018).
Fitzpatrick, Z. et al. Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol. Ther. Methods Clin. Dev. 9, 119–129 (2018).
Bevan, A. K. et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 19, 1971–1980 (2011).
Liu, Y. et al. Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol. Ther. Methods Clin. Dev. 1, 12 (2014).
Zhang, X., He, T., Chai, Z., Samulski, R. J. & Li, C. Blood–brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 176, 71–83 (2018).
Li, C. et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc. Natl Acad. Sci. USA 106, 10770–10774 (2009).
Sun, J. et al. An observational study from long-term AAV re-administration in two hemophilia dogs. Mol. Ther. Methods Clin. Dev. 10, 257–267 (2018).
Sun, J., Hua, B., Chen, X., Samulski, R. J. & Li, C. Gene delivery of activated factor VII using alternative adeno-associated virus serotype improves hemostasis in hemophiliac mice with FVIII inhibitors and adeno-associated virus neutralizing antibodies. Hum. Gene Ther. 28, 654–666 (2017).
Sack, B. K. & Herzog, R. W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 11, 493–503 (2009).
Ahn, K. et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6, 613–621 (1997).
Ahn, K. et al. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15, 3247–3255 (1996).
Shao, W., Chen, X., Samulski, R. J., Hirsch, M. L. & Li, C. Inhibition of antigen presentation during AAV gene therapy using virus peptides. Hum. Mol. Genet. 27, 601–613 (2018).
Xiao, Y. et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight 4, e99052 (2019).
Cooper, M. et al. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum. Gene Ther. 20, 767–776 (2009).
Mays, L. E. et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J. Immunol. 182, 6051–6060 (2009).
Zhu, J., Huang, X. & Yang, Y. The TLR9–MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 119, 2388–2398 (2009). This study shows that an innate immune response is triggered soon after AAV transduction.
Martino, A. T. et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 117, 6459–6468 (2011).
Faust, S. M. et al. CpG-depleted adeno-associated virus vectors evade immune detection. J. Clin. Invest. 123, 2994–3001 (2013).
Chan, Y. K. et al. Engineering AAV vectors to evade innate immune and inflammatory responses. Mol. Ther. 26, 457–458 (2018).
Reichel, F. F. et al. AAV8 can induce innate and adaptive immune response in the primate eye. Mol. Ther. 25, 2648–2660 (2017).
Louis Jeune, V., Joergensen, J. A., Hajjar, R. J. & Weber, T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods 24, 59–67 (2013).
Vandamme, C., Adjali, O. & Mingozzi, F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum. Gene Ther. 28, 1061–1074 (2017).
McCraw, D. M., O’Donnell, J. K., Taylor, K. A., Stagg, S. M. & Chapman, M. S. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology 431, 40–49 (2012).
Tseng, Y. S. et al. Adeno-associated virus serotype 1 (AAV1)– and AAV5–antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J. Virol. 89, 1794–1808 (2015).
Li, C. et al. Single amino acid modification of adeno-associated virus capsid changes transduction and humoral immune profiles. J. Virol. 86, 7752–7759 (2012).
Jose, A. et al. High resolution structural characterization of a new AAV5 antibody epitope toward engineering antibody resistant recombinant gene delivery vectors. J. Virol. https://doi.org/10.1128/JVI.01394-18 (2018).
Smith, J. K. & Agbandje-McKenna, M. Creating an arsenal of adeno-associated virus (AAV) gene delivery stealth vehicles. PLOS Pathog. 14, e1006929 (2018).
Tse, L. V. et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc. Natl Acad. Sci. USA 114, E4812–E4821 (2017).
Waterkamp, D. A., Muller, O. J., Ying, Y., Trepel, M. & Kleinschmidt, J. A. Isolation of targeted AAV2 vectors from novel virus display libraries. J. Gene Med. 8, 1307–1319 (2006).
Grimm, D. et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008).
Li, C. et al. Development of patient-specific AAV vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol. Ther. 24, 53–65 (2016).
Paulk, N. K. et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol. Ther. 26, 289–303 (2018).
Li, C. et al. Adeno-associated virus capsid antigen presentation is dependent on endosomal escape. J. Clin. Invest. 123, 1390–1401 (2013).
Pei, X. et al. Efficient capsid antigen presentation from adeno-associated virus empty virions. Vivo. Front. Immunol. 9, 844 (2018).
Sen, D. et al. Targeted modifications in adeno-associated virus serotype 8 capsid improves its hepatic gene transfer efficiency in vivo. Hum. Gene Ther. Methods 24, 104–116 (2013).
Martino, A. T. et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 121, 2224–2233 (2013).
Kaemmerer, W. F. How will the field of gene therapy survive its success? Bioeng. Transl Med. 3, 166–177 (2018).
Kotin, R. M. Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 20, R2–R6 (2011).
Grieger, J. C., Soltys, S. M. & Samulski, R. J. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol. Ther. 24, 287–297 (2016).
Penaud-Budloo, M., Francois, A., Clement, N. & Ayuso, E. Pharmacology of recombinant adeno-associated virus production. Mol. Ther. Methods Clin. Dev. 8, 166–180 (2018).
Kotin, R. M. & Snyder, R. O. Manufacturing clinical grade recombinant adeno-associated virus using invertebrate cell lines. Hum. Gene Ther. 28, 350–360 (2017).
Mietzsch, M. et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum. Gene Ther. 25, 212–222 (2014).
Kondratov, O. et al. Direct head-to-head evaluation of recombinant adeno-associated viral vectors manufactured in human versus insect cells. Mol. Ther. 25, 2661–2675 (2017).
Bosma, B. et al. Optimization of viral protein ratios for production of rAAV serotype 5 in the baculovirus system. Gene Ther. 25, 415–424 (2018).
Wang, Q. et al. A robust system for production of superabundant VP1 recombinant AAV vectors. Mol. Ther. Methods Clin. Dev. 7, 146–156 (2017).
Wang, Z., Cheng, F., Engelhardt, J. F., Yan, Z. & Qiu, J. Development of a novel recombinant adeno-associated virus production system using human bocavirus 1 helper genes. Mol. Ther. Methods Clin. Dev. 11, 40–51 (2018).
Wang, Z. et al. Human bocavirus 1 is a novel helper for adeno-associated virus replication. J. Virol. https://doi.org/10.1128/JVI.00710-17 (2017).
Zolotukhin, S. et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6, 973–985 (1999).
Auricchio, A., O’Connor, E., Hildinger, M. & Wilson, J. M. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol. Ther. 4, 372–374 (2001).
Grimm, D., Kern, A., Rittner, K. & Kleinschmidt, J. A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum. Gene Ther. 9, 2745–2760 (1998).
Smith, R. H., Levy, J. R. & Kotin, R. M. A simplified baculovirus–AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 17, 1888–1896 (2009).
Arnold, G. S., Sasser, A. K., Stachler, M. D. & Bartlett, J. S. Metabolic biotinylation provides a unique platform for the purification and targeting of multiple AAV vector serotypes. Mol. Ther. 14, 97–106 (2006).
Koerber, J. T., Jang, J. H., Yu, J. H., Kane, R. S. & Schaffer, D. V. Engineering adeno-associated virus for one-step purification via immobilized metal affinity chromatography. Hum. Gene Ther. 18, 367–378 (2007).
Wang, Q. et al. Identification of an adeno-associated virus binding epitope for AVB Sepharose affinity resin. Mol. Ther. Methods Clin. Dev. 2, 15040 (2015).
Qu, G. et al. Separation of adeno-associated virus type 2 empty particles from genome containing vectors by anion-exchange column chromatography. J. Virol. Methods 140, 183–192 (2007).
Mingozzi, F. et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl Med. 5, 194ra192 (2013).
Nass, S. A. et al. Universal method for the purification of recombinant AAV vectors of differing serotypes. Mol. Ther. Methods Clin. Dev. 9, 33–46 (2018).
Matsuzaki, Y. et al. Intravenous administration of the adeno-associated virus-PHP.B capsid fails to upregulate transduction efficiency in the marmoset brain. Neurosci. Lett. 665, 182–188 (2018).
Hordeaux, J. et al. The neurotropic properties of AAV-PHP.B are limited to C57BL/6J Mice. Mol. Ther. 26, 664–668 (2018).
Markusic, D. M. et al. Evaluation of engineered AAV capsids for hepatic factor IX gene transfer in murine and canine models. J. Transl Med. 15, 94 (2017).
Nietupski, J. B. et al. Systemic administration of AAV8–α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol. Ther. 19, 1999–2011 (2011).
Lisowski, L. et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014). This study uses chimeric humanized mice to select novel AAV mutants.
Li, S. et al. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol. Ther. 23, 1867–1876 (2015).
Wang, L. et al. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol. Ther. 23, 1877–1887 (2015).
Vercauteren, K. et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol. Ther. 24, 1042–1049 (2016).
Shao, W. et al. Superior human hepatocyte transduction with adeno-associated virus vector serotype 7. Gene Ther. 26, 504–514 (2019).
Kay, M. A. Selecting the best AAV capsid for human studies. Mol. Ther. 23, 1800–1801 (2015).
Zhang, Y. et al. Human skeletal muscle xenograft as a new preclinical model for muscle disorders. Hum. Mol. Genet. 23, 3180–3188 (2014).
Walsh, N. C. et al. Humanized mouse models of clinical disease. Annu. Rev. Pathol. 12, 187–215 (2017).
Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687 (2018).
Gonzalez-Cordero, A. et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat. Biotechnol. 31, 741–747 (2013).
Santos-Ferreira, T. et al. Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone–rod dystrophy. Invest. Ophthalmol. Vis. Sci. 57, 3509–3520 (2016).
Zaiss, A. K. et al. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 82, 2727–2740 (2008).
Moore, N. A., Morral, N., Ciulla, T. A. & Bracha, P. Gene therapy for inherited retinal and optic nerve degenerations. Expert. Opin. Biol. Ther. 18, 37–49 (2018).
Bainbridge, J. W. et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 372, 1887–1897 (2015). This paper presents findings that led to the first AAV-based drug, Luxturna, being approved by the FDA.
Jacobson, S. G. et al. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 372, 1920–1926 (2015).
Cideciyan, A. V. et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl Acad. Sci. USA 110, E517–E525 (2013).
Perrin, G. Q., Herzog, R. W. & Markusic, D. M. Update on clinical gene therapy for hemophilia. Blood 133, 407–414 (2019).
Miesbach, W. et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 131, 1022–1031 (2018).
Deverman, B. E., Ravina, B. M., Bankiewicz, K. S., Paul, S. M. & Sah, D. W. Y. Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug. Discov. 17, 641–659 (2018).
Chien, Y. H. et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet. Child. Adolesc. Health 1, 265–273 (2017).
Leone, P. et al. Long-term follow-up after gene therapy for canavan disease. Sci. Transl Med. 4, 165ra163 (2012).
Duan, D. & Systemic, A. A. V. Micro-dystrophin gene therapy for duchenne muscular dystrophy. Mol. Ther. 26, 2337–2356 (2018).
Aguti, S., Malerba, A. & Zhou, H. The progress of AAV-mediated gene therapy in neuromuscular disorders. Expert Opin. Biol. Ther. 18, 681–693 (2018).
Ishikawa, K., Weber, T. & Hajjar, R. J. Human cardiac gene therapy. Circ. Res. 123, 601–613 (2018).
Greenberg, B. et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387, 1178–1186 (2016).
Mueller, C. et al. 5 Year expression and neutrophil defect repair after gene therapy in α-1 antitrypsin deficiency. Mol. Ther. 25, 1387–1394 (2017).
D’Avola, D. et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 65, 776–783 (2016).
Lee, C. S. et al. Adenovirus-mediated gene delivery: potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 4, 43–63 (2017).
Zhang, W. W. et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther. 29, 160–179 (2018).
Lowenstein, P. R. & Castro, M. G. Evolutionary basis of a new gene- and immune-therapeutic approach for the treatment of malignant brain tumors: from mice to clinical trials for glioma patients. Clin. Immunol. 189, 43–51 (2018).
Hoggatt, J. Gene therapy for “Bubble Boy” disease. Cell 166, 263 (2016).
Sheridan, C. First approval in sight for Novartis’ CAR-T therapy after panel vote. Nat. Biotechnol. 35, 691–693 (2017).
Chow, V. A., Shadman, M. & Gopal, A. K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 132, 777–781 (2018).
Acknowledgements
The authors thank A. Dobbins for critical reading of the manuscript. This work was supported by National Institutes of Health Grants R01AI117408, R01HL125749, P01HL112761, R01AI072176 and R01HL144661. The authors apologize to any research group that feels their work was overlooked in this Review; we had to be extremely selective owing to space restrictions.
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
R.J.S. is the founder and a shareholder at Asklepios BioPharmaceutical and Bamboo Therapeutics, Inc. He holds patents that have been licensed by University of North Carolina to Asklepios Biopharmaceutical, for which he receives royalties. He has consulted for Baxter Healthcare and has received payment for speaking. C.L. is a cofounder of Bedrock Therapeutics, Inc. He holds patents licensed by University of North Carolina and has received royalties from Bedrock Therapeutics and Asklepios Biopharmaceutical.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FDA approves innovative gene therapy to treat pediatric patients with spinal muscular atrophy: https://www.fda.gov/news-events/press-announcements/fda-approves-innovative-gene-therapy-treat-pediatric-patients-spinal-muscular-atrophy-rare-disease
Pfizer presents initial clinical data on phase 1b gene therapy study for duchenne muscular dystrophy (DMD): https://www.pfizer.com/news/press-release/press-release-detail/pfizer_presents_initial_clinical_data_on_phase_1b_gene_therapy_study_for_duchenne_muscular_dystrophy_dmd
Solid Biosciences announces FDA removes clinical hold on SGT-001: https://investors.solidbio.com/news-releases/news-release-details/solid-biosciences-announces-fda-removes-clinical-hold-sgt-001
Statement by FDA Commissioner Scott Gottlieb, M.D., and Biologics Center Director Peter Marks, M.D., Ph.D. on FDA’s continued efforts to stop stem cell clinics and manufacturers from marketing unapproved products that put patients at risk: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-centre-biologics
Glossary
- Leber congenital amaurosis
-
A rare genetic eye disease caused by the deficiency of various genes.
- Choroideremia
-
A genetic disorder with progressive vision loss due to a deficiency in Rab escort protein-1 (REP-1) owing to mutations in the CHM gene.
- Retinitis pigmentosa
-
A genetic disorder that causes progressive vision loss due to inherited retinal degeneration.
- Leber’s hereditary optic neuropathy
-
An inherited mitochondrial disorder involving the loss of central vision caused by the degeneration of retinal ganglion cells and their axons owing to point mutations in mitochondrial DNA.
- Achromatopsia
-
Autosomal recessive congenital vision loss due to malfunction of the retinal phototransduction pathway.
- X-linked retinoschisis
-
A congenital eye disorder caused by mutations in the gene encoding retinoschisin, which plays a role in intercellular adhesion.
- Innate immune response
-
A general or non-specific defence mechanism that is the first-line defence against infection from viruses, bacteria, parasites and other foreign particles.
- Codon usage bias
-
A bias that results from background substitution biases and natural selection, and refers to the fact that, among species, some codons are more frequently used than other synonymous codons during translation.
- Stargardt disease
-
A common inherited retinal disease due to mutations in ABCA4, the gene encoding ATP-binding cassette transporter (ABCA4).
- Mini-dystrophin
-
A truncated form of dystrophin that retains its function despite deletion of ~75% of the central rod domain (19 of the 24 rods; two of the four hinges) and the distal C-terminal domain (exons 71–78).
- Usher syndrome
-
A genetic disease caused by a deficiency in various genes that results in partial or total hearing and vision loss.
- AAV helper plasmids
-
Plasmids containing adeno-associated virus (AAV) rep and cap genes without inverted terminal repeats.
- Alloantibodies
-
Antibodies produced from B cells after exposure to the individual’s own proteins.
- Major histocompatibility complex (MHC) class I pathway
-
MHC class I molecules are expressed on the cell surface of all nucleated cells. When peptide fragments generated from intracellular proteins bind MHC class I, the MHC class I–peptide complex is transported to the cell surface to induce the production of, and/or be recognized and killed by, cytotoxic T lymphocytes.
- Plasma apheresis
-
A procedure to remove the plasma from blood outside the body and reinfuse it back into patients.
- Adenovirus helper plasmid
-
A plasmid containing most adenovirus genes that helps the production of adeno-associated virus (AAV) Rep and AAV replication.
- Stable HeLa cell line–adenovirus method
-
A HeLa cell line contains adeno-associated virus (AAV) rep and cap genes, with or without integration of the AAV vector genome. When transduced with wild-type adenovirus and AAV vector, AAV Rep and Cap will be produced and the AAV vector genome will replicate and be packaged to produce a large amount of AAV vector.
- Herpesvirus helper method
-
Recombinant herpesvirus vectors (one contains adeno-associated virus (AAV) rep and cap, another contains AAV vector genome) are used to deliver the rep and cap genes, as well as the AAV vector genome, into HeLa cells for AAV vector production. Helper genes for helping AAV Rep and Cap production are provided by the herpes simplex virus genome.
- Kozak sequence
-
A sequence with the consensus ACCAUGG and a critical role in translation initiation.
- Leaky ribosomal scanning
-
A mechanism for regulating gene expression during the initiation phase of eukaryotic translation, in which a suboptimal translational initiation codon on mRNA is skipped by the small 40S ribosome subunit in translation initiation.
- Complement activation
-
Complement is a system made up of plasma proteins that can be activated by a pathogen or the antigen–antibody complex. Complement activation enhances the ability of antibodies or phagocytic cells to clear invading microorganisms or damaged cells.
Rights and permissions
About this article
Cite this article
Li, C., Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 21, 255–272 (2020). https://doi.org/10.1038/s41576-019-0205-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-019-0205-4
This article is cited by
-
Enhancing prime editor flexibility with coiled-coil heterodimers
Genome Biology (2024)
-
Advanced biomanufacturing and evaluation of adeno-associated virus
Journal of Biological Engineering (2024)
-
Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma
Virology Journal (2024)
-
Administration of an AAV vector coding for a P2X7-blocking nanobody-based biologic ameliorates colitis in mice
Journal of Nanobiotechnology (2024)
-
Nano-modified viruses prime the tumor microenvironment and promote the photodynamic virotherapy in liver cancer
Journal of Biomedical Science (2024)