Abstract
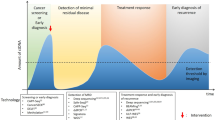
Precision oncology seeks to leverage molecular information about cancer to improve patient outcomes. Tissue biopsy samples are widely used to characterize tumours but are limited by constraints on sampling frequency and their incomplete representation of the entire tumour bulk. Now, attention is turning to minimally invasive liquid biopsies, which enable analysis of tumour components (including circulating tumour cells and circulating tumour DNA) in bodily fluids such as blood. The potential of liquid biopsies is highlighted by studies that show they can track the evolutionary dynamics and heterogeneity of tumours and can detect very early emergence of therapy resistance, residual disease and recurrence. However, the analytical validity and clinical utility of liquid biopsies must be rigorously demonstrated before this potential can be realized.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ashley, E. A. Towards precision medicine. Nat. Rev. Genet. 17, 507–522 (2016).
Kumar-Sinha, C. & Chinnaiyan, A. M. Precision oncology in the age of integrative genomics. Nat. Biotechnol. 36, 46–60 (2018).
Moscow, J. A., Fojo, T. & Schilsky, R. L. The evidence framework for precision cancer medicine. Nat. Rev. Clin. Oncol. 15, 183–192 (2018).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Cancer Genome Atlas Research Network. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 45, 1113–1120 (2013).
Cohen, J. D. et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl Acad. Sci. USA 114, 10202–10207 (2017).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018). This paper shows that a blood test can detect eight common cancer types through assessment of the levels of circulating proteins and mutations in cell-free DNA with a median diagnostic sensitivity of 70%.
Chan, K. C. A. et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N. Engl. J. Med. 377, 513–522 (2017). This prospective study investigates the use of EBV DNA in plasma samples to screen for early nasopharyngeal carcinoma in asymptomatic persons and achieves a sensitivity and specificity of 97.1% and 98.6%, respectively.
Pantel, K. & Alix-Panabieres, C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol. Med. 16, 398–406 (2010).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Heitzer, E., Ulz, P. & Geigl, J. B. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 61, 112–123 (2015).
Alix-Panabieres, C. & Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14, 623–631 (2014).
Alix-Panabieres, C. & Pantel, K. Clinical applications of circulating tumor cells and circulating tumor dna as liquid biopsy. Cancer Discov. 6, 479–491 (2016).
Bardelli, A. & Pantel, K. Liquid biopsies, what we do not know (yet). Cancer Cell 31, 172–179 (2017).
Amorim, M. G. et al. A total transcriptome profiling method for plasma-derived extracellular vesicles: applications for liquid biopsies. Sci. Rep. 7, 14395 (2017).
Chan, K. C. et al. Noninvasive detection of cancer-associated genome-wide hypomethylation and copy number aberrations by plasma DNA bisulfite sequencing. Proc. Natl Acad. Sci. USA 110, 18761–18768 (2013).
Kim, Y. et al. Targeted proteomics identifies liquid-biopsy signatures for extracapsular prostate cancer. Nat. Commun. 7, 11906 (2016).
Mayers, J. R. et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198 (2014).
Ko, J. et al. Machine learning to detect signatures of disease in liquid biopsies — a user’s guide. Lab. Chip 18, 395–405 (2018).
Teutsch, S. M. et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet. Med. 11, 3–14 (2009).
Hayes, D. F. Biomarker validation and testing. Mol. Oncol. 9, 960–966 (2015).
Hayes, D. F. Precision medicine and testing for tumor biomarkers — are all tests born equal? JAMA Oncol. 4, 773–774 (2017).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Cristofanilli, M. et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 23, 1420–1430 (2005).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 8, 329–340 (2008).
Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl Cancer Inst. 110, 560–567 (2018).
Bidard, F. C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414 (2014).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Antonarakis, E. S. et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 35, 2149–2156 (2017).
Lorente, D. et al. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur. Urol. 70, 985–992 (2016).
Heller, G. et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 36, 572–580 (2018).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Huang, X. et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 15, 202 (2015).
Riethdorf, S., O’Flaherty, L., Hille, C. & Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug. Deliv. Rev. 125, 102–121 (2018).
Sacher, A. G. et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2, 1014–1022 (2016).
Leighl, N. B. et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Guideline. J. Clin. Oncol. 32, 3673–3679 (2014).
Warren, J. D. et al. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 9, 133 (2011).
Song, L., Jia, J., Peng, X., Xiao, W. & Li, Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: a meta-analysis. Sci. Rep. 7, 3032 (2017).
Nian, J. et al. Diagnostic accuracy of methylated SEPT9 for blood-based colorectal cancer detection: a systematic review and meta-analysis. Clin. Transl Gastroenterol. 8, e216 (2017).
Wu, Y. L. et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 26, 1883–1889 (2015).
Lindeman, N. I. et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 142, 321–346 (2018).
Snyder, M. W., Kircher, M., Hill, A. J., Daza, R. M. & Shendure, J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell 164, 57–68 (2016).
Ulz, P. et al. Inferring expressed genes by whole-genome sequencing of plasma DNA. Nat. Genet. 48, 1273–1278 (2016). This paper reports the use of machine learning for gene classification based on differential read depth patterns at transcription start sites, which reflect nucleosome occupancy patterns.
De Mattos-Arruda, L. et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
De Mattos-Arruda, L. et al. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: a proof-of-principle. Ann. Oncol. 25, 1729–1735 (2014).
Jamal-Hanjani, M. et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann. Oncol. 27, 862–867 (2016).
Murtaza, M. et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat. Commun. 6, 8760 (2015).
Kuo, Y. B., Chen, J. S., Fan, C. W., Li, Y. S. & Chan, E. C. Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer. Clin. Chim. Acta 433, 284–289 (2014).
Oxnard, G. R. et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J. Clin. Oncol. 34, 3375–3382 (2016).
Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 21, 795 (2015).
Ulz, P. et al. Whole-genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat. Commun. 7, 12008 (2016).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl Med. 6, 224ra224 (2014). This study evaluates 640 patients with different cancer types and reports a high variability of ctDNA in patients with the same tumour entities and disease stages.
Carreira, S. et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl Med. 6, 254ra125 (2014).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014). This study demonstrates that the combination of UMIs with highly sophisticated bioinformatics algorithms can substantially improve the sensitivity of sequencing-based approaches.
Scherer, F. et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. 8, 364ra155 (2016).
Yu, M. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345, 216–220 (2014).
Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545, 446–451 (2017). This study uses a tumour-specific phylogenetic approach to track the subclonal nature of lung cancer relapse and metastasis. In addition, independent predictors of ctDNA release were identified and correlations of the tumour volume and ctDNA allele frequencies were described.
Parkinson, C. A. et al. Exploratory analysis of TP53 mutations in circulating tumour DNA as biomarkers of treatment response for patients with relapsed high-grade serous ovarian carcinoma: a retrospective study. PLOS Med. 13, e1002198 (2016). This study demonstrates that TP53 mutations can be used as personalized markers to monitor tumour burden and early changes as a predictor of response and time to progression in patients with HGSOC.
Chaudhuri, A. A. et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 12, 1394–1403 (2017).
Tie, J. et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 26, 1715–1722 (2015).
Tie, J. et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl Med. 8, 346ra392 (2016).
Olsson, E. et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol. Med. 7, 1034–1047 (2015).
Diaz, L. A. Jr. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Misale, S., Di Nicolantonio, F., Sartore-Bianchi, A., Siena, S. & Bardelli, A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 4, 1269–1280 (2014).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–U131 (2012).
Mohan, S. et al. Changes in colorectal carcinoma genomes under anti-EGFR therapy identified by whole-genome plasma DNA sequencing. PLOS Genet. 10, e1004271 (2014).
Beddowes, E., Sammut, S. J., Gao, M. & Caldas, C. Predicting treatment resistance and relapse through circulating DNA. Breast 34, S31–S35 (2017).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Del Monte, U. Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle 8, 505–506 (2009).
Heidary, M. et al. The dynamic range of circulating tumor DNA in metastatic breast cancer. Breast Cancer Res. 16, 421 (2014).
Heitzer, E. et al. Establishment of tumor-specific copy number alterations from plasma DNA of patients with cancer. Int. J. Cancer 133, 346–356 (2013).
Newman, A. M. et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 34, 547–555 (2016).
Park, G. et al. Characterization of background noise in capture-based targeted sequencing data. Genome Biol. 18, 136 (2017).
Kinde, I., Wu, J., Papadopoulos, N., Kinzler, K. W. & Vogelstein, B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl Acad. Sci. USA 108, 9530–9535 (2011).
Forshew, T. et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl Med. 4, 136ra168 (2012).
Schmitt, M. W. et al. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl Acad. Sci. USA 109, 14508–14513 (2012).
Phallen, J. et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl Med. 9, eaan2415 (2017). In this study, high-resolution gene panel profiling (TEC-Seq) is employed for non-invasive detection of early-stage tumours in 200 patients with stage I or II colorectal, breast, lung or ovarian cancer.
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in unique molecular identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Jiang, P. et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc. Natl Acad. Sci. USA 112, E1317–E1325 (2015).
Underhill, H. R. et al. Fragment length of circulating tumor DNA. PLOS Genet. 12, e1006162 (2016).
Jiang, P. Y. & Lo, Y. M. D. The long and short of circulating cell-free DNA and the ins and outs of molecular diagnostics. Trends Genet. 32, 360–371 (2016).
Lo, Y. M. D. et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl Med. 2, 61ra91 (2010).
Moser, T. et al. Single-stranded DNA library preparation does not preferentially enrich circulating tumor DNA. Clin. Chem. 63, 1656–1659 (2017).
Vong, J. S. L. et al. Single-stranded DNA library preparation preferentially enriches short maternal DNA in maternal plasma. Clin. Chem. 63, 1031–1037 (2017).
Sun, K. et al. Size-tagged preferred ends in maternal plasma DNA shed light on the production mechanism and show utility in noninvasive prenatal testing. Proc. Natl Acad. Sci. USA 115, E5106–E5114 (2018). The authors investigate whether preferred end sites might bear any relationship with fragment lengths of plasma DNA. Short and long plasma DNA molecules were associated with different preferred DNA end sites.
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Tomasetti, C. & Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015).
Adams, P. D., Jasper, H. & Rudolph, K. L. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell 16, 601–612 (2015).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Xie, M. C. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Ptashkin, R. N. et al. Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2018.2297(2018). This study demonstrates how clonal haematopoiesis-derived mutations could lead to erroneous reporting and treatment recommendations when tumour-only sequencing is used.
Coombs, C. C. et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-1201 (2018).
Hu, Y. et al. False positive plasma genotyping due to clonal hematopoiesis. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-0143 (2018).
Jacobs, K. B. et al. Detectable clonal mosaicism and its relationship to aging and cancer. Nat. Genet. 44, 651–668 (2012).
O’Huallachain, M., Karczewski, K. J., Weissman, S. M., Urban, A. E. & Snyder, M. P. Extensive genetic variation in somatic human tissues. Proc. Natl Acad. Sci. USA 109, 18018–18023 (2012).
Behjati, S. et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 513, 422–425 (2014).
Krimmel, J. D. et al. Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous tissues. Proc. Natl Acad. Sci. USA 113, 6005–6010 (2016).
Fernandez-Cuesta, L. et al. Identification of circulating tumor DNA for the early detection of small-cell lung cancer. EBioMedicine 10, 117–123 (2016).
Odegaard, J. I. et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin. Cancer Res. 24, 3539–3549 (2018).
Torga, G. & Pienta, K. J. Patient-paired sample congruence between 2 commercial liquid biopsy tests. JAMA Oncol. 4, 868–870 (2017).
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J. Clin. Oncol. 16, 1631–1641 (2018).
Grossman, R. L. et al. Collaborating to compete: Blood Profiling Atlas in Cancer (BloodPAC) Consortium. Clin. Pharmacol. Ther. 101, 589–592 (2017).
Inamdar, S., Nitiyanandan, R. & Rege, K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng. Transl Med. 2, 70–80 (2017).
Best, M. G. et al. Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell 32, 238–252 (2017).
Best, M. G. et al. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 28, 666–676 (2015).
Mayers, J. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Hao, X. et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc. Natl Acad. Sci. USA 114, 7414–7419 (2017).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome — biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
Warton, K., Mahon, K. L. & Samimi, G. Methylated circulating tumor DNA in blood: power in cancer prognosis and response. Endocr. Relat. Cancer 23, R157–R171 (2016).
Widschwendter, M. et al. Methylation patterns in serum DNA for early identification of disseminated breast cancer. Genome Med. 9, 115 (2017).
Widschwendter, M. et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med. 9, 116 (2017).
Li, M. et al. Sensitive digital quantification of DNA methylation in clinical samples. Nat. Biotechnol. 27, 858–863 (2009).
Guo, S. et al. Identification of methylation haplotype blocks aids in deconvolution of heterogeneous tissue samples and tumor tissue-of-origin mapping from plasma DNA. Nat. Genet. 49, 635–642 (2017).
Lehmann-Werman, R. et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc. Natl Acad. Sci. USA 113, E1826–E1834 (2016).
Pixberg, C. F., Schulz, W. A., Stoecklein, N. H. & Neves, R. P. Characterization of DNA methylation in circulating tumor cells. Genes 6, 1053–1075 (2015).
Mastoraki, S. et al. ESR1 methylation: a liquid biopsy-based epigenetic assay for the follow-up of patients with metastatic breast cancer receiving endocrine treatment. Clin. Cancer Res. 24, 1500–1510 (2018).
Kelsey, G., Stegle, O. & Reik, W. Single-cell epigenomics: recording the past and predicting the future. Science 358, 69–75 (2017).
Koh, W. et al. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl Acad. Sci. USA 111, 7361–7366 (2014).
Tsui, N. B. et al. Maternal plasma RNA sequencing for genome-wide transcriptomic profiling and identification of pregnancy-associated transcripts. Clin. Chem. 60, 954–962 (2014).
Silva, J. M. et al. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut 50, 530–534 (2002).
Collado, M. et al. Genomic profiling of circulating plasma RNA for the analysis of cancer. Clin. Chem. 53, 1860–1863 (2007).
Chen, X. et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 18, 997–1006 (2008).
Montani, F. & Bianchi, F. Circulating cancer biomarkers: the macro-revolution of the micro-RNA. EBioMedicine 5, 4–6 (2016).
Ng, E. K. O. et al. mRNA of placental origin is readily detectable in maternal plasma. Proc. Natl Acad. Sci. USA 100, 4748–4753 (2003).
Montani, F. et al. miR-Test: a blood test for lung cancer early detection. J. Natl. Cancer Inst. 107, djv063 (2015).
Sozzi, G. et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J. Clin. Oncol. 32, 768–773 (2014).
Cortez, M. A. et al. MicroRNAs in body fluids-the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 8, 467–477 (2011).
Schwarzenbach, H., da Silva, A. M., Calin, G. & Pantel, K. Data normalization strategies for microRNA quantification. Clin. Chem. 61, 1333–1342 (2015).
Schwarzenbach, H., Nishida, N., Calin, G. A. & Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 11, 145–156 (2014).
Maas, S. L., Breakefield, X. O. & Weaver, A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell. Biol. 27, 172–188 (2017).
Vlassov, A. V., Magdaleno, S., Setterquist, R. & Conrad, R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 1820, 940–948 (2012).
Yuan, T. Z. et al. Plasma extracellular RNA profiles in healthy and cancer patients. Sci. Rep. 6, 19413 (2016).
Khan, S. et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLOS ONE 7, e46737 (2012).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015).
Rupp, A. K. et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol. Oncol. 122, 437–446 (2011).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Thakur, B. K. et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 24, 766–769 (2014).
Allenson, K. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 28, 741–747 (2017).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Invest. 126, 1208–1215 (2016).
Im, H. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 32, 490–495 (2014).
Abels, E. R. & Breakefield, X. O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 36, 301–312 (2016).
Sharma, S. Tumor markers in clinical practice: general principles and guidelines. Indian J. Med. Paediatr. Oncol. 30, 1–8 (2009).
Attard, G. et al. Prostate cancer. Lancet 387, 70–82 (2016).
Aziz, D. C. Clinical use of tumor markers based on outcome analysis. Lab. Med. 27, 817–821 (1996).
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 (2016).
Crutchfield, C. A., Thomas, S. N., Sokoll, L. J. & Chan, D. W. Advances in mass spectrometry-based clinical biomarker discovery. Clin. Proteom. 13, 1 (2016).
Zahn, H. et al. Scalable whole-genome single-cell library preparation without preamplification. Nat. Methods 14, 167–173 (2017).
Vitak, S. A. et al. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat. Methods 14, 302–308 (2017).
Kalinich, M. et al. An RNA-based signature enables high specificity detection of circulating tumor cells in hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 114, 1123–1128 (2017).
Alix-Panabieres, C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 195, 69–76 (2012).
Clark, S. J., Lee, H. J., Smallwood, S. A., Kelsey, G. & Reik, W. Single-cell epigenomics: powerful new methods for understanding gene regulation and cell identity. Genome Biol. 17, 72 (2016).
Alix-Panabieres, C. & Pantel, K. Liquid biopsy in cancer patients: advances in capturing viable CTCs for functional studies using the EPISPOT assay. Expert Rev. Mol. Diagn. 15, 1411–1417 (2015).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Hodgkinson, C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20, 897–903 (2014).
Cayrefourcq, L. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 75, 892–901 (2015).
Heitzer, E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013).
Malaney, P., Nicosia, S. V. & Dave, V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 344, 1–12 (2014).
Lallo, A., Schenk, M. W., Frese, K. K., Blackhall, F. & Dive, C. Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl Lung Cancer Res. 6, 397–408 (2017).
Chicard, M. et al. Genomic copy number profiling using circulating free tumor DNA highlights heterogeneity in neuroblastoma. Clin. Cancer Res. 22, 5564–5573 (2016).
Chim, S. S. C. et al. Detection and characterization of placental MicroRNAs in maternal plasma. Clin. Chem. 54, 482–490 (2008).
Wang, K. et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc. Natl Acad. Sci. USA 106, 4402–4407 (2009).
Sun, K. et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc. Natl Acad. Sci. USA 112, E5503–E5512 (2015). In this study, the tissue of origin of circulating cell-free DNA is tracked using methylation deconvolution in different cohorts.
Wong, F. C. et al. Cell-free DNA in maternal plasma and serum: a comparison of quantity, quality and tissue origin using genomic and epigenomic approaches. Clin. Biochem. 49, 1379–1386 (2016).
Lam, W. K. J. et al. DNA of erythroid origin is present in human plasma and informs the types of anemia. Clin. Chem. 63, 1614–1623 (2017).
Lui, Y. N. Y. N. et al. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin. Chem. 48, 421–427 (2002).
Wu, D. C. & Lambowitz, A. M. Facile single-stranded DNA sequencing of human plasma DNA via thermostable group II intron reverse transcriptase template switching. Sci. Rep. 7, 8421 (2017).
Mohme, M., Riethdorf, S. & Pantel, K. Circulating and disseminated tumour cells — mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 14, 155–167 (2017).
Mazel, M. et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 9, 1773–1782 (2015).
Ricklefs, F. L. et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018).
Gibney, G. T., Weiner, L. M. & Atkins, M. B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 17, E542–E551 (2016).
Khagi, Y. et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor-based immunotherapy. Clin. Cancer Res. 23, 5729–5736 (2017).
Kim, S. T. et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. https://doi.org/10.1038/s41591-018-0101-z (2018).
Gandara, D. R. et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 24, 1441–1448 (2018).
Sefrioui, D. et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer 117, 1017–1025 (2017).
Kalinich, M. & Haber, D. A. Cancer detection: Seeking signals in blood. Science 359, 866–867 (2018).
Springer, S. U. et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife 7, e32143 (2018).
Wang, Y. et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl Med. 10, eaap8793 (2018).
Chen, C. L. et al. Deep learning in label-free cell classification. Sci. Rep. 6, 21471 (2016).
Elias, K. M. et al. Diagnostic potential for a serum miRNA neural network for detection of ovarian cancer. eLife 6, e28932 (2017).
Yuan, Y. et al. DeepGene: an advanced cancer type classifier based on deep learning and somatic point mutations. BMC Bioinformatics 17, 476 (2016).
Kang, S. L. et al. CancerLocator: non-invasive cancer diagnosis and tissue-of-origin prediction using methylation profiles of cell-free DNA. Genome Biol. 18, 53 (2017). This study exploits the diagnostic potential of genome-wide DNA methylation data from cfDNA to determine not only the presence but also the location of tumours. CancerLocator simultaneously infers the proportions and the tissue of origin of tumour-derived cell-free DNA in a blood sample.
Mohassel, P. SecureML: a system for scalable privacy-preserving machine learning. IEEE https://doi.org/10.1109/SP.2017.12 (2017).
Acknowledgements
The authors thank P. Ulz for his assistance in drafting the figures for this article and S. Perakis for her assistance in revising the text. The work in the authors’ laboratory is supported by CANCER-ID, a project funded by the Innovative Medicines Joint Undertaking; the Austrian National Bank (grant 16917); the Austrian Science Fund (grant P28949-B28); the BioTechMed-Graz flagship project ‘EPIAge’; and the Christian Doppler Research Fund for Liquid Biopsies for Early Detection of Cancer.
Reviewer information
Nature Reviews Genetics thanks P. Hofman and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
E.H. and M.R.S. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission. I.S.H. and C.E.S.R. wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
I.S.H. and C.E.S.R. are employees of Freenome. Freenome is the industrial partner of the Christian Doppler Research Fund for Liquid Biopsies for Early Detection of Cancer, which is headed by E.H. The authors declare that no other competing interests exist.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BloodPAC: www.bloodpac.org
Blueprint Epigenome consortium: www.blueprint-epigenome.eu/
Cancer-ID: www.cancer-id.eu
CEN/TS 16835-3:2015: https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT:41040&cs=171988FF551BF281CD5E65F5D59C82961
International Human Epigenome Consortium (IHEC): http://ihec-epigenomes.org/
Standardization of generic pre-analytical procedures for in vitro diagnostics for personalized medicine (SPIDIA4P): http://www.spidia.eu/about-the-projects/
The European Committee for Standardization (CEN): https://standards.cen.eu
Glossary
- Precision oncology
-
Molecular profiling of a tumour with the aim to detect somatic alterations that can be targeted for therapy.
- Next-generation sequencing
-
(NGS). A high-throughput method used to determine the nucleotide sequence of DNA or RNA.
- The Cancer Genome Atlas
-
(TCGA). A comprehensive and coordinated effort to accelerate our understanding of the molecular basis of cancer through the application of genome analysis technologies.
- Epigenetic
-
A biochemical change in the genome, such as DNA methylation or histone modification, that does not alter the DNA sequence but may affect gene activity and expression.
- Druggable targets
-
Somatic mutations involved in cancer development and progression that can be exploited with a therapeutic intent.
- Pleural effusions
-
Excessive accumulations of fluid in the space surrounding the lung (pleural cavity).
- Circulating tumour cells
-
(CTCs). Cells that have been shed into the vasculature or lymphatics from a primary tumour and/or metastasis and are carried around the body in the blood circulation.
- Circulating cell-free DNA
-
(cfDNA). DNA circulating in the bloodstream that is not associated with cells.
- Circulating tumour DNA
-
(ctDNA). Tumour-derived, cell-free DNA that is thought to be representative of the entire tumour genome.
- Circulating cell-free RNA
-
(cfRNA). Circulating gene transcripts (mRNA and non-coding RNAs) that are partly protected from degradation by their packaging into exosomes.
- Extracellular vesicles
-
(EVs). Generic term for vesicles, including exosomes, microvesicles or apoptotic bodies, that are secreted from all cells and carry complex cargoes such as proteins, lipids and nucleic acids across biological membranes.
- Exosomes
-
Cell-derived vesicles likely present in all body fluids, which contain nucleic acids, lipids and metabolites and are involved in intercellular signalling and communication.
- Tumour-educated platelets
-
(TEPs). Platelets with altered functions that interact with tumour cells via different signalling molecules, thereby promoting tumour cell survival and metastasis.
- Copy number alterations
-
Loss (deletion) or gain (ranging from duplication to high-level amplification) of genomic regions resulting in a copy number that deviates from two.
- Transcriptome
-
The full range of mRNA molecules expressed in a cell, tissue or organism at a certain time.
- Epigenome
-
The full complement of epigenetic marks within a genome, which helps to determine the activity of genes in any particular cell and its lineage. The epigenome is prone to change during ageing and in cancer cells.
- Proteome
-
The entire set of proteins expressed in a cell, tissue or organism at a certain time.
- Metabolome
-
The complete set of small-molecule chemicals found within a cell, tissue or organism at a certain time.
- Laboratory-developed tests
-
(LDTs). According to US Food and Drug Administration regulations, these tests are in vitro diagnostic tests designed, manufactured and used within a single laboratory.
- Epithelial cell adhesion molecules
-
(EpCAMs). Transmembrane glycoproteins that are expressed solely in epithelia and epithelial-derived cancer and are commonly used as a diagnostic marker.
- Variant allele frequency
-
(VAF). The frequency of a particular allele of a gene relative to all other alleles in a DNA sample.
- Metabolic tumour volume
-
The proportion of a tumour, measured by volume, that is hypermetabolic. Often measured by positron emission tomography.
- Minimal residual disease
-
Cancer cells that remain in the patient’s body during and after treatment, frequently escape detection by routine diagnostic procedures and are critically involved in relapse.
- Genome equivalents
-
(GE). The amount of DNA that corresponds to the diploid genome of a single cell.
- Sequencing sensitivity
-
The analytical sensitivity of a sequencing method; that is, the ability to detect a low concentration of a particular sequence in a biological sample. The sequencing sensitivity of an approach determines its ability to accurately measure the variant allele frequency of a variant.
- Molecular barcodes
-
Degenerate sequence tags consisting of random or specified bases to label DNA fragments in order to track individual molecules after PCR; they are also referred to as unique molecular identifiers.
- Cancer driver gene
-
A gene that, when mutated, is essential for cancer to develop and progress and that is under positive selection during tumour evolution.
- Clonal expansion
-
A process in which acquisition of somatic mutations drives the production of daughter cells all arising from a single cell.
- Methylomes
-
All nucleic acid methylation modifications in the genomes of cells.
- Bisulfite sequencing
-
A method for detecting DNA methylation patterns. DNA is treated with bisulfite, which converts cytosine but not 5-methylcytosine to uracil, enabling methylated and unmethylated cytosines to be discriminated from sequencing data.
- Classifier
-
A means to group data into categories on the basis of certain characteristics, such as inherent similarity.
- Tumour mutational burden
-
(TMB). A biomarker that measures the number of mutations present in a tumour of a patient with cancer. Usually, given as the number of coding somatic mutations per megabase of DNA.
- Diagnostic sensitivity
-
The likelihood that a diagnostic test will be positive when testing a person with the disease.
- Diagnostic specificity
-
The likelihood that a diagnostic test will be negative when testing a person without the disease.
- Positive predictive value
-
(PPV). The proportion of positive results in diagnostic tests. PPV depends on the sensitivity and specificity of the test and on the prevalence of the disease within the general population.
- Deconvolution
-
The process of extracting cell type-specific information from heterogeneous samples. In liquid biopsies, this might involve resolving plasma DNA fragments into their constituent elements (for example, determining their tissue of origin on the basis of methylation markers).
- Hidden layers
-
The intermediate layers of information generated when an artificial neural network breaks down and processes input data to generate the output.
Rights and permissions
About this article
Cite this article
Heitzer, E., Haque, I.S., Roberts, C.E.S. et al. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 20, 71–88 (2019). https://doi.org/10.1038/s41576-018-0071-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-018-0071-5
This article is cited by
-
Clinical applications and perspectives of circulating tumor DNA in gastric cancer
Cancer Cell International (2024)
-
Circulating cell-free DNA as a biomarker for diagnosis of Schistosomiasis japonica
Parasites & Vectors (2024)
-
Soluble Periostin is a potential surveillance biomarker for early and long-term response to chemotherapy in advanced breast cancer
Cancer Cell International (2024)
-
Incidental pathogenic germline alterations detected through liquid biopsy in patients with solid tumors: prevalence, clinical utility and implications
British Journal of Cancer (2024)
-
NMR and MS reveal characteristic metabolome atlas and optimize esophageal squamous cell carcinoma early detection
Nature Communications (2024)