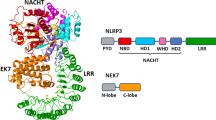
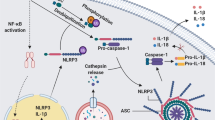
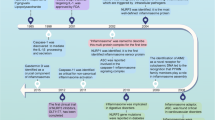
Abstract
Diseases associated with chronic inflammation constitute a major health burden across the world. As central instigators of the inflammatory response to infection and tissue damage, inflammasomes — and the NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome in particular — have emerged as key regulators in diverse rheumatic, metabolic and neurodegenerative diseases. Similarly to other inflammasome sensors, NLRP3 assembles a cytosolic innate immune complex that activates the cysteine protease caspase-1, which in turn cleaves gasdermin D (GSDMD) to induce pyroptosis, a regulated mode of lytic cell death. Pyroptosis is highly inflammatory, partly because of the concomitant extracellular release of the inflammasome-dependent cytokines IL-1β and IL-18 along with a myriad of additional danger signals and intracellular antigens. Here, we discuss how NLRP3 and downstream inflammasome effectors such as GSDMD, apoptosis-associated speck-like protein containing a CARD (ASC) and nerve injury-induced protein 1 (NINJ1) have gained significant traction as therapeutic targets. We highlight the recent progress in developing small-molecule and biologic inhibitors that are advancing into the clinic and serving to harness the broad therapeutic potential of modulating the NLRP3 inflammasome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Newton, K. & Dixit, V. M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 4, a006049 (2012).
Meissner, F., Scheltema, R. A., Mollenkopf, H. J. & Mann, M. Direct proteomic quantification of the secretome of activated immune cells. Science 340, 475–478 (2013).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Paerewijck, O. & Lamkanfi, M. The human inflammasomes. Mol. Asp. Med. 88, 101100 (2022).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). This report describes the identification of GSDMD as an essential effector of pyroptosis and IL-1β release.
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). This report describes the identification of GSDMD as an essential effector of pyroptosis and IL-1β release.
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 25, 1285–1298 (2015).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Sborgi, L. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778 (2016).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021). This paper demonstrates that pyroptotic cell lysis is a controlled event regulated by NINJ1.
Nadkarni, R. et al. Viral proteases activate the CARD8 inflammasome in the human cardiovascular system. J. Exp. Med. 219, e0212117 (2022).
Su, S. et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages. Cell 175, 442–457 e423 (2018).
Kumari, P., Russo, A. J., Shivcharan, S. & Rathinam, V. A. AIM2 in health and disease: inflammasome and beyond. Immunol. Rev. 297, 83–95 (2020).
Fidler, T. P. et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592, 296–301 (2021).
Zhou, J. Y. et al. Activation of the NLRP1 inflammasome in human keratinocytes by the dsDNA mimetic poly(dA:dT). Proc. Natl Acad. Sci. USA 120, e2213777120 (2023).
Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell 171, 1110–1124 e1118 (2017).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Gross, C. J. et al. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45, 761–773 (2016).
Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–846 (2016).
Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
McKee, C. M. & Coll, R. C. NLRP3 inflammasome priming: a riddle wrapped in a mystery inside an enigma. J. Leukoc. Biol. 108, 937–952 (2020).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Nunez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258 (2016).
Schmid-Burgk, J. L. et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J. Biol. Chem. 291, 103–109 (2016).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113, 2324–2335 (2009).
O’Brien, M. et al. A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. J. Immunol. methods 447, 1–13 (2017).
Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Andreeva, L. et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 184, 6299–6312 e6222 (2021).
Dekker, C. et al. Crystal structure of NLRP3 NACHT domain with an inhibitor defines mechanism of inflammasome inhibition. J. Mol. Biol. 433, 167309 (2021).
Hochheiser, I. V. et al. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 604, 184–189 (2022). Report of a cryo-EM structure of full-length human NLRP3 bound to an NLRP3 inhibitor.
Ohto, U. et al. Structural basis for the oligomerization-mediated regulation of NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA 119, e2121353119 (2022).
Xiao, L., Magupalli, V. G. & Wu, H. Cryo-EM structures of the active NLRP3 inflammasome disk. Nature 613, 595–600 (2022). Report of a cryo-EM structure of the active NLRP3 inflammasome complex.
Hochheiser, I. V. et al. Directionality of PYD filament growth determined by the transition of NLRP3 nucleation seeds to ASC elongation. Sci. Adv. 8, eabn7583 (2022).
Brinkschulte, R. et al. ATP-binding and hydrolysis of human NLRP3. Commun. Biol. 5, 1176 (2022).
Hu, Z. et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013).
Zhang, L. et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science 350, 404–409 (2015).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Akbal, A. et al. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol. Immunol. 19, 1201–1214 (2022).
Chen, J. & Chen, Z. J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76 (2018).
Daussy, C. F. et al. The inflammasome components NLRP3 and ASC act in concert with IRGM to rearrange the Golgi apparatus during hepatitis C virus infection. J. Virol. 95, e00826–e00920 (2021).
Gao, W., Yang, J., Liu, W., Wang, Y. & Shao, F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl Acad. Sci. USA 113, E4857–E4866 (2016).
Van Gorp, H. et al. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc. Natl Acad. Sci. USA 113, 14384–14389 (2016).
Zhang, Z. et al. Distinct changes in endosomal composition promote NLRP3 inflammasome activation. Nat. Immunol. 24, 30–41 (2022).
Lee, B. et al. Disruptions in endocytic traffic contribute to the activation of the NLRP3 inflammasome. Sci. Signal. 16, eabm7134 (2023).
Mangan, M. S. J. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug. Discov. 17, 588–606 (2018).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Wree, A. et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 92, 1069–1082 (2014).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Gordon, R. et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10, eaah4066 (2018).
Gris, D. et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 (2010).
Zeng, J. et al. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine 75, 103803 (2022).
Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Van Gorp, H. & Lamkanfi, M. The emerging roles of inflammasome-dependent cytokines in cancer development. EMBO Rep. 20, e47575 (2019).
Van Gijn, M. E. et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J. Med. Genet. 55, 530–537 (2018).
Hamon, Y. et al. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90, 2911–2915 (1997).
Laliberte, R. E. et al. Glutathione S-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing. J. Biol. Chem. 278, 16567–16578 (2003).
Perregaux, D. G. et al. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197 (2001).
Lamkanfi, M. et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009). This paper demonstrates inhibition of the NLRP3 inflammasome by small-molecule inhibitors.
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015). This important paper identifies CRID3 as a potent and selective NLRP3 inhibitor.
Tapia-Abellan, A. et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 15, 560–564 (2019).
Coll, R. C. et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15, 556–559 (2019).
Vande Walle, L. et al. MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 17, e3000354 (2019).
Weber, A. N. R. et al. Effective ex vivo inhibition of cryopyrin-associated periodic syndrome (CAPS)-associated mutant NLRP3 inflammasome by MCC950/CRID3. Rheumatology 61, e299–e313 (2022).
Shah, F. et al. Setting clinical exposure levels of concern for drug-induced liver injury (DILI) using mechanistic in vitro assays. Toxicol. Sci. 147, 500–514 (2015).
Kennedy, C. R. et al. A probe for NLRP3 inflammasome inhibitor MCC950 identifies carbonic anhydrase 2 as a novel target. ACS Chem. Biol. 16, 982–990 (2021).
Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug. Discov. 7, 168–181 (2008).
Parmar, D. V. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral NLRP3 inflammasome inhibitor ZYIL1: first-in-human phase 1 studies (single ascending dose and multiple ascending dose). Clin. Pharmacol. Drug. Dev. 12, 202–211 (2023).
Mullard, A. Roche snaps up another NLRP3 contender. Nat. Rev. Drug. Discov. 19, 744 (2020).
Madurka, I. et al. DFV890: a new oral NLRP3 inhibitor-tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection 51, 641–654 (2022).
Ventyx Biosciences, Inc. Ventyx Biosciences announces positive topline phase 1 data for its peripheral NLRP3 inhibitor VTX2735, https://ir.ventyxbio.com/node/7071/html (2022).
Schwaid, A. G. & Spencer, K. B. Strategies for targeting the NLRP3 inflammasome in the clinical and preclinical space. J. Med. Chem. 64, 101–122 (2021).
F. Hoffmann-La Roche Ltd. Roche Group product development portfolio. https://www.roche.com/solutions/pipeline/ (2023).
F. Hoffmann-La Roche Ltd. Roche Group development pipeline. https://assets.cwp.roche.com/f/126832/x/8eaa872b21/irp220721-annex.pdf (2022).
F. Hoffmann-La Roche Ltd. Roche HY 2022 results. https://assets.cwp.roche.com/f/126832/x/c92c6c03a9/irp220721.pdf (2022).
NodThera, I. NodThera announces positive phase 1 study readouts for the NLRP3 inflammasome inhibitors NT-0796 and NT-0249. https://www.nodthera.com/news/nodthera-announces-positive-phase-1-study-readouts-for-the-nlrp3-inflammasome-inhibitors-nt-0796-and-nt-0249/ (2022).
Kluck, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
Marchetti, C. et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Darakhshan, S. & Pour, A. B. Tranilast: a review of its therapeutic applications. Pharmacol. Res. 91, 15–28 (2015).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426 (2002).
Huang, Y. et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10, e8689 (2018).
Chen, Y. et al. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell. Mol. Immunol. 18, 1425–1436 (2021).
Oronsky, B. et al. Discovery of RRx-001, a Myc and CD47 downregulating small molecule with tumor targeted cytotoxicity and healthy tissue cytoprotective properties in clinical development. J. Med. Chem. 64, 7261–7271 (2021).
Hill, J. R. et al. Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem 12, 1449–1457 (2017).
Salla, M. et al. Identification, synthesis, and biological evaluation of the major human metabolite of NLRP3 inflammasome inhibitor MCC950. ACS Med. Chem. Lett. 7, 1034–1038 (2016).
Hsu, P. L., Ma, J. K. & Luzzi, L. A. Interactions of sulfonylureas with plasma proteins. J. Pharm. Sci. 63, 570–573 (1974).
Primiano, M. J. et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J. Immunol. 197, 2421–2433 (2016).
Agarwal, S. et al. Discovery of N-cyano-sulfoximineurea derivatives as potent and orally bioavailable NLRP3 inflammasome inhibitors. ACS Med. Chem. Lett. 11, 414–418 (2020).
Harrison, D. et al. Discovery of a series of ester-substituted NLRP3 inflammasome inhibitors. Bioorg. Med. Chem. Lett. 30, 127560 (2020).
Harrison, D. et al. Discovery and optimization of triazolopyrimidinone derivatives as selective NLRP3 inflammasome inhibitors. ACS Med. Chem. Lett. 13, 1321–1328 (2022).
Narros-Fernandez, P. et al. Synthesis and pharmacological evaluation of new N-sulfonylureas as NLRP3 inflammasome inhibitors: identification of a hit compound to treat gout. J. Med. Chem. 65, 6250–6260 (2022).
Agarwal, S. et al. Identification of a novel orally bioavailable NLRP3 inflammasome inhibitor. Bioorg. Med. Chem. Lett. 30, 127571 (2020).
Fulp, J. et al. Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J. Med. Chem. 61, 5412–5423 (2018).
Marchetti, C. et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J. Cardiovasc. Pharmacol. 63, 316–322 (2014).
Yin, J. et al. NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 1977–1987 (2018).
Guo, C. et al. Development and characterization of a hydroxyl-sulfonamide analogue, 5-chloro-N-[2-(4-hydroxysulfamoyl-phenyl)-ethyl]-2-methoxy-benzamide, as a novel NLRP3 inflammasome inhibitor for potential treatment of multiple sclerosis. ACS Chem. Neurosci. 8, 2194–2201 (2017).
Jiang, Y. et al. Discovery of second-generation NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J. Med. Chem. 62, 9718–9731 (2019).
Xu, Y. et al. Development of sulfonamide-based NLRP3 inhibitors: further modifications and optimization through structure–activity relationship studies. Eur. J. Med. Chem. 238, 114468 (2022).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Zhang, X., Hu, L., Xu, S., Ye, C. & Chen, A. Erianin: a direct NLRP3 inhibitor with remarkable anti-inflammatory activity. Front. Immunol. 12, 739953 (2021).
He, H. et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9, 2550 (2018).
Liu, X., Xu, J., Zhou, J. & Shen, Q. Oridonin and its derivatives for cancer treatment and overcoming therapeutic resistance. Genes. Dis. 8, 448–462 (2021).
Pang, L., Liu, H., Quan, H., Sui, H. & Jia, Y. Development of novel oridonin analogs as specifically targeted NLRP3 inflammasome inhibitors for the treatment of dextran sulfate sodium-induced colitis. Eur. J. Med. Chem. 245, 114919 (2022).
Zhang, Z. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Sheth, R. et al. First in human phase I study of BMS-986299 as monotherapy and combined with nivolumab and ipilimumab in advanced solid tumors. J. Clin. Oncol. 41, e14584 (2023).
Baroja-Mazo, A. et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 15, 738–748 (2014).
Franklin, B. S. et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Desu, H. L. et al. IC100: a novel anti-ASC monoclonal antibody improves functional outcomes in an animal model of multiple sclerosis. J. Neuroinflammation 17, 143 (2020).
Bertheloot, D. et al. Nanobodies dismantle post-pyroptotic ASC specks and counteract inflammation in vivo. EMBO Mol. Med. 14, e15415 (2022).
Soriano-Teruel, P. M. et al. Identification of an ASC oligomerization inhibitor for the treatment of inflammatory diseases. Cell Death Dis. 12, 1155 (2021).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug. Discov. 20, 384–405 (2021).
Mulvihill, E. et al. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 37, e98321 (2018).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021). A report on a cryo-EM structure of the GSDMD pore.
Zhu, F. et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity 56, 753–767 e758 (2023).
Huang, L. S. et al. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52, 475–486 e475 (2020).
Weindel, C. G. et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 185, 3214–3231 e3223 (2022).
de Vasconcelos, N. M., Van Opdenbosch, N., Van Gorp, H., Parthoens, E. & Lamkanfi, M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 26, 146–161 (2019).
Xiao, J. et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 16, e3000047 (2018).
Kanneganti, A. et al. GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J. Exp. Med. 215, 1519–1529 (2018). This presents in vivo evidence that GSDMD blockade can be effective in a chronic autoinflammatory disease model.
Xu, B. et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 68, 773–782 (2018).
Gao, H. et al. Dysregulated microbiota-driven gasdermin D activation promotes colitis development by mediating IL-18 release. Front. Immunol. 12, 750841 (2021).
Li, S. et al. Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J. Exp. Med. 216, 2562–2581 (2019).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, eaat2738 (2018).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Lu, C., Li, X., Ren, Y. & Zhang, X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother. Pharmacol. 87, 159–172 (2021).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Kornberg, M. D. et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 (2018).
Cao, R. et al. Identification of a small molecule with strong anti-inflammatory activity in experimental autoimmune encephalomyelitis and sepsis through blocking gasdermin D activation. J. Immunol. 209, 820–828 (2022).
Schiffelers, L. D. J. et al. Antagonistic nanobodies reveal mechanism of GSDMD pore formation and unexpected therapeutic potential. Preprint at https://doi.org/10.1101/2023.04.20.537718 (2023).
Kopp, A. et al. Pyroptosis inhibiting nanobodies block gasdermin D pore formation. Preprint at https://doi.org/10.1101/2023.04.20.537705 (2023).
Liu, Z. et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49.e44 (2019). This paper reports the crystal structure of murine and human GSDMD, demonstrating the molecular mechanism of GSDMD CT-mediated autoinhibition.
Liu, Z. et al. Caspase-1 engages full-length gasdermin d through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity 53, 106–114 e105 (2020).
Wang, K. et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 180, 941–955 e920 (2020).
Li, Z. et al. Shigella evades pyroptosis by arginine ADP-riboxanation of caspase-11. Nature 599, 290–295 (2021).
Luchetti, G. et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–1530 e1510 (2021).
Ma, J. et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking gasdermin D cleavage. EMBO J. 40, e108249 (2021).
Lei, X. et al. Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J. Virol. 91, e01069–e010117 (2017).
Chai, Q. et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 378, eabq0132 (2022).
de Vasconcelos, N. M. et al. An apoptotic caspase network safeguards cell death induction in pyroptotic macrophages. Cell Rep. 32, 107959 (2020).
Mahib, M. R. et al. Caspase-7 mediates caspase-1-induced apoptosis independently of Bid. Microbiol. Immunol. 64, 143–152 (2020).
Taabazuing, C. Y., Okondo, M. C. & Bachovchin, D. A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–514 e504 (2017).
Doerflinger, M. et al. Flexible usage and interconnectivity of diverse cell death pathways protect against intracellular infection. Immunity 53, 533–547 e537 (2020).
Newton, K. et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 (2019).
Fritsch, M. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 (2019).
Wang, C. et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 6, eabj3859 (2021).
Zhou, B. & Abbott, D. W. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 35, 108998 (2021).
Degen, M. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature 618, 1065–1071 (2023). This report details the structural mechanisms by which NINJ1 promotes cell lysis.
Choi, S. et al. Ninjurin1 plays a crucial role in pulmonary fibrosis by promoting interaction between macrophages and alveolar epithelial cells. Sci. Rep. 8, 17542 (2018).
Ahn, B. J. et al. Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice. J. Biol. Chem. 289, 3328–3338 (2014).
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Le, H. et al. Disruption of ninjurin1 leads to repetitive and anxiety-like behaviors in mice. Mol. Neurobiol. 54, 7353–7368 (2017).
Bruchard, M. et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat. Immunol. 16, 859–870 (2015).
Liu, Y. et al. NLRP3 regulates macrophage M2 polarization through up-regulation of IL-4 in asthma. Biochem. J. 475, 1995–2008 (2018).
Zheng, J. et al. A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis. Cell Death Dis. 12, 871 (2021).
Van Opdenbosch, N. & Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 50, 1352–1364 (2019).
Li, Z. et al. Novel sulfonylurea-based NLRP3 inflammasome inhibitor for efficient treatment of nonalcoholic steatohepatitis, endotoxic shock, and colitis. J. Med. Chem. 66, 12966–12989 (2023).
Van Gorp, H., Van Opdenbosch, N. & Lamkanfi, M. Inflammasome-dependent cytokines at the crossroads of health and autoinflammatory disease. Cold Spring Harb. Perspect. Biol. 11, a028563 (2019).
Voet, S., Srinivasan, S., Lamkanfi, M. & van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 11, e10248 (2019).
Shao, S. et al. Therapeutic potential of the target on NLRP3 inflammasome in multiple sclerosis. Pharmacol. Ther. 227, 107880 (2021).
Malhotra, S. et al. NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain 143, 1414–1430 (2020).
Inoue, M., Williams, K. L., Gunn, M. D. & Shinohara, M. L. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 10480–10485 (2012).
Jha, S. et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 30, 15811–15820 (2010).
Sanchez-Fernandez, A., Skouras, D. B., Dinarello, C. A. & Lopez-Vales, R. OLT1177 (Dapansutrile), a selective NLRP3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 10, 2578 (2019).
Griffin, W. S. et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl Acad. Sci. USA 86, 7611–7615 (1989).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol., 9, 857–865 (2008).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 552, 355–361 (2017).
Dempsey, C. et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 61, 306–316 (2017).
Codolo, G. et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE 8, e55375 (2013).
Lee, E. et al. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 26, 213–228 (2019).
Yan, Y. et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015).
Huang, S. et al. A selective NLRP3 inflammasome inhibitor attenuates behavioral deficits and neuroinflammation in a mouse model of Parkinson’s disease. J. Neuroimmunol. 354, 577543 (2021).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017). This important paper describes a large-scale clinical trial demonstrating that IL-1β inhibition confers protection against atherosclerotic disease.
Ridker, P. M. et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Solomon, D. H. et al. Relationship of interleukin-1beta blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann. Intern. Med. 169, 535–542 (2018).
Schieker, M. et al. Effects of interleukin-1beta inhibition on incident hip and knee replacement : exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 173, 509–515 (2020).
Vallurupalli, M. et al. Effects of interleukin-1beta inhibition on incident anemia: exploratory analyses from a randomized trial. Ann. Intern. Med. 172, 523–532 (2020).
Acknowledgements
The authors apologize to colleagues whose work was not cited because of space constraints. This work was supported by the Fund for Scientific Research (FWO)-Flanders grant G017121N and European Research Council Grant 101101075 (PyroScreen) to M.L.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.L. serves as a scientific consultant for Ventyx Biosciences and Novo Nordisk. L.V.W. declares no conflict of interest.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Rongbin Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
clinicaltrials.gov: https://clinicaltrials.gov
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vande Walle, L., Lamkanfi, M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat Rev Drug Discov 23, 43–66 (2024). https://doi.org/10.1038/s41573-023-00822-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00822-2
This article is cited by
-
Cell death as an architect of adult skin stem cell niches
Cell Death & Differentiation (2024)