Abstract
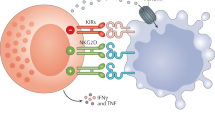
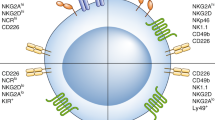
Natural killer (NK) cells have crucial roles in the innate immunosurveillance of cancer and viral infections. They are ‘first responders’ that can spontaneously recognize abnormal cells in the body, rapidly eliminate them through focused cytotoxicity mechanisms and potently produce pro-inflammatory cytokines and chemokines that recruit and activate other immune cells to initiate an adaptive response. From the initial discovery of the diverse cell surface receptors on NK cells to the characterization of regulatory events that control their function, our understanding of the basic biology of NK cells has improved dramatically in the past three decades. This advanced knowledge has revealed increased mechanistic complexity, which has opened the doors to the development of a plethora of exciting new therapeutics that can effectively manipulate and target NK cell functional responses, particularly in cancer patients. Here, we summarize the basic mechanisms that regulate NK cell biology, review a wide variety of drugs, cytokines and antibodies currently being developed and used to stimulate NK cell responses, and outline evolving NK cell adoptive transfer approaches to treat cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ljunggren, H.-G. & Kärre, K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
Campbell, K. S. & Hasegawa, J. Natural killer cell biology: an update and future directions. J. Allergy Clin. Immunol. 132, 536–544 (2013).
Zitti, B. & Bryceson, Y. T. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 42, 37–46 (2018).
Freud, A. G., Mundy-Bosse, B. L., Yu, J. & Caligiuri, M. A. The broad spectrum of human natural killer cell diversity. Immunity 47, 820–833 (2017).
Imai, K., Matsuyama, S., Miyake, S., Suga, K. & Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799 (2000).
Orange, J. S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 132, 515–525 (2013).
MacFarlane, A. W. 4th et al. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology 6, e1330235 (2017).
Pazina, T. et al. Alterations of NK cell phenotype in the disease course of multiple myeloma. Cancers 13, 226 (2021).
Laughney, A. M. et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat. Med. 26, 259–269 (2020). These authors report that metastasizing lung adenocarcinoma cells that express high levels of SOX9 (alveolar epithelial progenitor state) have improved NK cell-mediated killing evasion, owing to increased MHC-I expression, whereas SOX2hi cells (regenerative state) are more sensitive to NK cell attack, through downregulation of MHC-I and upregulation of activating receptor ligands.
Lo, H. C. et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat. Cancer 1, 709–722 (2020). This article reports that individual circulating tumour cells with mesenchymal characteristics are more sensitive to NK cell-mediated elimination than polyclonal clusters of circulating tumour cells, which are more likely to escape owing to more epithelial phenotype, lower expression of NK cell activating ligands and higher expression of adhesion molecules.
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Ivashkiv, L. B. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18, 545–558 (2018).
Collins, P. L. et al. Gene regulatory programs conferring phenotypic identities to human NK cells. Cell 176, 348–360.e12 (2019).
Fehniger, T. A. et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell derived IL-2: a potential new link between adaptive and innate immunity. Blood 100, 1935–1947 (2003).
Milush, J. M. et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood 114, 4823–4831 (2009).
Dogra, P. et al. Tissue determinants of human NK cell development, function, and residence. Cell 180, 749–763.e13 (2020).
Melsen, J. E., Lugthart, G., Lankester, A. C. & Schilham, M. W. Human circulating and tissue-resident cd56bright natural killer cell populations. Front. Immunol. 7, 262 (2016).
Cooper, M. A., Fehniger, T. A. & Caligiuri, M. A. The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 (2001).
Crinier, A. et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK Cell subsets in humans and mice. Immunity 49, 971–986.e5 (2018).
Yudanin, N. A. et al. Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity. Immunity 50, 505–519.e4 (2019).
Brownlie, D. et al. Expansions of adaptive-like NK cells with a tissue-resident phenotype in human lung and blood. Proc. Natl Acad. Sci. USA 118, e2016580118 (2021).
Freud, A. G. & Caligiuri, M. A. Human natural killer cell development. Immunol. Rev. 214, 56–72 (2006).
Carson, W. E. et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99, 937–943 (1997).
Cooper, M. A. et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100, 3633–3638 (2002).
Vosshenrich, C. A. et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 174, 1213–1221 (2005).
MacFarlane, A. W. 4th & Campbell, K. S. Signal transduction in natural killer cells. Curr. Top. Microbiol. Immunol. 298, 23–57 (2006).
Dhatchinamoorthy, K., Colbert, J. D. & Rock, K. L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 12, 636568 (2021).
Campbell, K. S. & Purdy, A. K. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 132, 315–325 (2011).
Poznanski, S. M. et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 33, 1205–1220.e5 (2021). This study reports that expansion of NK cells with IL-21 or treatment with an activator of the NRF2 antioxidant pathway promotes Warburg-like metabolism with increased glycolysis and reduced oxidative phosphorylation, resulting in substantially improved resistance to the oxidative stressed and nutrient-deprived TME and enhanced antitumour responses.
Pazina, T., Shemesh, A., Brusilovsky, M., Porgador, A. & Campbell, K. S. Regulation of the functions of natural cytotoxicity receptors by interactions with diverse ligands and alterations in splice variant expression. Front. Immunol. 8, 369 (2017).
Gorvel, L. & Olive, D. Targeting the “PVR-TIGIT axis” with immune checkpoint therapies. F1000Res. 9, 354 (2020).
Li, J. et al. PVRIG is a novel NK cell immune checkpoint receptor in acute myeloid leukemia. Haematologica 106, 3115–3124 (2021).
Horowitz, A. et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl Med. 5, 208ra145 (2013). This study is the first to apply mass cytometry to examine expression of 35 receptors on peripheral blood NK cells in healthy donors, including monozygotic twins, and found incredibly high diversity with up to 30,000 different NK cell clones within an individual, based on unique receptor expression phenotypes.
Strauss-Albee, D. M. et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl Med. 7, 297ra115 (2015).
Cerwenka, A. & Lanier, L. L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 16, 112–123 (2016).
Foley, B. et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 119, 2665–2674 (2012).
Lopez-Verges, S. et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl Acad. Sci. USA 108, 14725–14732 (2011).
Kulkarni, S., Martin, M. P. & Carrington, M. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 20, 343–352 (2008).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Schönberg, K., Sribar, M., Enczmann, J., Fischer, J. C. & Uhrberg, M. Analyses of HLA-C–specific KIR repertoires in donors with group A and B haplotypes suggest a ligand-instructed model of NK cell receptor acquisition. Blood 117, 98–107 (2011).
Anfossi, N. et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 (2006).
Kim, S. et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl Acad. Sci. USA 105, 3053–3058 (2008).
Furukawa, H. et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (bare lymphocyte syndrome type I). Hum. Immunol. 60, 32–40 (1999).
Kim, S. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005).
Boudreau, J. E. et al. Cell-extrinsic MHC class I molecule engagement augments human NK cell education programmed by cell-intrinsic MHC class I. Immunity 45, 280–291 (2016).
MacFarlane, A. W.4th et al. Enhanced NK-cell development and function in BCAP-deficient mice. Blood 112, 131–140 (2008). This article reports that BCAP facilitates activation signalling and BCAP-deficient mice have defects in B cell development and function, but finds that these mice paradoxically exhibit enhanced NK cell development to a more terminally mature and functional state, indicating that loss of activation signalling through BCAP promotes NK cell maturation and function.
Bald, T., Krummel, M. F., Smyth, M. J. & Barry, K. C. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 21, 835–847 (2020).
Cooper, M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood 97, 3146–3151 (2001).
Hauser, M. A. & Legler, D. F. Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes. J. Leukoc. Biol. 99, 869–882 (2016).
Edsparr, K., Basse, P. H., Goldfarb, R. H. & Albertsson, P. Matrix metalloproteinases in cytotoxic lymphocytes impact on tumour infiltration and immunomodulation. Cancer Microenviron. 4, 351–360 (2011).
Carrega, P. et al. CD56brightperforinlow noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 192, 3805–3815 (2014).
Fionda, C. et al. Hitting more birds with a stone: impact of TGF-beta on ILC activity in cancer. J. Clin. Med. 9, 143 (2020).
Slattery, K. & Gardiner, C. M. NK Cell Metabolism and TGFbeta - implications for immunotherapy. Front. Immunol. 10, 2915 (2019).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Cortez, V. S. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 18, 995–1003 (2017).
Balsamo, M. et al. Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur. J. Immunol. 43, 2756–2764 (2013).
Ni, J. et al. Single-Cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity 52, 1075–1087.e8 (2020).
Zheng, X. et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 20, 1656–1667 (2019).
Bonavita, E. et al. Antagonistic inflammatory phenotypes dictate tumor fate and response to immune checkpoint blockade. Immunity 53, 1215–1229.e8 (2020).
Jacobs, B. et al. The oncometabolite 5′-deoxy-5′-methylthioadenosine blocks multiple signaling pathways of NK cell activation. Front. Immunol. 11, 2128 (2020).
Della Chiesa, M. et al. The tryptophan catabolite l-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125 (2006).
Harmon, C. et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol. Res. 7, 335–346 (2019).
Chambers, A. M. et al. Adenosinergic signaling alters natural killer cell functional responses. Front. Immunol. 9, 2533 (2018).
O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 19, 282–290 (2019).
Molfetta, R., Zingoni, A., Santoni, A. & Paolini, R. Post-translational mechanisms regulating NK cell activating receptors and their ligands in cancer: potential targets for therapeutic intervention. Front. Immunol. 10, 2557 (2019).
Pan, B. & Lentzsch, S. The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol. Ther. 136, 56–68 (2012).
Lacy, M. Q. et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia 24, 1934–1939 (2010).
Lagrue, K., Carisey, A., Morgan, D. J., Chopra, R. & Davis, D. M. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood 126, 50–60 (2015).
Fionda, C. et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget 6, 23609–23630 (2015).
Ito, T. et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010).
Kronke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309 (2014).
Asatsuma-Okumura, T., Ito, T. & Handa, H. Molecular mechanisms of cereblon-based drugs. Pharmacol. Ther. 202, 132–139 (2019).
Hideshima, T. et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia 35, 177–188 (2021).
Armeanu, S. et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clin. Cancer Res. 14, 3520–3528 (2008).
Niu, C. et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and gammadelta T cell-mediated lysis in multiple myeloma. Oncotarget 8, 5954–5964 (2017).
Gras Navarro, A. et al. Pretreatment of glioblastoma with bortezomib potentiates natural killer cell cytotoxicity through TRAIL/DR5 mediated apoptosis and prolongs animal survival. Cancers 11, 996 (2019).
Jinushi, M. et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc. Natl Acad. Sci. USA 105, 1285–1290 (2008).
Shi, J. et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood 111, 1309–1317 (2008).
Chen, Q., Sun, L. & Chen, Z. J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Marcus, A. et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity 49, 754–763.e4 (2018).
Sivick, K. E. et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor immunity. Cell Rep. 29, 785–789 (2019).
Nicolai, C. J. et al. NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists. Sci. Immunol. 5, eaaz2738 (2020). This study reports that injection of various CTL-resistant tumours in mice with a cyclic dinucleotide STING agonist substantially enhanced antitumour responses by NK cells towards the injected tumours, as well as non-injected tumours, by promoting type I interferon production and stimulating IL-15 production by DCs.
Francica, B. J. et al. TNFalpha and radioresistant stromal cells are essential for therapeutic efficacy of cyclic dinucleotide STING agonists in nonimmunogenic tumors. Cancer Immunol. Res. 6, 422–433 (2018).
Demaria, O. et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl Acad. Sci. USA 112, 15408–15413 (2015).
Lotze, M. T., Grimm, E. A., Mazumder, A., Strausser, J. L. & Rosenberg, S. A. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 41, 4420–4425 (1981).
Grimm, E. A., Mazumder, A., Zhang, H. Z. & Rosenberg, S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 155, 1823–1841 (1982).
Pisani, R. J., Krco, C. J., Wold, L. E. & McKean, D. J. Lymphokine-activated killer (LAK) cell activity in tumor-infiltrating lymphocytes from non-small cell lung cancer. Am. J. Clin. Pathol. 92, 435–446 (1989).
Phillips, J. H., Gemlo, B. T., Myers, W. W., Rayner, A. A. & Lanier, L. L. In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J. Clin. Oncol. 5, 1933–1941 (1987).
Wu, Y. et al. Natural killer cells as a double-edged sword in cancer immunotherapy: a comprehensive review from cytokine therapy to adoptive cell immunotherapy. Pharmacol. Res. 155, 104691 (2020).
Sim, G. C. et al. IL2 variant circumvents ICOS+ regulatory T-cell expansion and promotes NK cell activation. Cancer Immunol. Res. 4, 983–994 (2016).
Lopes, J. E. et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer 8, e000673 (2020).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Bergamaschi, C. et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood 120, e1–e8 (2012).
Kobayashi, H. et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727 (2005).
Yang, Y. et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J. Clin. Invest. 130, 5508–5522 (2020).
Cooley, S. et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 3, 1970–1980 (2019).
Miller, J. S. et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res. 24, 1525–1535 (2018).
Watson, D. C. et al. Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J. Extracell. Vesicles 7, 1442088 (2018).
Bergamaschi, C. et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-gamma, CXCL9 and CXCL10. J. Immunother. Cancer 8, e000599 (2020).
Han, K. P. et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 56, 804–810 (2011).
Xu, W. et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor alphaSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 73, 3075–3086 (2013).
Kim, P. S. et al. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 7, 16130–16145 (2016).
Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018).
Romee, R. et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 131, 2515–2527 (2018).
Felices, M. et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3, e96219 (2018).
Merino, A. et al. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J. Clin. Invest. 129, 3770–3785 (2019).
Mirlekar, B. & Pylayeva-Gupta, Y. IL-12 family cytokines in cancer and immunotherapy. Cancers 13, 167 (2021).
Zundler, S. & Neurath, M. F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 26, 559–568 (2015).
Lasek, W., Zagozdzon, R. & Jakobisiak, M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 63, 419–435 (2014).
Nguyen, K. G. et al. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 11, 575597 (2020).
Gillies, S. D. et al. Antibody-IL-12 fusion proteins are effective in SCID mouse models of prostate and colon carcinoma metastases. J. Immunol. 160, 6195–6203 (1998).
Peng, L. S., Penichet, M. L., Dela Cruz, J. S., Sampogna, S. L. & Morrison, S. L. Mechanism of antitumor activity of a single-chain interleukin-12 IgG3 antibody fusion protein (mscIL-12.her2.IgG3). J. Interferon Cytokine Res. 21, 709–720 (2001).
Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin. Cancer Res. 26, 2827–2837 (2020).
Lewis, N. D. et al. Exosome surface display of IL12 results in tumor-retained pharmacology with superior potency and limited systemic exposure compared with recombinant IL12. Mol. Cancer Ther. 20, 523–534 (2021).
Kaplanski, G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol. Rev. 281, 138–153 (2018).
Konjevic, G. M., Vuletic, A. M., Mirjacic Martinovic, K. M., Larsen, A. K. & Jurisic, V. B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 117, 30–40 (2019).
Ma, Z. et al. Augmentation of immune checkpoint cancer immunotherapy with IL18. Clin. Cancer Res. 22, 2969–2980 (2016).
Robertson, M. J. et al. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin. Cancer Res. 14, 3462–3469 (2008).
Robertson, M. J. et al. A dose-escalation study of recombinant human interleukin-18 in combination with rituximab in patients with non-Hodgkin lymphoma. J. Immunother. 36, 331–341 (2013).
Robertson, M. J. et al. A dose-escalation study of recombinant human interleukin-18 in combination with ofatumumab after autologous peripheral blood stem cell transplantation for lymphoma. J. Immunother. 41, 151–157 (2018).
Fabbi, M., Carbotti, G. & Ferrini, S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J. Leukoc. Biol. 97, 665–675 (2015).
Molgora, M. et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114 (2017).
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020). These authors report on the engineering of a novel form of IL-18 that can stimulate potent antitumour responses by NK and CD8+ T cells, but is incapable of interacting with the negative regulator IL-18BP, which is produced in many cancers and binds to IL-18 with high affinity to prevent its biological activity, limiting the efficacy of IL-18 therapies in cancer patients.
Skak, K., Frederiksen, K. S. & Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 123, 575–583 (2008).
Burgess, S. J., Marusina, A. I., Pathmanathan, I., Borrego, F. & Coligan, J. E. IL-21 down-regulates NKG2D/DAP10 expression on human NK and CD8+ T cells. J. Immunol. 176, 1490–1497 (2006).
Strengell, M. et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J. Immunol. 170, 5464–5469 (2003).
French, A. R., Holroyd, E. B., Yang, L., Kim, S. & Yokoyama, W. M. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine 35, 229–234 (2006).
Bhatt, S., Sarosiek, K. A. & Lossos, I. S. Interleukin 21- its potential role in the therapy of B-cell lymphomas. Leuk. Lymphoma 58, 17–29 (2017).
Huynh, L. K., Hipolito, C. J. & Ten Dijke, P. A perspective on the development of TGF-beta inhibitors for cancer treatment. Biomolecules 9, 743 (2019).
Morris, J. C. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 9, e90353 (2014).
Formenti, S. C. et al. Focal irradiation and systemic TGFbeta blockade in metastatic breast cancer. Clin. Cancer Res. 24, 2493–2504 (2018).
Tran, H. C. et al. TGFbetaR1 blockade with galunisertib (LY2157299) enhances anti-neuroblastoma activity of the anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin. Cancer Res. 23, 804–813 (2017).
Faivre, S. et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int. 39, 1468–1477 (2019).
Melisi, D. et al. TGFbeta receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother. Pharmacol. 83, 975–991 (2019).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
Zemek, R. M. et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci. Transl Med. 11, eaav7816 (2019).
Bezman, N. A. et al. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv. 1, 753–765 (2017).
Siebert, N., Zumpe, M., Jüttner, M., Troschke-Meurer, S. & Lode, H. N. PD-1 blockade augments anti-neuroblastoma immune response induced by anti-GD(2) antibody ch14.18/CHO. Oncoimmunology 6, e1343775 (2017).
Yamashita, K. et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. Br. J. Cancer 124, 595–603 (2021).
Pesce, S. et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J. Allergy Clin. Immunol. 139, 335–346.e3 (2017).
Davis, Z. et al. Low-density PD-1 expression on resting human natural killer cells is functional and upregulated after transplantation. Blood Adv. 5, 1069–1080 (2021).
Judge, S. J. et al. Minimal PD-1 expression in mouse and human NK cells under diverse conditions. J. Clin. Invest. 130, 3051–3068 (2020).
Caumartin, J. et al. Trogocytosis-based generation of suppressive NK cells. EMBO J. 26, 1423–1433 (2007).
Nakamura, K. et al. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl Acad. Sci. USA 110, 9421–9426 (2013).
Mariotti, F. R. et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology 8, 1557030 (2019).
Quatrini, L. et al. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat. Immunol. 19, 954–962 (2018).
Oyer, J. L., Gitto, S. B., Altomare, D. A. & Copik, A. J. PD-L1 blockade enhances anti-tumor efficacy of NK cells. Oncoimmunology 7, e1509819 (2018).
Dong, W. et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 9, 1422–1437 (2019). This study reports that direct contact with susceptible tumours can promote PDL1 expression on NK cells as a marker of their activation, including in patients with AML, and an anti-PDL1 antibody can surprisingly stimulate antitumour responses by these PDL1+ NK cells via the induction of p38 signalling.
Tallerico, R. et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology 6, e1261242 (2017).
Sanseviero, E. et al. Anti–CTLA-4 activates intratumoral NK cells and combined with IL15/IL15Rα complexes enhances tumor control. Cancer Immunol. Res. 7, 1371–1380 (2019).
Russick, J. et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J. Immunother. Cancer 8, e001054 (2020).
Lougaris, V. et al. CTLA-4 regulates human natural killer cell effector functions. Clin. Immunol. 194, 43–45 (2018).
Lanuza, P. M. et al. Recalling the biological significance of immune checkpoints on NK cells: a chance to overcome LAG3, PD1, and CTLA4 inhibitory pathways by adoptive NK cell transfer? Front. Immunol. 10, 3010 (2020).
Romagne, F. et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 (2009).
Kohrt, H. E. et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood 123, 678–686 (2014).
Mamessier, E. et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 121, 3609–3622 (2011).
Borst, L., van der Burg, S. H. & van Hall, T. The NKG2A–HLA-E axis as a novel checkpoint in the tumor microenvironment. Clin. Cancer Res. 26, 5549–5556 (2020).
van Montfoort, N. et al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell 175, 1744–1755.e15 (2018).
Galot, R. et al. A phase II study of monalizumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: the I1 cohort of the EORTC-HNCG-1559 UPSTREAM trial. Eur. J. Cancer 158, 17–26 (2021).
Thijs, W. H. F. et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 105, e76–e79 (2020).
Binyamin, L. et al. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J. Immunol. 180, 6392–6401 (2008).
Carlsten, M. et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin. Cancer Res. 22, 5211–5222 (2016). This study reports a significant reduction in expression of KIR2D on NK and T cells of patients treated with IPH2101; the remaining KIR2D+ NK cells are found to be hyporesponsive towards MHC-I-deficient tumour target cells, suggesting that blocking KIR2D by lirilumab can disrupt the education/licensing process.
Chauvin, J.-M. et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin. Cancer Res. 26, 5520–5533 (2020).
Ohs, I. et al. Restoration of natural killer cell antimetastatic activity by IL12 and checkpoint blockade. Cancer Res. 77, 7059–7071 (2017).
Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018).
da Silva, I. P. et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2, 410–422 (2014).
Farkas, A. M. et al. Tim-3 and TIGIT mark NK and T cells susceptible to effector dysfunction in human bladder cancer [abstract 4745]. Cancer Res. 78, 4745 (2018).
Davar, D. & Zarour, H. M. Immunological targets for immunotherapy: inhibitory T cell receptors. Methods Mol. Biol. 2055, 23–60 (2020).
He, Y. et al. Contribution of inhibitory receptor TIGIT to NK cell education. J. Autoimmun. 81, 1–12 (2017).
Rosental, B. et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J. Immunol. 187, 5693–5702 (2011).
Cantoni, C. et al. NKp44, a triggering receptor involved in tumor cell lysis by activating human natural killer cells, is a novel member of the immunoglobulin superfamily. J. Exp. Med. 189, 787–796 (1999).
Gonzalez-Magana, A. & Blanco, F. J. Human PCNA structure, function and interactions. Biomolecules 10, 570 (2020).
Kundu, K. et al. Inhibition of the NKp44-PCNA immune checkpoint using a mAb to PCNA. Cancer Immunol. Res. 7, 1120–1134 (2019). This study reports the development of a mAb that interacts with a portion of PCNA that is exposed on tumour cell surfaces, which disrupts binding to the NK cell receptor NKp44 and prevents inhibitory signalling; this antibody substantially potentiates NK cell responses towards PCNA+ tumour cells, both in vitro and in mouse models.
Cheng, H. et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1–negative human lung cancers. Clin. Cancer Res. 24, 1954–1964 (2018).
Jing, C. Y. et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J. Immunother. Cancer 7, 77 (2019).
Wang, B., Ran, Z., Liu, M. & Ou, Y. Prognostic significance of potential immune checkpoint member HHLA2 in human tumors: a comprehensive analysis. Front. Immunol. 10, 1573 (2019).
Trundley, A. E. et al. Molecular characterization of KIR3DL3. Immunogenetics 57, 904–916 (2006).
Leaton, L. A. et al. Conservation, extensive heterozygosity, and convergence of signaling potential all indicate a critical role for KIR3DL3 in higher primates. Front. Immunol. 10, 24 (2019).
Janakiram, M. et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin. Cancer Res. 21, 2359–2366 (2015).
Zhuang, X. & Long, E. O. CD28 homolog is a strong activator of natural killer cells for Lysis of B7H7+ tumor cells. Cancer Immunol. Res. 7, 939–951 (2019).
Bhatt, R. S. et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol. Res. 9, 156–169 (2021).
Veillette, A. & Chen, J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends immunol. 39, 173–184 (2018).
Deuse, T. et al. The SIRPα-CD47 immune checkpoint in NK cells. J. Exp. Med. 218, e20200839 (2021).
Temming, A. R. et al. Functional attributes of antibodies, effector cells, and target cells affecting NK cell-mediated antibody-dependent cellular cytotoxicity. J. Immunol. 203, 3126–3135 (2019).
Veeramani, S. et al. Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 118, 3347–3349 (2011).
Treon, S. P. et al. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenström’s macroglobulinemia. J. Clin. Oncol. 23, 474–481 (2005).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99, 754–758 (2002).
Bang, Y. J. et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 28, 855–861 (2017).
Chenoweth, A. M., Wines, B. D., Anania, J. C. & Mark Hogarth, P. Harnessing the immune system via FcγR function in immune therapy: a pathway to next-gen mAbs. Immunol. Cell Biol. 98, 287–304 (2020).
Vitolo, U. et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J. Clin. Oncol. 35, 3529–3537 (2017).
Marcus, R. et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N. Engl. J. Med. 377, 1331–1344 (2017).
Lunning, M. et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 134, 1811–1820 (2019).
Kumar, A., Planchais, C., Fronzes, R., Mouquet, H. & Reyes, N. Binding mechanisms of therapeutic antibodies to human CD20. Science 369, 793–799 (2020).
Chan, H. T. et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into Triton X-100 insoluble membrane rafts. Cancer Res. 63, 5480–5489 (2003).
Campbell, K. S., Cohen, A. D. & Pazina, T. Mechanisms of NK cell activation and clinical activity of the therapeutic SLAMF7 antibody, elotuzumab in multiple myeloma. Front. Immunol. 9, 2551 (2018).
Pazina, T. et al. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and -independent mechanisms. Oncoimmunology 6, e1339853 (2017).
Pazina, T. et al. Enhanced SLAMF7 homotypic interactions by elotuzumab improves NK cell killing of multiple myeloma. Cancer Immunol. Res. 7, 1633–1646 (2019).
Dimopoulos, M. A. et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N. Engl. J. Med. 379, 1811–1822 (2018).
Dimopoulos, M. A. et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 22, 801–812 (2021).
Casneuf, T. et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 1, 2105–2114 (2017).
Boyerinas, B. et al. Antibody-dependent cellular cytotoxicity activity of a novel anti–PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol. Res. 3, 1148–1157 (2015).
Juliá, E. P., Amante, A., Pampena, M. B., Mordoh, J. & Levy, E. M. Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front. Immunol. 9, 2140 (2018).
Ferrari de Andrade, L. et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 359, 1537–1542 (2018).
Du, C. et al. MICA immune complex formed with alpha 3 domain-specific antibody activates human NK cells in a Fc-dependent manner. J. Immunother. Cancer 7, 207 (2019).
Kaur, K., Safaie, T., Ko, M. W., Wang, Y. & Jewett, A. ADCC against MICA/B Is mediated against differentiated oral and pancreatic and not stem-like/poorly differentiated tumors by the NK cells; loss in cancer patients due to down-modulation of CD16 receptor. Cancers 13, 239 (2021).
Romee, R. et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 121, 3599–3608 (2013).
Srpan, K. et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 217, 3267–3283 (2018).
Chew, H. Y. et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell 180, 895–914.e27 (2020).
Goebeler, M.-E. & Bargou, R. C. T cell-engaging therapies — BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Ellwanger, K. et al. Redirected optimized cell killing (ROCK®): a highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. mAbs 11, 899–918 (2019).
Dahlén, E., Veitonmäki, N. & Norlén, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 6, 3–17 (2018).
Oberg, H. H. et al. Tribody [(HER2)2xCD16] is more effective than trastuzumab in enhancing γδ T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells. Front. Immunol. 9, 814 (2018).
Glorius, P. et al. The novel tribody [(CD20)2xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia 27, 190–201 (2013).
Davis, Z. B., Vallera, D. A., Miller, J. S. & Felices, M. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin. Immunol. 31, 64–75 (2017).
Schmohl, J. U. et al. Engineering of anti-CD133 trispecific molecule capable of inducing NK expansion and driving antibody-dependent cell-mediated cytotoxicity. Cancer Res. Treat. 49, 1140–1152 (2017).
Felices, M. et al. Novel CD19-targeted TriKE restores NK cell function and proliferative capacity in CLL. Blood Adv. 3, 897–907 (2019).
Arvindam, U. S. et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia 35, 1586–1596 (2021).
Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177, 1701–1713.e16 (2019). This report describes systematic engineering of trispecific antibodies, which they call NK cell engagers, that consist of a tumour surface antigen binding element along with optimally spaced interaction sites for NKp46 and CD16 to trigger highly potent NK cell-mediated antitumour responses in vitro and in mouse models.
Au, K. M., Park, S. I. & Wang, A. Z. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci. Adv. 6, eaba8564 (2020).
Srivastava, R. M. et al. CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin. Cancer Res. 23, 707–716 (2017).
Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021).
Cooley, S. et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116, 2411–2419 (2010).
Venstrom, J. M. et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 367, 805–816 (2012).
Knorr, D. A. et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cell Transl. Med. 2, 274–283 (2013).
Hermanson, D. L. et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cell 34, 93–101 (2016).
Shereck, E. et al. Immunophenotypic, cytotoxic, proteomic and genomic characterization of human cord blood vs. peripheral blood CD56dim NK cells. Innate Immun. 25, 294–304 (2019).
Herrera, L. et al. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci. Rep. 9, 18729 (2019).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Ewen, E. M., Pahl, J. H. W., Miller, M., Watzl, C. & Cerwenka, A. KIR downregulation by IL-12/15/18 unleashes human NK cells from KIR/HLA-I inhibition and enhances killing of tumor cells. Eur. J. Immunol. 48, 355–365 (2018).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl Med. 8, 357ra123 (2016). The authors report that a combination of IL-12, IL-15 and IL-18 induces expansion of memory-like NK cells with robust IFNγ and cytotoxicity responses towards primary AML cells in vitro and in xenograft mouse models that are independent of KIR inhibition. In a small clinical trial, adoptive transfer of memory-like NK cells into patients with AML results in clinical responses in five of nine evaluable patients.
Dong, H. et al. The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol. 20, 865–878 (2019).
Kerbauy, L. N. et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30+ malignancies. Clin. Cancer Res. 27, 3744–3756 (2021). This article shows that AFM13, the tetravalent anti-CD16/CD30 bispecific antibody currently in clinical trials for CD30+ malignancies, has specific applications for adoptive transfer therapy; first, for enhanced cytotoxicity when paired with cytokine-induced memory-like NK cells, and second, for its high avidity for CD16, which allows it to be pre-loaded onto NK cells before adoptive transfer.
Kweon, S. et al. Expansion of human NK cells using K562 cells expressing OX40 ligand and short exposure to IL-21. Front. Immunol. 10, 879 (2019).
Fujisaki, H. et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 69, 4010–4017 (2009).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020). This report describes a clinical trial in which patients with B cell tumours were treated with MHC-I-mismatched NK cells bearing an anti-CD19 CAR, IL-15 and an inducible caspase 9 suicide gene, and 8 of 11 patients experienced rapid clinical responses and long-term survival of CAR-bearing NK cells without cytokine release syndrome or other autoimmune toxicities.
Li, Y., Hermanson, D. L., Moriarity, B. S. & Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192.e5 (2018).
Oei, V. Y. S. et al. Intrinsic functional potential of NK-cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol. Res. 6, 467–480 (2018).
Jochems, C. et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 7, 86359–86373 (2016).
Mitwasi, N. et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci. Rep. 10, 2141 (2020).
Williams, B. A. et al. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 8, 89256–89268 (2017).
Bonanni, V., Antonangeli, F., Santoni, A. & Bernardini, G. Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells. J. Immunother. cancer 7, 290 (2019).
Carlsten, M. et al. Efficient mRNA-based genetic engineering of human NK cells with high-affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-associated chemokine CCL19. Front. Immunol. 7, 105 (2016).
Jing, Y. et al. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS ONE 10, e0121788 (2015).
Zhu, H. et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 135, 399–410 (2020). The authors generate iPSC-derived NK cells expressing a modified CD16 that was high-affinity (158V) and ADAM17 cleavage resistant (S197P), which are noteworthy for their more robust ADCC response and more stable CD16 expression than unmodified iPSC-derived NK cells.
Zhu, H. et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 27, 224–237.e6 (2020).
Naeimi Kararoudi, M. et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 136, 2416–2427 (2020).
van der Horst, H. J., Nijhof, I. S., Mutis, T. & Chamuleau, M. E. D. Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies. Cancers 12, 3041 (2020).
Horton, H. M. et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 68, 8049–8057 (2008).
Purdy, A. K. & Campbell, K. S. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR). Cancer Biol. Ther. 8, 2211–2220 (2009).
Acknowledgements
The authors appreciate funding from a Health Research Formula Fund grant from the Commonwealth of Pennsylvania (CURE; to K.S.C.), NIH grants AI148117 (K.S.C.) and CA194263 (K.S.C.), NCI NIH training grant T32 CA9035 (N.A.M.) and NCI Comprehensive Cancer Center support grant CA06927 (FCCC).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
K.S.C. has received research support from Janssen Pharma, Genentech, Horizon Pharma, ImmunityBio and Immune Oncology Biosciences, consulting fees from Immunitas and Tavotec, and has patents with ImmunityBio. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Jeffrey Miller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Opsonization
-
The binding of antibodies to the surface of a pathogen or tumour cell, which are recognized by Fc receptors on phagocytes to facilitate antibody-dependent cellular phagocytosis or by CD16 on natural killer cells to trigger antibody-dependent cellular cytotoxicity.
- Tolerize
-
The process by which normal cells induce tolerance towards natural killer cell-mediated attack by expressing major histocompatibility complex class I molecules that engage inhibitory receptors, such as killer cell inhibitory receptor (KIR).
- Fucosylation
-
The process of covalently attaching the sugar fucose to IgG molecules as a post-translational event, which reduces the affinity of IgG1 or IgG3 to bind CD16 and thereby decreases their potency in stimulating antibody-dependent cellular cytotoxicity by natural killer cells.
- Fratricide
-
Classically defined as killing one’s sibling, this term has been used to define the process by which natural killer cells sometimes aberrantly attack and kill each other.
- Feeder cell lines
-
Cell lines that are used to stimulate the ex vivo proliferation of natural killer (NK) cells and are generally chosen for low surface expression of major histocompatibility complex class I and/or engineered to express surface bound cytokines or ligands for NK cell-activating receptors.
Rights and permissions
About this article
Cite this article
Maskalenko, N.A., Zhigarev, D. & Campbell, K.S. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov 21, 559–577 (2022). https://doi.org/10.1038/s41573-022-00413-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-022-00413-7
This article is cited by
-
Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy
Journal of Hematology & Oncology (2024)
-
Comprehensive characterization of immunogenic cell death in acute myeloid leukemia revealing the association with prognosis and tumor immune microenvironment
BMC Medical Genomics (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)
-
Characterization of a novel anti-PVRIG antibody with Fc-competent function that exerts strong antitumor effects via NK activation in preclinical models
Cancer Immunology, Immunotherapy (2024)
-
Prognostic risk factors of serous ovarian carcinoma based on mesenchymal stem cell phenotype and guidance for therapeutic efficacy
Journal of Translational Medicine (2023)