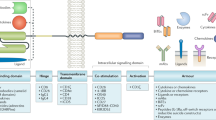
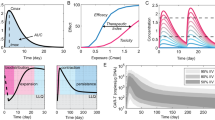
Abstract
Cell therapy is one of the fastest growing areas in the pharmaceutical industry, with considerable therapeutic potential. However, substantial challenges regarding the utility of these therapies will need to be addressed before they can become mainstream medicines with applicability similar to that of small molecules or monoclonal antibodies. Engineered T cells have achieved success in the treatment of blood cancers, with four chimeric antigen receptor (CAR)-T cell therapies now approved for the treatment of B cell malignancies based on their unprecedented efficacy in clinical trials. However, similar results have not yet been achieved in the treatment of the much larger patient population with solid tumours. For cell therapies to become mainstream medicines, they may need to offer transformational clinical effects for patients and be applicable in disease settings that remain unaddressed by simpler approaches. This Perspective provides an industry perspective on the progress achieved by engineered T cell therapies to date and the opportunities and current barriers for accessing broader patient populations, and discusses the solutions and new development strategies required to fully industrialize the therapeutic potential of engineered T cells as medicines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hoos, A. Development of immuno-oncology drugs — from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 15, 235–247 (2016).
Tang, J., Pearce, L., O’Donnell-Tormey, J. & Hubbard-Lucey, V. M. Trends in the global immuno-oncology landscape. Nat. Rev. Drug Discov. 17, 783–784 (2018).
Xin Yu, J., Hubbard-Lucey, V. M. & Tang, J. The global pipeline of cell therapies for cancer. Nat. Rev. Drug Discov. 18, 821–822 (2019).
Guedan, S., Ruella, M. & June, C. H. Emerging cellular therapies for cancer. Annu. Rev. Immunol. 37, 145–171 (2019).
Robbins, P. F. et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019–1027 (2015).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Salmikangas, P., Kinsella, N. & Chamberlain, P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy — moving target for industry? Pharm. Res. 35, 152 (2018).
Delhove, J. & Qasim, W. Genome-edited T cell therapies. Curr. Stem Cell Rep. 3, 124–136 (2017).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Grupp, S. A. et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia. Blood 132, 895–895 (2018).
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20, 31–42 (2019).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Liu, J., Zhou, G., Zhang, L. & Zhao, Q. Building potent chimeric antigen receptor T cells with CRISPR genome editing. Front. Immunol. 10, 456 (2019).
Kazi, S. et al. Long-term follow up after third-party viral-specific cytotoxic lymphocytes for immunosuppression- and Epstein–Barr virus-associated lymphoproliferative disease. Haematologica 104, e356–e359 (2019).
Rosenberg, S. A. et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 17, 4550–4557 (2011).
Andersen, R. et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin. Cancer Res. 22, 3734–3745 (2016).
Stevanovic, S. et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin. Cancer Res. 25, 1486–1493 (2019).
Stevanovic, S. et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356, 200–205 (2017).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Goff, S. L. et al. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J. Immunother. 33, 840–847 (2010).
Yarchoan, M., Johnson, B. A. 3rd, Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 17, 569 (2017).
Garber, K. Pursuit of tumor-infiltrating lymphocyte immunotherapy speeds up. Nat. Biotechnol. 37, 969–971 (2019).
Mikkilineni, L. & Kochenderfer, J. N. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 130, 2594–2602 (2017).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl Med. 7, 303ra139 (2015).
Locke, F. L. et al. Durability of response in ZUMA-1, the pivotal phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with refractory large B-cell lymphoma. J. Clin. Oncol. 36, 3003 (2018).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Abramson, J. S. et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J. Clin. Oncol. 36, 7505 (2018).
Abramson, J. S. et al. Lisocabtagene maraleucel (liso-cel) treatment of patients (pts) with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL) and secondary CNS lymphoma: initial results from TRANSCEND NHL 001. J. Clin. Oncol. 37, 7515 (2019).
Jacobson, C. A. et al. Interim analysis of ZUMA-5: a phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL). J. Clin. Oncol. 38, 8008 (2020).
Zhao, W. H. et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 11, 141 (2018).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: safety and preliminary efficacy in combination with anti-PD-1 agent. J. Clin. Oncol. 37, 2511 (2019).
TCR2 Therapeutics. TCR2 announces recist response in ovarian cancer from ongoing phase 1/2 trial of TC-210 in treatment refractory mesothelin-expressing solid tumors — press release. TCR2 Therapeutics https://investors.tcr2.com/news-releases/news-release-details/tcr2-announces-recist-response-ovarian-cancer-ongoing-phase-12 (2020).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Brudno, J. N. & Kochenderfer, J. N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 34, 45–55 (2019).
Ying, Z. et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 25, 947–953 (2019).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
D’Angelo, S. P. et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1c259T cells in synovial sarcoma. Cancer Discov. 8, 944–957 (2018).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Stadtmauer, E. A. et al. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv. 3, 2022–2034 (2019).
Hong, D. S. et al. Phase I dose escalation and expansion trial to assess the safety and efficacy of ADP-A2M4 SPEAR T cells in advanced solid tumors. J. Clin. Oncol. 38 (Suppl. 15), 102 (2020).
Van Tine, B. A. et al. Durable responses in patients with synovial sarcoma in the phase I trials of ADP-A2M4 (MAGE-A4). CTOS https://www.eventscribe.net/2020/CTOS/agenda.asp?pfp=ON (2020).
Barrett, D. M., Grupp, S. A. & June, C. H. Chimeric antigen receptor- and TCR-modified T cells enter main street and wall street. J. Immunol. 195, 755–761 (2015).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
Duinkerken, C. W. et al. Sensorineural hearing loss after adoptive cell immunotherapy for melanoma using MART-1 specific T cells: a case report and its pathophysiology. Otol. Neurotol. 40, e674–e678 (2019).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
van Zelm, M. C. et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Invest. 120, 1265–1274 (2010).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Shalabi, H. et al. Sequential loss of tumor surface antigens following chimeric antigen receptor T-cell therapies in diffuse large B-cell lymphoma. Haematologica 103, e215–e218 (2018).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Rosenthal, R. et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 (2019).
Brown, C. E. et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 21, 4062–4072 (2015).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl Med. 9, eaaa0984 (2017).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Crespo, J., Sun, H., Welling, T. H., Tian, Z. & Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Loffek, S. Transforming of the tumor microenvironment: implications for TGF-β inhibition in the context of immune-checkpoint therapy. J. Oncol. 2018, 9732939 (2018).
Whiteside, T. L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets 22, 353–363 (2018).
Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 14, 1014–1022 (2013).
Irving, M., Vuillefroy de Silly, R., Scholten, K., Dilek, N. & Coukos, G. Engineering chimeric antigen receptor T-cells for racing in solid tumors: don’t forget the fuel. Front. Immunol. 8, 267 (2017).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, eaau0135 (2019).
O’Sullivan, D., Sanin, D. E., Pearce, E. J. & Pearce, E. L. Metabolic interventions in the immune response to cancer. Nat. Rev. Immunol. 19, 324–335 (2019).
Bengsch, B. et al. Epigenomic-guided mass cytometry profiling reveals disease-specific features of exhausted CD8 T cells. Immunity 48, 1029–1045.e5 (2018).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Gazon, H., Barbeau, B., Mesnard, J. M. & Peloponese, J. M. Jr. Hijacking of the AP-1 signaling pathway during development of ATL. Front. Microbiol. 8, 2686 (2017).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Klebanoff, C. A., Rosenberg, S. A. & Restifo, N. P. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat. Med. 22, 26–36 (2016).
Maus, M. V. & June, C. H. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 22, 1875–1884 (2016).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Schneider, D. et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J. Immunother. Cancer 5, 42 (2017).
Zhao, J., Song, Y. & Liu, D. Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. J. Hematol. Oncol. 12, 17 (2019).
Achkova, D. & Pule, M. CAR T-cell integration of multiple input signals allows for precise targeting of cancer. Cancer Discov. 8, 918–920 (2018).
Srivastava, S. et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 35, 489–503.e8 (2019).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167, 419–432.e16 (2016).
Mardiana, S., Solomon, B. J., Darcy, P. K. & Beavis, P. A. Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci. Transl Med. 11, eaaw2293 (2019).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Rupp, L. J. et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737 (2017).
Yin, Y. et al. Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol. Ther. Oncolytics 11, 20–38 (2018).
Curran, K. J. et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol. Ther. 23, 769–778 (2015).
Huang, Y. et al. Interleukin-armed chimeric antigen receptor-modified T cells for cancer immunotherapy. Gene Ther. 25, 192–197 (2018).
Koneru, M., Purdon, T. J., Spriggs, D., Koneru, S. & Brentjens, R. J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4, e994446 (2015).
Chmielewski, M., Kopecky, C., Hombach, A. A. & Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–5706 (2011).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Wang, L. C. et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2, 154–166 (2014).
Barrett, J. A. et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 25, 106–116 (2018).
Rodgers, D. T. et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl Acad. Sci. USA 113, E459–E468 (2016).
Wu, C. et al. Development of an inducible caspase-9 safety switch for pluripotent stem cell-based therapies. Mol. Ther. Methods Clin. Dev. 1, 14053 (2014).
Stavrou, M. et al. A rapamycin-activated caspase 9-based suicide gene. Mol. Ther. 26, 1266–1276 (2018).
Greco, R. et al. Improving the safety of cell therapy with the TK-suicide gene. Front. Pharmacol. 6, 95 (2015).
Voorwerk, L. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928 (2019).
Borghaei, H. et al. 24-month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 14, 124–129 (2019).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Al-Hadidi, S. A., Chuang, H. H., Miranda, R. N. & Lee, H. J. Programmed cell death-one inhibition therapy in classical Hodgkin lymphoma. Clin. Lymphoma Myeloma Leuk. 21, e105–e111 (2021).
Nagpal, P., Descalzi-Montoya, D. B. & Lodhi, N. The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes. Cancer Rep. https://doi.org/10.1002/cnr2.1311 (2020).
Ferrarini, I., Rigo, A., Visco, C., Krampera, M. & Vinante, F. The evolving knowledge on T and NK cells in classic Hodgkin lymphoma: insights into novel subsets populating the immune microenvironment. Cancers 12, 3757 (2020).
Lees, C., Keane, C., Gandhi, M. K. & Gunawardana, J. Biology and therapy of primary mediastinal B-cell lymphoma: current status and future directions. Br. J. Haematol. 185, 25–41 (2019).
Ma, J., Setton, J., Lee, N. Y., Riaz, N. & Powell, S. N. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Nat. Commun. 9, 3292 (2018).
Richman, S. Deficient mismatch repair: read all about it (Review). Int. J. Oncol. 47, 1189–1202 (2015).
Hargadon, J. M., Johnson, C. E. & Williams, C. J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 62, 29–39 (2018).
Chesney, J. et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 36, 1658–1667 (2018).
Conry, R. M., Westbrook, B., McKee, S. & Norwood, T. G. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum. Vaccin. Immunother. 14, 839–846 (2018).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Andre, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Merck. Keytruda (pembrolizumab) prescribing information. Merck https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (2020).
Bristol Myers Squibb. Opdivo (nivolumab) prescribing information. BMS https://packageinserts.bms.com/pi/pi_opdivo.pdf (2020).
Ribas, A. et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622 (2013).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet. Oncol. 15, 700–712 (2014).
Wang, M. et al. Safety and preliminary efficacy in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL) receiving lisocabtagene maraleucel (Liso-cel) in TRANSCEND NHL 001. J. Clin. Oncol. 37, 7516 (2019).
Schmid, P. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 379, 2108–2121 (2018).
Cortes, J. et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 38, 1000 (2020).
US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer (FDA, 2020).
Meister, A., Hentrich, M., Wyen, C. & Hubel, K. Malignant lymphoma in the HIV-positive patient. Eur. J. Haematol. 101, 119–126 (2018).
Goncalves, P. H., Ziegelbauer, J., Uldrick, T. S. & Yarchoan, R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Curr. Opin. HIV AIDS 12, 47–56 (2017).
Engels, E. A. et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306, 1891–1901 (2011).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Salles, G. et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv. Ther. 34, 2232–2273 (2017).
Demichelis-Gomez, R., Perez-Samano, D. & Bourlon, C. Bispecific antibodies in hematologic malignancies: when, to whom, and how should be best used? Curr. Oncol. Rep. 21, 17 (2019).
Trudel, S. et al. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): a dose escalation and expansion phase 1 trial. Lancet Oncol. 19, 1641–1653 (2018).
Trudel, S. et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 9, 37 (2019).
OK, C. Y. & Young, K. H. Checkpoint inhibitors in hematological malignancies. J. Hematol. Oncol. 10, 103 (2017).
Fischbach, M. A., Bluestone, J. A. & Lim, W. A. Cell-based therapeutics: the next pillar of medicine. Sci. Transl Med. 5, 179ps177 (2013).
Martinez, M. & Moon, E. K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front. Immunol. 10, 128 (2019).
Yu, J. X., Upadhaya, S., Tatake, R., Barkalow, F. & Hubbard-Lucey, V. M. Cancer cell therapies: the clinical trial landscape. Nat. Rev. Drug Discov. 19, 583–584 (2020).
US Food and Drug Administration. Statement from FDA commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies (FDA, 2019).
Seitz, K. & Zhou, H. Pharmacokinetic drug–drug interaction potentials for therapeutic monoclonal antibodies: reality check. J. Clin. Pharmacol. 47, 1104–1118 (2007).
Baruch, E. N., Berg, A. L., Besser, M. J., Schachter, J. & Markel, G. Adoptive T cell therapy: an overview of obstacles and opportunities. Cancer 123, 2154–2162 (2017).
Morrow, T. & Felcone, L. H. Defining the difference: what makes biologics unique. Biotechnol. Healthc. 1, 24–29 (2004).
Ramachandran, I. et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J. Immunother. Cancer 7, 276 (2019).
Brudno, J. N. et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 34, 1112–1121 (2016).
US Food and Drug Administration. Exploratory IND studies. Guidance for industry, investigators, and reviewers (FDA, 2006).
Friends of Cancer Research & Parker Institute for Cancer Immunotherapy. Designing the future of cell therapies. Friends of Cancer Research https://www.focr.org/sites/default/files/pdf/Friends_Cellular_Therapies_White_Paper.pdf (2019).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377, 62–70 (2017).
Gottlieb, S. Remarks to the Alliance for Regenerative Medicine’s annual board meeting (FDA, 2018).
Moutsatsou, P., Ochs, J., Schmitt, R. H., Hewitt, C. J. & Hanga, M. P. Automation in cell and gene therapy manufacturing: from past to future. Biotechnol. Lett. 41, 1245–1253 (2019).
US Food and Drug Administration. Current good manufacturing practice for phase I investigational drugs (FDA, 2008).
Stewart, M. D. et al. Accelerating the development of innovative cellular therapy products for the treatment of cancer. Cytotherapy 22, 239–246 (2020).
McCarron, A., Donnelley, M., McIntyre, C. & Parsons, D. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 240, 23–30 (2016).
Papathanasiou, M. M. et al. Autologous CAR T-cell therapies supply chain: challenges and opportunities? Cancer Gene Ther. 27, 799–809 (2020).
Wang, X. & Riviere, I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics 3, 16015 (2016).
O’Connor, R. S. et al. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 189, 1330–1339 (2012).
McGuirk, J. et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy 19, 1015–1024 (2017).
Perica, K., Curran, K. J., Brentjens, R. J. & Giralt, S. A. Building a CAR garage: preparing for the delivery of commercial CAR T cell products at Memorial Sloan Kettering Cancer Center. Biol. Blood Marrow Transpl. 24, 1135–1141 (2018).
Iyer, R. K., Bowles, P. A., Kim, H. & Dulgar-Tulloch, A. Industrializing autologous adoptive immunotherapies: manufacturing advances and challenges. Front. Med. 5, 150 (2018).
Kaiser, A. D. et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 22, 72–78 (2015).
Buhl, T. et al. Controlled-rate freezer cryopreservation of highly concentrated peripheral blood mononuclear cells results in higher cell yields and superior autologous T-cell stimulation for dendritic cell-based immunotherapy. Cancer Immunol. Immunother. 61, 2021–2031 (2012).
Massie, I. et al. GMP cryopreservation of large volumes of cells for regenerative medicine: active control of the freezing process. Tissue Eng Part C Methods 20, 693–702 (2014).
Priesner, C. et al. Automated enrichment, transduction, and expansion of clinical-scale CD62L+ T cells for manufacturing of gene therapy medicinal products. Hum. Gene Ther. 27, 860–869 (2016).
Mock, U. et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy 18, 1002–1011 (2016).
US Food and Drug Administration. Chemistry, manufacturing and control (CMC) information for human gene therapy investigational new drug applications (INDs) (FDA, 2020).
European Commission. Guidelines on good manufacturing practice specific to advanced therapy medicinal products (EC, 2017).
Cauchon, N. S., Oghamian, S., Hassanpour, S. & Abernathy, M. Innovation in chemistry, manufacturing, and controls — a regulatory perspective from industry. J. Pharm. Sci. 108, 2207–2504 (2019).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987).
Maher, J., Brentjens, R. J., Gunset, G., Riviere, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Anderson, V. E. et al. Abstract 2313: enhanced activity of second-generation MAGE-A4 SPEAR T-cells through co-expression of a CD8α homodimer. Cancer Res. 79, 2313–2313 (2019).
Yu, S., Yi, M., Qin, S. & Wu, K. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol. Cancer 18, 125 (2019).
Aftab, B. T. et al. Toward “off-the-shelf” allogeneic CAR T cells. Adv. Cell Gene Ther. 3, e86 (2020).
Zhao, J., Lin, Q., Song, Y. & Liu, D. Universal CARs, universal T cells, and universal CAR T cells. J. Hematol. Oncol. 11, 132–132 (2018).
Acknowledgements
This work was funded by GlaxoSmithKline (GSK). Editorial support was provided by F. Woodward and G. Corr of Fishawack Indicia Ltd, UK, and was funded by GSK.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.S. is an employee of GlaxoSmithKline (GSK) and owns stocks/shares. C.M.B. was an employee of GSK and owns stocks/shares; he recently moved to Immatics Biotechnologies GmbH. A.H. is an employee of GSK and holds stocks/shares, and is a non-executive director and shareholder of Imugene and TCR2 Therapeutics.
Additional information
Peer review information
Nature Reviews Drug Discovery thanks Piotr Pierog, David Gilham and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alliance for Regenerative Medicine: https://alliancerm.org/
American Society for Transplantation and Cellular Therapy: https://www.astct.org/home
American Society of Gene and Cell Therapy: https://www.asgct.org/
European Society for Blood and Marrow Transplantation: https://www.ebmt.org/
Friends of Cancer Research: https://friendsofcancerresearch.org/
Supplementary information
Rights and permissions
About this article
Cite this article
Britten, C.M., Shalabi, A. & Hoos, A. Industrializing engineered autologous T cells as medicines for solid tumours. Nat Rev Drug Discov 20, 476–488 (2021). https://doi.org/10.1038/s41573-021-00175-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00175-8
This article is cited by
-
Advancing CAR T cell therapy through the use of multidimensional omics data
Nature Reviews Clinical Oncology (2023)
-
A digital platform for the design of patient-centric supply chains
Scientific Reports (2022)
-
Biomarker correlates with response to NY-ESO-1 TCR T cells in patients with synovial sarcoma
Nature Communications (2022)