Abstract
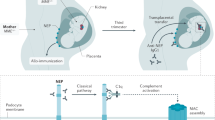
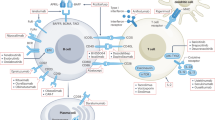
IgA nephropathy (IgAN), the most prevalent primary glomerulonephritis worldwide, carries a considerable lifetime risk of kidney failure. Clinical manifestations of IgAN vary from asymptomatic with microscopic or intermittent macroscopic haematuria and stable kidney function to rapidly progressive glomerulonephritis. IgAN has been proposed to develop through a ‘four-hit’ process, commencing with overproduction and increased systemic presence of poorly O-glycosylated galactose-deficient IgA1 (Gd-IgA1), followed by recognition of Gd-IgA1 by antiglycan autoantibodies, aggregation of Gd-IgA1 and formation of polymeric IgA1 immune complexes and, lastly, deposition of these immune complexes in the glomerular mesangium, leading to kidney inflammation and scarring. IgAN can only be diagnosed by kidney biopsy. Extensive, optimized supportive care is the mainstay of therapy for patients with IgAN. For those at high risk of disease progression, the 2021 KDIGO Clinical Practice Guideline suggests considering a 6-month course of systemic corticosteroid therapy; however, the efficacy of systemic steroid treatment is under debate and serious adverse effects are common. Advances in understanding the pathophysiology of IgAN have led to clinical trials of novel targeted therapies with acceptable safety profiles, including SGLT2 inhibitors, endothelin receptor blockers, targeted-release budesonide, B cell proliferation and differentiation inhibitors, as well as blockade of complement components.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pattrapornpisut, P., Avila-Casado, C. & Reich, H. N. IgA nephropathy: core curriculum 2021. Am. J. Kidney Dis. 78, 429–441 (2021).
Berthoux, F. et al. Predicting the risk for dialysis or death in IgA nephropathy. J. Am. Soc. Nephrol. 22, 752–761 (2011).
Jarrick, S. et al. Mortality in IgA nephropathy: a nationwide population-based cohort study. J. Am. Soc. Nephrol. 30, 866–876 (2019).
Moriyama, T. et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS ONE 9, e91756 (2014).
Okonogi, H. et al. A grading system that predicts the risk of dialysis induction in IgA nephropathy patients based on the combination of the clinical and histological severity. Clin. Exp. Nephrol. 23, 16–25 (2019).
Suzuki, H. et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J. Clin. Invest. 118, 629–639 (2008).
Suzuki, H. et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 22, 1795–1803 (2011).
Xin, G. et al. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J. Nephrol. 26, 683–690 (2013).
Zhai, Y. L. et al. Increased APRIL expression induces IgA1 aberrant glycosylation in IgA nephropathy. Medicine 95, e3099 (2016).
Lafayette, R. et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet 402, 859–870 (2023).
Wheeler, D. C. et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 100, 215–224 (2021).
Heerspink, H. J. L. et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 401, 1584–1594 (2023).
Rodrigues, J. C., Haas, M. & Reich, H. N. IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12, 677–686 (2017).
Xie, Y. & Chen, X. Epidemiology, major outcomes, risk factors, prevention and management of chronic kidney disease in China. Am. J. Nephrol. 28, 1–7 (2008).
Schena, F. P. & Nistor, I. Epidemiology of IgA nephropathy: a global perspective. Semin. Nephrol. 38, 435–442 (2018).
Okpechi, I. G. et al. Epidemiology of histologically proven glomerulonephritis in Africa: a systematic review and meta-analysis. PLoS ONE 11, e0152203 (2016).
Nair, R. & Walker, P. D. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 69, 1455–1458 (2006).
Kiryluk, K., Novak, J. & Gharavi, A. G. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu. Rev. Med. 64, 339–356 (2013).
Sanchez-Rodriguez, E., Southard, C. T. & Kiryluk, K. GWAS-based discoveries in IgA nephropathy, membranous nephropathy, and steroid-sensitive nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 16, 458–466 (2021).
Feehally, J. et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J. Am. Soc. Nephrol. 21, 1791–1797 (2010).
Gharavi, A. G. et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 43, 321–327 (2011).
Kiryluk, K. & Novak, J. The genetics and immunobiology of IgA nephropathy. J. Clin. Invest. 124, 2325–2332 (2014).
Kiryluk, K. et al. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat. Genet. 55, 1091–1105 (2023).
Magistroni, R., D’Agati, V. D., Appel, G. B. & Kiryluk, K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 88, 974–989 (2015).
Lai, K. N. et al. IgA nephropathy. Nat. Rev. Dis. Primers 2, 16001 (2016).
Shen, P. et al. Clinicopathological characteristics and outcome of adult patients with hematuria and/or proteinuria found during routine examination. Nephron Clin. Pract. 103, c149–c156 (2006).
Moldoveanu, Z. et al. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 71, 1148–1154 (2007).
Allen, A. C., Bailey, E. M., Barratt, J., Buck, K. S. & Feehally, J. Analysis of IgA1 O-glycans in IgA nephropathy by fluorophore-assisted carbohydrate electrophoresis. J. Am. Soc. Nephrol. 10, 1763–1771 (1999).
Hiki, Y. et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 59, 1077–1085 (2001).
Xu, L. X. & Zhao, M. H. Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int. 68, 167–172 (2005).
Barratt, J., Smith, A. C. & Feehally, J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology 12, 275–284 (2007).
Dotz, V. et al. O- and N-glycosylation of serum immunoglobulin A is associated with IgA nephropathy and glomerular function. J. Am. Soc. Nephrol. 32, 2455–2465 (2021).
Ohyama, Y., Renfrow, M. B., Novak, J. & Takahashi, K. Aberrantly glycosylated IgA1 in IgA nephropathy: what we know and what we don’t know. J. Clin. Med. 10, 3467 (2021).
Gesualdo, L., Di Leo, V. & Coppo, R. The mucosal immune system and IgA nephropathy. Semin. Immunopathol. 43, 657–668 (2021). A very good review of the role of the mucosal immune system in IgAN.
Franc, V. et al. Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: role of cysteine alkylation during sample processing. J. Proteom. 92, 299–312 (2013).
Yamada, K. et al. Down-regulation of core 1 β1,3-galactosyltransferase and Cosmc by Th2 cytokine alters O-glycosylation of IgA1. Nephrol. Dial. Transpl. 25, 3890–3897 (2010).
Suzuki, H. et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J. Biol. Chem. 289, 5330–5339 (2014).
Xing, Y. et al. C1GALT1 expression is associated with galactosylation of IgA1 in peripheral B lymphocyte in immunoglobulin a nephropathy. BMC Nephrol. 21, 18 (2020).
Serino, G., Sallustio, F., Cox, S. N., Pesce, F. & Schena, F. P. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J. Am. Soc. Nephrol. 23, 814–824 (2012).
Serino, G. et al. Role of let-7b in the regulation of N-acetylgalactosaminyltransferase 2 in IgA nephropathy. Nephrol. Dial. Transpl. 30, 1132–1139 (2015).
Serino, G. et al. In a retrospective international study, circulating miR-148b and let-7b were found to be serum markers for detecting primary IgA nephropathy. Kidney Int. 89, 683–692 (2016).
Wang, Z. et al. Small RNA deep sequencing reveals novel miRNAs in peripheral blood mononuclear cells from patients with IgA nephropathy. Mol. Med. Rep. 22, 3378–3386 (2020).
Gale, D. P. et al. Galactosylation of IgA1 is associated with common variation in C1GALT1. J. Am. Soc. Nephrol. 28, 2158–2166 (2017). This study demonstrates that common variation at C1GALT1 influences the Gd-IgA1 level in the population, which is independently associated with risk of progressive IgAN.
Gharavi, A. G. et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J. Am. Soc. Nephrol. 19, 1008–1014 (2008).
Xie, Y., Chen, X., Nishi, S., Narita, I. & Gejyo, F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 65, 1135–1144 (2004).
Floege, J. & Feehally, J. The mucosa–kidney axis in IgA nephropathy. Nat. Rev. Nephrol. 12, 147–156 (2016).
Harper, S. J. et al. Expression of J chain mRNA in duodenal IgA plasma cells in IgA nephropathy. Kidney Int. 45, 836–844 (1994).
Yeo, S. C., Cheung, C. K. & Barratt, J. New insights into the pathogenesis of IgA nephropathy. Pediatr. Nephrol. 33, 763–777 (2018).
Batra, A., Smith, A. C., Feehally, J. & Barratt, J. T-cell homing receptor expression in IgA nephropathy. Nephrol. Dial. Transpl. 22, 2540–2548 (2007).
Buren, M., Yamashita, M., Suzuki, Y., Tomino, Y. & Emancipator, S. N. Altered expression of lymphocyte homing chemokines in the pathogenesis of IgA nephropathy. Contrib. Nephrol. 157, 50–55 (2007).
Zachova, K. et al. Galactose-deficient IgA1 B cells in the circulation of IgA nephropathy patients carry preferentially lambda light chains and mucosal homing receptors. J. Am. Soc. Nephrol. 33, 908–917 (2022).
Kano, T. et al. Mucosal immune system dysregulation in the pathogenesis of IgA nephropathy. Biomedicines 10, 3027 (2022).
Kawabe, M. et al. Association between galactose-deficient IgA1 derived from the tonsils and recurrence of IgA nephropathy in patients who underwent kidney transplantation. Front. Immunol. 11, 2068 (2020).
Rovin, B. H. et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 100, 753–779 (2021). This is the KDIGO practice guideline recommended for IgAN.
Schmitt, R. et al. Tissue deposits of IgA-binding streptococcal M proteins in IgA nephropathy and Henoch–Schönlein purpura. Am. J. Pathol. 176, 608–618 (2010).
Watanabe, H. et al. Comprehensive microbiome analysis of tonsillar crypts in IgA nephropathy. Nephrol. Dial. Transpl. 32, 2072–2079 (2017).
Park, J. I. et al. Comparative analysis of the tonsillar microbiota in IgA nephropathy and other glomerular diseases. Sci. Rep. 10, 16206 (2020).
Kiryluk, K. et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 46, 1187–1196 (2014). A large GWA study that suggests that interactions between the host and intestinal pathogens may influence the genetics of IgAN.
Rehnberg, J., Symreng, A., Ludvigsson, J. F. & Emilsson, L. Inflammatory bowel disease is more common in patients with IgA nephropathy and predicts progression of ESKD: a Swedish population-based cohort study. J. Am. Soc. Nephrol. 32, 411–423 (2021).
Barratt, J. et al. Why target the gut to treat IgA nephropathy? Kidney Int. Rep. 5, 1620–1624 (2020).
De Angelis, M. et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS ONE 9, e99006 (2014).
Hobby, G. P. et al. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Ren. Physiol. 316, F1211–F1217 (2019).
Sallustio, F. et al. High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol. Dial. Transpl. 36, 452–464 (2021).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Donadio, M. E. et al. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch–Schönlein purpura and primary IgA nephropathy. Pediatr. Nephrol. 29, 1545–1551 (2014).
Zheng, N. et al. TLR7 in B cells promotes renal inflammation and Gd-IgA1 synthesis in IgA nephropathy. JCI Insight 5, e136965 (2020).
Ciferska, H. et al. Does the renal expression of Toll-like receptors play a role in patients with IgA nephropathy? J. Nephrol. 33, 307–316 (2020).
Li, W. et al. TLR9 and BAFF: their expression in patients with IgA nephropathy. Mol. Med. Rep. 10, 1469–1474 (2014).
Qin, W. et al. External suppression causes the low expression of the Cosmc gene in IgA nephropathy. Nephrol. Dial. Transpl. 23, 1608–1614 (2008).
Cheema, G. S., Roschke, V., Hilbert, D. M. & Stohl, W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 44, 1313–1319 (2001).
McCarthy, D. D. et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J. Clin. Invest. 121, 3991–4002 (2011).
Goto, T. et al. Increase in B-cell-activation factor (BAFF) and IFN-γ productions by tonsillar mononuclear cells stimulated with deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in patients with IgA nephropathy. Clin. Immunol. 126, 260–269 (2008).
Ye, M. et al. Vibration induces BAFF overexpression and aberrant O-glycosylation of IgA1 in cultured human tonsillar mononuclear cells in IgA nephropathy. Biomed. Res. Int. 2016, 9125960 (2016).
Shao, J. et al. Capsaicin induces high expression of BAFF and aberrantly glycosylated IgA1 of tonsillar mononuclear cells in IgA nephropathy patients. Hum. Immunol. 75, 1034–1039 (2014).
Xin, G. et al. Serum BAFF and APRIL might be associated with disease activity and kidney damage in patients with anti-glomerular basement membrane disease. Nephrology 18, 209–214 (2013).
Muto, M. et al. Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J. Am. Soc. Nephrol. 28, 1227–1238 (2017).
Takahara, M. et al. A proliferation-inducing ligand (APRIL) induced hyper-production of IgA from tonsillar mononuclear cells in patients with IgA nephropathy. Cell Immunol. 341, 103925 (2019).
Makita, Y. et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 97, 340–349 (2020).
Suzuki, H. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 119, 1668–1677 (2009).
Tomana, M. et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Invest. 104, 73–81 (1999).
Tomana, M. et al. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 52, 509–516 (1997).
Suzuki, H. & Novak, J. IgA glycosylation and immune complex formation in IgAN. Semin. Immunopathol. 43, 669–678 (2021).
Knoppova, B. et al. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front. Immunol. 7, 117 (2016).
Knoppova, B. et al. Pathogenesis of IgA nephropathy: current understanding and implications for development of disease-specific treatment. J. Clin. Med. 10, 4051 (2021).
Moldoveanu, Z. et al. Experimental evidence of pathogenic role of IgG autoantibodies in IgA nephropathy. J. Autoimmun. 118, 102593 (2021).
Matsumoto, Y. et al. Identification and characterization of circulating immune complexes in IgA nephropathy. Sci. Adv. 8, eabm8783 (2022).
Xu, B. et al. Mass spectrometry-based screening identifies circulating immunoglobulinA-α1-microglobulin complex as potential biomarker in immunoglobulin A nephropathy. Nephrol. Dial. Transpl. 36, 782–792 (2021).
van Zandbergen, G. et al. Crosslinking of the human Fc receptor for IgA (FcαRI/CD89) triggers FcR γ-chain-dependent shedding of soluble CD89. J. Immunol. 163, 5806–5812 (1999).
Launay, P. et al. Fcα receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J. Exp. Med. 191, 1999–2009 (2000).
van Dijk, T. B. et al. Cloning and characterization of Fc alpha Rb, a novel Fc alpha receptor (CD89) isoform expressed in eosinophils and neutrophils. Blood 88, 4229–4238 (1996).
Cambier, A. et al. Soluble CD89 is a critical factor for mesangial proliferation in childhood IgA nephropathy. Kidney Int. 101, 274–287 (2022).
Berthelot, L. et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J. Exp. Med. 209, 793–806 (2012).
Jhee, J. H. et al. Circulating CD89-IgA complex does not predict deterioration of kidney function in Korean patients with IgA nephropathy. Clin. Chem. Lab. Med. 56, 75–85 (2017).
Moura, I. C. et al. Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J. Am. Soc. Nephrol. 16, 2667–2676 (2005).
Ikee, R. et al. Involvement of transglutaminase-2 in pathological changes in renal disease. Nephron Clin. Pract. 105, c139–c146 (2007).
Moresco, R. N. et al. Urinary myeloid IgA Fc alpha receptor (CD89) and transglutaminase-2 as new biomarkers for active IgA nephropathy and Henoch-Schönlein purpura nephritis. BBA Clin. 5, 79–84 (2016).
Barratt, J., Feehally, J. & Smith, A. C. Pathogenesis of IgA nephropathy. Semin. Nephrol. 24, 197–217 (2004).
Molyneux, K. et al. β1,4-galactosyltransferase 1 is a novel receptor for IgA in human mesangial cells. Kidney Int. 92, 1458–1468 (2017).
Mocsai, A., Ruland, J. & Tybulewicz, V. L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 (2010).
McAdoo, S. P. et al. Correlation of disease activity in proliferative glomerulonephritis with glomerular spleen tyrosine kinase expression. Kidney Int. 88, 52–60 (2015).
Kim, M. J. et al. Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J. Immunol. 189, 3751–3758 (2012).
Tamouza, H. et al. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 82, 1284–1296 (2012).
Makita, Y. et al. Glomerular deposition of galactose-deficient IgA1-containing immune complexes via glomerular endothelial cell injuries. Nephrol. Dial. Transpl. 37, 1629–1636 (2022).
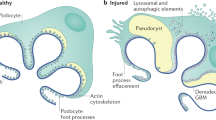
Lai, K. N. et al. Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrol. Dial. Transpl. 24, 62–72 (2009).
Wang, C. et al. Effect of aggregated immunoglobulin A1 from immunoglobulin A nephropathy patients on nephrin expression in podocytes. Nephrology 14, 213–218 (2009).
Lai, K. N. et al. Activation of podocytes by mesangial-derived TNF-α: glomerulo-podocytic communication in IgA nephropathy. Am. J. Physiol. Ren. Physiol. 294, F945–F955 (2008).
Chan, L. Y., Leung, J. C., Tsang, A. W., Tang, S. C. & Lai, K. N. Activation of tubular epithelial cells by mesangial-derived TNF-α: glomerulotubular communication in IgA nephropathy. Kidney Int. 67, 602–612 (2005).
Zambrano, S. et al. Molecular insights into the early stage of glomerular injury in IgA nephropathy using single-cell RNA sequencing. Kidney Int. 101, 752–765 (2022).
Wu, D. et al. Mesangial C3 deposition and serum C3 levels predict renal outcome in IgA nephropathy. Clin. Exp. Nephrol. 25, 641–651 (2021).
Nam, K. H. et al. Predictive value of mesangial C3 and C4d deposition in IgA nephropathy. Clin. Immunol. 211, 108331 (2020).
Zwirner, J. et al. Activated complement C3: a potentially novel predictor of progressive IgA nephropathy. Kidney Int. 51, 1257–1264 (1997).
Kim, S. J. et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS ONE 7, e40495 (2012).
Mizerska-Wasiak, M. et al. Relationship between serum IgA/C3 ratio and severity of histological lesions using the Oxford classification in children with IgA nephropathy. Pediatr. Nephrol. 30, 1113–1120 (2015).
Evans, D. J. et al. Glomerular deposition of properdin in Henoch-Schönlein syndrome and idiopathic focal nephritis. Br. Med. J. 3, 326–328 (1973).
Rauterberg, E. W., Lieberknecht, H. M., Wingen, A. M. & Ritz, E. Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int. 31, 820–829 (1987).
Miyazaki, R. et al. Glomerular deposition and serum levels of complement control proteins in patients with IgA nephropathy. Clin. Nephrol. 21, 335–340 (1984).
Tomino, Y., Endoh, M., Nomoto, Y. & Sakai, H. Double immunofluorescence studies of immunoglobulins, complement components and their control proteins in patients with IgA nephropathy. Acta Pathol. Jpn. 32, 251–256 (1982).
Segarra, A. et al. Mesangial C4d deposits in early IgA nephropathy. Clin. J. Am. Soc. Nephrol. 13, 258–264 (2018).
Lee, H. J. et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin. Nephrol. 80, 98–104 (2013).
Tan, L. et al. A multicenter, prospective, observational study to determine association of mesangial C1q deposition with renal outcomes in IgA nephropathy. Sci. Rep. 11, 5467 (2021).
Chang, S. & Li, X. K. The role of immune modulation in pathogenesis of IgA nephropathy. Front. Med. 7, 92 (2020).
Hiemstra, P. S., Gorter, A., Stuurman, M. E., Es, L. A.van & Daha, M. R. Activation of the alternative pathway of complement by human serum IgA. Eur. J. Immunol. 17, 321–326 (1987).
Russell, M. W. & Mansa, B. Complement-fixing properties of human IgA antibodies. Alternative pathway complement activation by plastic-bound, but not specific antigen-bound, IgA. Scand. J. Immunol. 30, 175–183 (1989).
Poppelaars, F., Faria, B., Schwaeble, W. & Daha, M. R. The contribution of complement to the pathogenesis of IgA nephropathy: are complement-targeted therapies moving from rare disorders to more common diseases? J. Clin. Med. 10, 4715 (2021). A very good review of the role of complement in IgAN.
Guo, W. Y. et al. Glomerular complement factor H-related protein 5 is associated with histologic injury in immunoglobulin A nephropathy. Kidney Int. Rep. 6, 404–413 (2021).
Poppelaars, F. et al. A family affair: addressing the challenges of factor H and the related proteins. Front. Immunol. 12, 660194 (2021).
Tortajada, A. et al. Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 92, 953–963 (2017).
Roos, A. et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J. Am. Soc. Nephrol. 17, 1724–1734 (2006).
Barratt, J. et al. IgA nephropathy: the lectin pathway and implications for targeted therapy. Kidney Int. 104, 254–264 (2023). A very good review of the role of the lectin pathway in IgAN.
Faria, B. et al. Arteriolar C4d in IgA nephropathy: a cohort study. Am. J. Kidney Dis. 76, 669–678 (2020).
Liu, L. L., Jiang, Y., Wang, L. N. & Liu, N. Urinary mannose-binding lectin is a biomarker for predicting the progression of immunoglobulin (Ig)A nephropathy. Clin. Exp. Immunol. 169, 148–155 (2012).
Medjeral-Thomas, N. R. et al. Progressive IgA nephropathy is associated with low circulating mannan-binding lectin-associated serine protease-3 (MASP-3) and increased glomerular factor H-related protein 5 (FHR5) deposition. Kidney Int. Rep. 3, 426–438 (2018).
Paunas, T. I. F. et al. Glomerular abundance of complement proteins characterized by proteomic analysis of laser-captured microdissected glomeruli associates with progressive disease in IgA nephropathy. Clin. Proteom. 14, 30 (2017).
Koopman, J. J. E., van Essen, M. F., Rennke, H. G., de Vries, A. P. J. & van Kooten, C. Deposition of the membrane attack complex in healthy and diseased human kidneys. Front. Immunol. 11, 599974 (2020).
Selvaskandan, H., Shi, S., Twaij, S., Cheung, C. K. & Barratt, J. Monitoring immune responses in IgA nephropathy: biomarkers to guide management. Front. Immunol. 11, 572754 (2020).
Cho, B. S., Kim, S. D., Choi, Y. M. & Kang, H. H. School urinalysis screening in Korea: prevalence of chronic renal disease. Pediatr. Nephrol. 16, 1126–1128 (2001).
Murakami, M. Screening for proteinuria and hematuria in school children – methods and results. Acta Paediatr. Jpn. 32, 682–689 (1990).
Rivera, F., Lopez-Gomez, J. M. & Perez-Garcia, R., Spanish Registry of Glomerulonephritis. Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 66, 898–904 (2004).
Gutierrez, E. et al. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2, 51–57 (2007).
Tissandie, E. et al. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int. 80, 1352–1363 (2011).
Saha, M. K., Julian, B. A., Novak, J. & Rizk, D. V. Secondary IgA nephropathy. Kidney Int. 94, 674–681 (2018).
Suzuki, K. et al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 63, 2286–2294 (2003).
Waldherr, R., Rambausek, M., Duncker, W. D. & Ritz, E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol. Dial. Transpl. 4, 943–946 (1989).
Axelsen, R. A. et al. Renal glomerular lesions in unselected patients with cirrhosis undergoing orthotopic liver transplantation. Pathology 27, 237–246 (1995).
Calmus, Y. et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J. Hepatol. 57, 572–576 (2012).
Suzuki, H. et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 93, 700–705 (2018). A study suggesting that IgAN and IgA vasculitis with nephritis have a shared feature regarding galactose-deficient IgA1-oriented pathogenesis.
Kauffmann, R. H., Herrmann, W. A., Meyer, C. J., Daha, M. R. & Van, Es,L. A. Circulating IgA-immune complexes in Henoch-Schönlein purpura. A longitudinal study of their relationship to disease activity and vascular deposition of IgA. Am. J. Med. 69, 859–866 (1980).
Kiryluk, K. et al. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int. 80, 79–87 (2011).
Pillebout, E. & Sunderkotter, C. IgA vasculitis. Semin. Immunopathol. 43, 729–738 (2021).
Ambruzs, J. M., Walker, P. D. & Larsen, C. P. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin. J. Am. Soc. Nephrol. 9, 265–270 (2014).
Ventura, A., Magazzu, G. & Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 117, 297–303 (1999).
Welander, A., Sundelin, B., Fored, M. & Ludvigsson, J. F. Increased risk of IgA nephropathy among individuals with celiac disease. J. Clin. Gastroenterol. 47, 678–683 (2013).
Rollino, C., Vischini, G. & Coppo, R. IgA nephropathy and infections. J. Nephrol. 29, 463–468 (2016).
Bu, R. et al. Clinicopathologic features of IgA-dominant infection-associated glomerulonephritis: a pooled analysis of 78 cases. Am. J. Nephrol. 41, 98–106 (2015).
Huang, Z. et al. Clinicopathological and prognostic study of IgA-dominant postinfectious glomerulonephritis. BMC Nephrol. 22, 248 (2021).
Champtiaux, N. et al. Spondyloarthritis-associated IgA nephropathy. Kidney Int. Rep. 5, 813–820 (2020).
Makino, H. et al. Renal involvement in rheumatoid arthritis: analysis of renal biopsy specimens from 100 patients. Mod. Rheumatol. 12, 148–154 (2002).
Grewal, S. K. et al. The risk of IgA nephropathy and glomerular disease in patients with psoriasis: a population-based cohort study. Br. J. Dermatol. 176, 1366–1369 (2017).
Le, W. et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol. Dial. Transpl. 27, 1479–1485 (2012).
Reich, H. N., Troyanov, S., Scholey, J. W. & Cattran, D. C. Remission of proteinuria improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 18, 3177–3183 (2007).
Inker, L. A. et al. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am. J. kidney Dis. 68, 392–401 (2016).
Knoop, T., Vikse, B. E., Mwakimonga, A., Leh, S. & Bjorneklett, R. Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol. Dial. Transpl. 32, 1841–1850 (2017).
Pitcher, D. et al. Long-term outcomes in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 18, 727–738 (2023).
Roberts, I. S. et al. the Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 76, 546–556 (2009).
Trimarchi, H. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 91, 1014–1021 (2017).
Coppo, R. et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 86, 828–836 (2014).
Itami, S., Moriyama, T., Miyabe, Y., Karasawa, K. & Nitta, K. A novel scoring system based on Oxford classification indicating steroid therapy use for IgA nephropathy. Kidney Int. Rep. 7, 99–107 (2022).
Cambier, A., Troyanov, S., Tesar, V. & Coppo, R., Validation Study of Oxford Classification (VALIGA) Group. Indication for corticosteroids in IgA nephropathy: validation in the European VALIGA cohort of a treatment score based on the Oxford classification. Nephrol. Dial. Transpl. 37, 1195–1197 (2022).
Barbour, S. J. et al. Updating the international IgA nephropathy prediction tool for use in children. Kidney Int. 99, 1439–1450 (2021).
Barbour, S. J. et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern. Med. 179, 942–952 (2019). This study reports a new risk prediction tool in IgAN.
Barbour, S. J. et al. Application of the International IgA Nephropathy Prediction Tool one or two years post-biopsy. Kidney Int. 102, 160–172 (2022). This study reports an updated version of the risk prediction tool in IgAN that can be applied up to 2 years after biopsy.
Haaskjold, Y. L. et al. Validation of two IgA nephropathy risk-prediction tools using a cohort with a long follow-up. Nephrol. Dial. Transpl. 38, 1183–1191 (2023).
Rauen, T. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N. Engl. J. Med. 373, 2225–2236 (2015). To our knowledge, this is the first of the latest clinical trials studying the value of corticosteroids in IgAN.
Floege, J. & Feehally, J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat. Rev. Nephrol. 9, 320–327 (2013).
Cheng, J. et al. ACEI/ARB therapy for IgA nephropathy: a meta analysis of randomised controlled trials. Int. J. Clin. Pract. 63, 880–888 (2009). This meta-analysis investigated the effects of RAASi in IgAN.
Lie, D. N. W. et al. Long-term outcomes of add-on direct renin inhibition in IgA nephropathy: a propensity score-matched cohort study. J. Nephrol. 36, 407–416 (2023).
Hayashi, K., Nagahama, T., Oka, K., Epstein, M. & Saruta, T. Disparate effects of calcium antagonists on renal microcirculation. Hypertens. Res. 19, 31–36 (1996).
Ruggenenti, P., Perna, A., Benini, R. & Remuzzi, G. Effects of dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibition, and blood pressure control on chronic, nondiabetic nephropathies. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). J. Am. Soc. Nephrol. 9, 2096–2101 (1998).
Park, H. C. et al. Effect of losartan and amlodipine on proteinuria and transforming growth factor-β1 in patients with IgA nephropathy. Nephrol. Dial. Transpl. 18, 1115–1121 (2003).
Demarie, B. K. & Bakris, G. L. Effects of different calcium antagonists on proteinuria associated with diabetes mellitus. Ann. Intern. Med. 113, 987–988 (1990).
Mickisch, O. et al. Calcium antagonists in chronic renal failure. Undesirable effects on glomerular hemodynamics? [German]. Dtsch. Med. Wochenschr. 113, 1546–1548 (1988).
Berthoux, F., Mariat, C. & Maillard, N. Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol. Dial. Transpl. 28, iv160–iv166 (2013).
Wang, Q. et al. Impact of body mass index on primary immunoglobulin A nephropathy prognosis: a systematic review and meta-analysis. Int. Urol. Nephrol. 54, 1067–1078 (2022).
Wang, S. et al. Cigarette smoking may accelerate the progression of IgA nephropathy. BMC Nephrol. 22, 239 (2021).
Nuffield Department of Population Health Renal Studies, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet 400, 1788–1801 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04573478 (2023).
Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383, 2219–2229 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05047263 (2023).
Rauen, T. et al. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int. 98, 1044–1052 (2020).
Lv, J. et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 327, 1888–1898 (2022). This is the latest cinical trial studying the value of corticosteroids in IgAN.
Seikrit, C., Stamellou, E., Rauen, T. & Floege, J. TESTING the effects of corticosteroids in patients with IgA nephropathy. Nephrol. Dial. Transpl. 37, 1786–1788 (2022).
Zhang, Y. M., Lv, J. C., Wong, M. G., Zhang, H. & Perkovic, V. Glucocorticoids for IgA nephropathy-pro. Kidney Int. 103, 666–669 (2023).
Cheung, C. K. & Barratt, J. First do no harm: systemic glucocorticoids should not be used for the treatment of progressive IgA nephropathy. Kidney Int. 103, 669–673 (2023).
Fellstrom, B. C. et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389, 2117–2127 (2017).
Barratt, J. et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 103, 391–402 (2023). This is the clinical trial testing TRF-budesonide in patients with IgAN, the first drug approved for IgAN.
Ismail, G. et al. Budesonide versus systemic corticosteroids in IgA nephropathy: a retrospective, propensity-matched comparison. Medicine 99, e21000 (2020).
Sun, L., Zi, X., Wang, Z. & Zhang, X. The clinical efficacy of fluticasone propionate combined with ACEI/ARB in the treatment of immunoglobulin A nephropathy. BMC Nephrol. 24, 63 (2023).
Maes, B. D. et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 65, 1842–1849 (2004).
Frisch, G. et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol. Dial. Transpl. 20, 2139–2145 (2005).
Tang, S. C. et al. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 77, 543–549 (2010).
Hou, J. H. et al. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am. J. Kidney Dis. 69, 788–795 (2017).
Huerta, A. et al. Corticosteroids and mycophenolic acid analogues in immunoglobulin A nephropathy with progressive decline in kidney function. Clin. Kidney J. 15, 771–777 (2022).
Hou, F. F. et al. Effectiveness of mycophenolate mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Netw. Open. 6, e2254054 (2023).
Liu, L. J. et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am. J. Kidney Dis. 74, 15–22 (2019).
Tang, C. et al. Long-term safety and efficacy of hydroxychloroquine in patients with IgA nephropathy: a single-center experience. J. Nephrol. 35, 429–440 (2022).
Lafayette, R. A. et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J. Am. Soc. Nephrol. 28, 1306–1313 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05065970 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05799287 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04716231 (2021).
Lv, J. et al. Randomized phase 2 trial of telitacicept in patients with IgA nephropathy with persistent proteinuria. Kidney Int. Rep. 8, 499–506 (2023).
Barratt, J. et al. Randomized phase II JANUS study of atacicept in patients with IgA nephropathy and persistent proteinuria. Kidney Int. Rep. 7, 1831–1841 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04287985 (2023).
US National Library of Medicine. Clinicaltrials.gov https://clinicaltrials.gov/study/NCT03945318 (2023).
Barratt, J. et al. POS-109 interim results of phase 1 and 2 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of BION-1301 in patients with IgA nephropathy [abstract POS-109]. Kidney Int. Rep. 7 (Suppl. 2), S46 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02062684 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02112838?tab=history&a=10 (2019).
Tam, W. K. F. et al. Spleen tyrosine kinase (SYK) inhibition in IgA nephropathy: a global, phase II, randomised placebo-controlled trial of fostamatinib [abstract SUN-036]. Kidney Int. Rep. 7 (Suppl. 7), S168 (2019).
Kateifides, A., Garlo, K., Rice, K., Spoerri, N. & Najafian, N. A phase 2 study evaluating the efficacy and safety of ravulizumab in patients with immunoglobulin A (IgA) nephropathy or proliferative lupus nephritis (LN) [abstract WCN23-0140]. Kidney Int. Rep. 8 (Suppl. 3), S54–S55 (2023).
Barratt, J. Exploratory results from the phase 2 study of cemdisiran in patients with IgA nephropathy [abstract FR-OR67]. Presented at Kidney Week 2022 www.asn-online.org/education/kidneyweek/2022/program-abstract.aspx?controlId=3797891 (2022).
Barratt, J. Efficacy and safety of iptacopan in IgA nephropathy: results of a randomized double-blind placebo-controlled phase 2 study at 6 months [abstract POS-546]. Kidney Int. Rep. 7 (Suppl. 2), S236 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03373461 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04578834 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03453619 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04014335 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02682407 (2020).
Lafayette, R. A. et al. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of IgA nephropathy. Kidney Int. Rep. 5, 2032–2041 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03608033 (2019).
Chemouny, J. M. et al. Modulation of the microbiota by oral antibiotics treats immunoglobulin A nephropathy in humanized mice. Nephrol. Dial. Transpl. 34, 1135–1144 (2019).
Di Leo, V. et al. Rifaximin as a potential treatment for IgA nephropathy in a humanized mice model. J. Pers. Med. 11, 309 (2021).
Tan, J. et al. Probiotics ameliorate IgA nephropathy by improving gut dysbiosis and blunting NLRP3 signaling. J. Transl. Med. 20, 382 (2022).
Lauriero, G. et al. Fecal microbiota transplantation modulates renal phenotype in the humanized mouse model of IgA nephropathy. Front. Immunol. 12, 694787 (2021).
Zhao, J. et al. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: the first case reports. Ren. Fail. 43, 928–933 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05182775 (2022).
Lv, J. et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J. Am. Soc. Nephrol. 24, 2118–2125 (2013).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100, S1–S276 (2021).
Audemard-Verger, A. et al. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis Rheumatol. 69, 1862–1870 (2017).
Leung, A. K. C., Barankin, B. & Leong, K. F. Henoch-Schönlein purpura in children: an updated review. Curr. Pediatr. Rev. 16, 265–276 (2020).
Ozen, S. et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis – the SHARE initiative. Rheumatology 58, 1607–1616 (2019).
Coppo, R. Treatment of IgA nephropathy in children: a land without KDIGO guidance. Pediatr. Nephrol. 36, 491–496 (2021). A very good review about the treatment of IgAN in children.
Clayton, P., McDonald, S. & Chadban, S. Steroids and recurrent IgA nephropathy after kidney transplantation. Am. J. Transpl. 11, 1645–1649 (2011).
Vasilica, C. et al. Identifying information needs of patients with IgA nephropathy using an innovative social media-stepped analytical approach. Kidney Int. Rep. 6, 1317–1325 (2021).
Feldman, D. L et al. Voice of the patient: report on the externally led patient-focused drug development meeting on IgA nephropathy. National Kidney Foundation https://www.igan.org/wp-content/uploads/2021/01/VOP_IgAN_12-7-20__FNL.pdf (2019).
Zaour, N., Mayländer, M., Walda, S, George, A.T. Patient journey, perceptions, and burden associated with immunoglobulin a nephropathy (IgAN): a qualitative study [abstract PUB159]. Presented at Kidney Week 2020 www.asn-online.org/education/kidneyweek/2020/program-abstract.aspx?controlId=3444184 (2020).
Davis, R. S. Fc receptor-like molecules. Annu. Rev. Immunol. 25, 525–560 (2007).
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010).
Lu, M. M. et al. Association of TNFSF4 polymorphisms with systemic lupus erythematosus: a meta-analysis. Mod. Rheumatol. 23, 686–693 (2013).
Lucientes-Continente, L., Marquez-Tirado, B. & Goicoechea de Jorge, E. The factor H protein family: the switchers of the complement alternative pathway. Immunol. Rev. 313, 25–45 (2023).
Ruben, S. M. et al. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes. Dev. 6, 745–760 (1992).
Esensten, J. H., Helou, Y. A., Chopra, G., Weiss, A. & Bluestone, J. A. CD28 costimulation: from mechanism to therapy. Immunity 44, 973–988 (2016).
Nam, S. & Lim, J. S. Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch. Pharm. Res. 39, 1548–1555 (2016).
Shaffer, A. L., Emre, N. C., Romesser, P. B. & Staudt, L. M. IRF4: immunity. malignancy! therapy? Clin. Cancer Res. 15, 2954–2961 (2009).
Khodadadi, L., Cheng, Q., Radbruch, A. & Hiepe, F. The maintenance of memory plasma cells. Front. Immunol. 10, 721 (2019).
Adamaki, M. et al. Implication of IRF4 aberrant gene expression in the acute leukemias of childhood. PLoS ONE 8, e72326 (2013).
Lynch, R. C., Gratzinger, D. & Advani, R. H. Clinical impact of the 2016 update to the WHO lymphoma classification. Curr. Treat. Options Oncol. 18, 45 (2017).
Redondo, M. J., Fain, P. R. & Eisenbarth, G. S. Genetics of type 1A diabetes. Recent. Prog. Horm. Res. 56, 69–89 (2001).
Murray, J. A. et al. HLA DQ gene dosage and risk and severity of celiac disease. Clin. Gastroenterol. Hepatol. 5, 1406–1412 (2007).
Dyment, D. A., Ebers, G. C. & Sadovnick, A. D. Genetics of multiple sclerosis. Lancet Neurol. 3, 104–110 (2004).
Varney, M. D. et al. HLA DPA1, DPB1 alleles and haplotypes contribute to the risk associated with type 1 diabetes: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes 59, 2055–2062 (2010).
Yang, L., Lu, P., Yang, X., Li, K. & Qu, S. Annexin A3, a calcium-dependent phospholipid-binding protein: implication in cancer. Front. Mol. Biosci. 8, 716415 (2021).
Marin, N. D. & Garcia, L. F. The role of CD30 and CD153 (CD30L) in the anti-mycobacterial immune response. Tuberculosis 102, 8–15 (2017).
Kennedy, M. K., Willis, C. R. & Armitage, R. J. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology 118, 143–152 (2006).
Picornell, Y. et al. TNFSF15 is an ethnic-specific IBD gene. Inflamm. Bowel Dis. 13, 1333–1338 (2007).
Bertin, J. et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-κB. J. Biol. Chem. 275, 41082–41086 (2000).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
Evans, D. M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Fan, S., Liu, H. & Li, L. The REEP family of proteins: molecular targets and role in pathophysiology. Pharmacol. Res. 185, 106477 (2022).
Ellinghaus, D. et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet. 90, 636–647 (2012).
Human Protein Atlas. ZMIZ1. Human Protein Atlas https://www.proteinatlas.org/ENSG00000108175-ZMIZ1 (2019).
Jia, D. et al. OVOL guides the epithelial-hybrid-mesenchymal transition. Oncotarget 6, 15436–15448 (2015).
Roca, H. et al. Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS ONE 8, e76773 (2013).
Lecerf, K. et al. Case report and review of the literature: immune dysregulation in a large familial cohort due to a novel pathogenic RELA variant. Rheumatology 62, 347–359 (2022).
Garrett-Sinha, L. A. Review of Ets1 structure, function, and roles in immunity. Cell Mol. Life Sci. 70, 3375–3390 (2013).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
Holtschke, T. et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 (1996).
Hu, X. et al. IRF8 regulates acid ceramidase expression to mediate apoptosis and suppresses myelogeneous leukemia. Cancer Res. 71, 2882–2891 (2011).
Maecker, H. et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell 123, 931–944 (2005).
Burkly, L. C. TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complexities. Semin. Immunol. 26, 229–236 (2014).
Tangye, S. G., Bryant, V. L., Cuss, A. K. & Good, K. L. BAFF, APRIL and human B cell disorders. Semin. Immunol. 18, 305–317 (2006).
Corneth, O. B. J., Neys, S. F. H. & Hendriks, R. W. Aberrant B cell signaling in autoimmune diseases. Cells 11, 3391 (2022).
Byrjalsen, A. et al. Nationwide germline whole genome sequencing of 198 consecutive pediatric cancer patients reveals a high incidence of cancer prone syndromes. PLoS Genet. 16, e1009231 (2020).
Bakema, J. E. & van Egmond, M. The human immunoglobulin A Fc receptor FcαRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol. 4, 612–624 (2011).
Wang, J. et al. Leukemia inhibitory factor, a double-edged sword with therapeutic implications in human diseases. Mol. Ther. 31, 331–343 (2023).
Sun, X., Zhan, M., Sun, X., Liu, W. & Meng, X. C1GALT1 in health and disease. Oncol. Lett. 22, 589 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05097989 (2023).
Floege, J., Johnson, R. J. & Feehally, J. Comprehensive Clinical Nephrology 4th edn (Elsevier, 2010).
Wehbi, B., Pascal, V., Zawil, L., Cogne, M. & Aldigier, J. C. History of IgA nephropathy mouse models. J. Clin. Med. 10, 3142 (2021).
Monteiro, R. C. & Suzuki, Y. Are there animal models of IgA nephropathy? Semin. Immunopathol. 43, 639–648 (2021).
Suzuki, H. & Suzuki, Y. Murine models of human IgA nephropathy. Semin. Nephrol. 38, 513–520 (2018).
Wyatt, R. J. et al. IgA nephropathy: long-term prognosis for pediatric patients. J. Pediatr. 127, 913–919 (1995).
Nozawa, R. et al. Clinicopathological features and the prognosis of IgA nephropathy in Japanese children on long-term observation. Clin. Nephrol. 64, 171–179 (2005).
Coppo, R. Pediatric IgA nephropathy: clinical and therapeutic perspectives. Semin. Nephrol. 28, 18–26 (2008).
Fassbinder, W. et al. Combined report on regular dialysis and transplantation in Europe, XX, 1989. Nephrol. Dial. Transpl. 6, 5–35 (1991).
Coppo, R. & Robert, T. IgA nephropathy in children and in adults: two separate entities or the same disease? J. Nephrol. 33, 1219–1229 (2020).
Zhong, X. & Ding, J. Diagnosis and treatment of IgA nephropathy and IgA vasculitis nephritis in Chinese children. Pediatr. Nephrol. 38, 1707–1715 (2023).
Coppo, R. Pediatric IgA nephropathy in Europe. Kidney Dis. 5, 182–188 (2019).
Uffing, A., Hullekes, F., Riella, L. V. & Hogan, J. J. Recurrent glomerular disease after kidney transplantation: diagnostic and management dilemmas. Clin. J. Am. Soc. Nephrol. 16, 1730–1742 (2021).
Briganti, E. M., Russ, G. R., McNeil, J. J., Atkins, R. C. & Chadban, S. J. Risk of renal allograft loss from recurrent glomerulonephritis. N. Engl. J. Med. 347, 103–109 (2002).
O’Shaughnessy, M. M. et al. Kidney transplantation outcomes across GN subtypes in the United States. J. Am. Soc. Nephrol. 28, 632–644 (2017).
Cosio, F. G. & Cattran, D. C. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 91, 304–314 (2017).
Moroni, G. et al. The long-term outcome of renal transplantation of IgA nephropathy and the impact of recurrence on graft survival. Nephrol. Dial. Transpl. 28, 1305–1314 (2013).
Floege, J. & Gröne, H. J. Recurrent IgA nephropathy in the renal allograft: not a benign condition. Nephrol. Dial. Transpl. 28, 1070–1073 (2013).
Uffing, A. et al. Recurrence of IgA nephropathy after kidney transplantation in adults. Clin. J. Am. Soc. Nephrol. 16, 1247–1255 (2021).
Alachkar, N. et al. Evaluation of the modified Oxford score in recurrent IgA nephropathy in North American kidney transplant recipients: the Banff Recurrent Glomerulonephritis Working Group Report. Transplantation 107, 2055–2063 (2023).
Author information
Authors and Affiliations
Contributions
Introduction (R.K. and E.S.); Epidemiology (R.K., C.S. and E.S.); Mechanisms/pathophysiology (E.S., J.B. and J.F.); Diagnosis/screening/prevention (E.S., C.S., S.C.W.T., V.T., J.B., J.F. and P.B.); Management (E.S., C.S., S.C.W.T., V.T. and J.F.); Quality of life (J.B.); Outlook (J.F.); Overview of the Primer (R.K.).
Corresponding author
Ethics declarations
Competing interests
S.C.W.T. has acted as a consultant for Boehringer Ingelheim, Eledon Pharmaceuticals and Travere, and has received speaker’s honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, GSK and Novartis. He is an Executive Committee member of KDIGO. P.B. has received consulting fees from Bristol Myers Squibb, lecture fees from Novo Nordisk, Novartis, Astellas and research funding from Ergonex Pharma GmbH and Affiris AG, but none in association with this work. V.T. has acted as a consultant for or has received honoraria from AstraZeneca, Bayer, Boehringer-Ingelheim, Calliditas, GSK, Novartis, Omeros, Otsuka and Travere. J.F. has acted as a consultant for or has received honoraria from AstraZeneca, Boehringer, Calliditas, Chinook, Novartis, Omeros, Roche, Stadapharm, Travere and Vifor. He was also chair of the data safety monitoring committee of a trial by Visterra. J.B. has acted as a consultant for or has received honoraria from Alnylam, Argenx, Astellas, BioCryst, Calliditas, Chinook, Dimerix, Galapagos, Novartis, Omeros, Travere Therapeutics, Vera Therapeutics and Visterra; has received grant support from Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics and Visterra; and has been involved in the following clinical trials: ADU-CL-19 & ALIGN (Chinook), APPLAUSE (Novartis), ARTEMIS-IGAN (Omeros), ENVISION (Visterra), NefIgARD (Calliditas) and ORIGIN (Vera Therapeutics). His research projects include Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics, Visterra. R.K. is a founder, shareholder and board member of Sequantrix GmbH, a member of the scientific advisory board of Hybridize Therapeutics, has received honoraria for advisory boards and talks from Bayer, Chugai, Pfizer, Roche, Genentech, Lilly and GSK and has research funding from Travere Therapeutics, Galapagos, Novo Nordisk and Ask Bio. R.K. was supported by grants from the German Research Foundation (DFG; SFBTRR219: CRU344 428857858 and CRU5011 445703531), by two grants from the European Research Council (ERC-StG 677448, ERC-CoG 101043403), a grant from the Else Kroener Fresenius Foundation (EKFS), the Dutch Kidney Foundation (DKF), TASKFORCE EP1805 and Kolff Grant no. 113351, the NWO VIDI 09150172010072 and a grant from the Leducq Foundation, and the BMBF eMed Consortium Fibromap and the BMBF Consortium CureFib. C.S. has received honoraria from Stadapharm and is supported by the clinical research unit InteraKD consortium CRU5011 (project ID 45703531) of the DFG and by the Dr. Werner Jackstädt Stiftung. E.S. is supported by the clinical research unit InteraKD consortium CRU5011 (project ID 45703531) of the DFG and by STOP-FSGS by the German Ministry for Science and Education (STOP-FSGS-01GM2202C).
Peer review
Peer reviewer information
Nature Reviews Disease Primers thanks J.-C. Lv; R. Monteiro, who co-reviewed with J. Gleeson; L. Peruzzi; and H. Suzuki for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Alternative complement pathway
-
The evolutionarily oldest of the three complement pathways. It undergoes constant low-level self-activation, which is rapidly amplified in the presence of a microorganism, a damaged host cell, or a deficiency in complement regulatory proteins.
- BAFF
-
A key cytokine and member of the TNF family of ligands that regulates B cell survival, maturation and differentiation through binding with its receptors: BAFF receptor (BAFF-R), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA).
- Class switch recombination
-
A process by which a mature B cell switches from the production of IgM to the production of IgG, IgE or IgA in response to antigen stimulation and costimulatory signals.
- Complement C3
-
A pivotal protein in the activation of the complement system, with its processing and activation by C3 convertase being essential for both the classic and alternative pathways of complement activation.
- Crescent
-
Inflammatory collection of cells in Bowman’s space resulting from severe glomerular injury and localized rupture of the capillary wall or Bowman’s capsule, which enables entry of plasma proteins and inflammatory material into Bowman’s space.
- eGFR
-
The estimated overall rate at which fluid is filtered through the kidney; it is commonly measured using renal clearance techniques and typically averages around 120 ml/min per 1.73 m2 of surface area.
- Glomerulosclerosis
-
Scarring of glomeruli that disrupts the filtration process and leads to leakage of protein from the blood into the urine.
- Lectin pathway
-
One of the three complement pathways. It is activated by pattern recognition molecules of two major protein families, the ficolins and the collectins. This pathway has a crucial role in innate immunity and host defence against microbial infections.
- Minimal change disease
-
A podocytopathy that is characterized by the absence of histological abnormalities except for ultrastructural evidence of podocyte foot process fusion and, clinically, by the manifestation of nephrotic syndrome.
- Nephrotic syndrome
-
A clinical syndrome characterized by the presence of heavy proteinuria (>3.5 g per 24 h), hypoalbuminaemia, hypercholesterolaemia and peripheral oedema due to increased filtration of macromolecules across the glomerular capillary wall.
- TLRs
-
Cell surface and intracellular molecules of eukaryotic cells that recognize structurally conserved molecules derived from microorganisms, activating innate immunity serving as a first line of defence against foreign pathogens.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stamellou, E., Seikrit, C., Tang, S.C.W. et al. IgA nephropathy. Nat Rev Dis Primers 9, 67 (2023). https://doi.org/10.1038/s41572-023-00476-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-023-00476-9