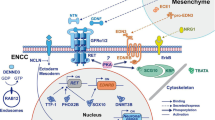
Abstract
Hirschsprung disease (HSCR) is a rare congenital intestinal disease that occurs in 1 in 5,000 live births. HSCR is characterized by the absence of ganglion cells in the myenteric and submucosal plexuses of the intestine. Most patients present during the neonatal period with the first meconium passage delayed beyond 24 h, abdominal distension and vomiting. Syndromes associated with HSCR include trisomy 21, Mowat–Wilson syndrome, congenital central hypoventilation syndrome, Shah–Waardenburg syndrome and cartilage–hair hypoplasia. Multiple putative genes are involved in familial and isolated HSCR, of which the most common are the RET proto-oncogene and EDNRB. Diagnosis consists of visualization of a transition zone on contrast enema and confirmation via rectal biopsy. HSCR is typically managed by surgical removal of the aganglionic bowel and reconstruction of the intestinal tract by connecting the normally innervated bowel down to the anus while preserving normal sphincter function. Several procedures, namely Swenson, Soave and Duhamel procedures, can be undertaken and may include a laparoscopically assisted approach. Short-term and long-term comorbidities include persistent obstructive symptoms, enterocolitis and soiling. Continued research and innovation to better understand disease mechanisms holds promise for developing novel techniques for diagnosis and therapy, and improving outcomes in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Best, K. E. et al. Hirschsprung’s disease prevalence in Europe: a register based study. Birth Defects Res. A Clin. Mol. Teratol. 100, 695–702 (2014).
Kawaguchi, A. L. et al. Management and outcomes for long-segment Hirschsprung disease: a systematic review from the APSA Outcomes and Evidence Based Practice Committee. J. Pediatr. Surg. 56, 1513–1523 (2021).
Fusaro, F. et al. Autologous intestinal reconstructive surgery in the management of total intestinal aganglionosis. J. Pediatr. Gastroenterol. Nutr. 68, 635–641 (2019).
Saxton, M. L., Ein, S. H., Hoehner, J. & Kim, P. C. W. Near-total intestinal aganglionosis: long-term follow-up of a morbid condition. J. Pediatr. Surg. 35, 669–672 (2000).
Chatterjee, S. & Chakravarti, A. A gene regulatory network explains RET–EDNRB epistasis in Hirschsprung disease. Hum. Mol. Genet. 28, 3137–3147 (2019).
De Lorijn, F. et al. Diagnosis of Hirschsprung’s disease: a prospective, comparative accuracy study of common tests. J. Pediatr. 146, 787–792 (2005).
Swenson, O., Rheinlander, H. F. & Diamond, I. Hirschsprung’s disease; a new concept of the etiology – operative results in 34 patients. N. Engl. J. Med. 241, 551–556 (1949). This article is the first to report a successful surgical reconstruction technique for Hirschsprung disease, that is still the most performed technique nowadays.
Duhamel, B. New operation for congenital megacolon: retrorectal and transanal lowering of the colon, and its possible application to the treatment of various other malformations [French]. Presse Med. 64, 2249–2250 (1956).
Yancey, A. G., Cromartie, J. E., Ford, J. R., Nichols, R. R. & Saville, A. F. A modification of the Swenson technique for congenital megacolon. J. Natl Med. Assoc. 44, 356–363 (1952).
Woode, D. et al. Asa G Yancey: The first to describe a modification of the Swenson technique for Hirschsprung disease. J. Pediatr. Surg. 57, 1701–1703 (2022).
Soave, F. Hirschsprung’s disease: a new surgical technique. Arch. Dis. Child. 39, 116–124 (1964).
Rehbein, F. & Von Zimmermann, H. Results with abdominal resection in Hirschsprung’s disease. Arch. Dis. Child. 35, 29–37 (1960).
Smith, B. M., Steiner, R. B. & Lobe, T. E. Laparoscopic Duhamel pullthrough procedure for Hirschsprung’s disease in childhood. J. Laparoendosc. Surg. 4, 273–276 (1994).
Georgeson, K. E., Fuenfer, M. M. & Hardin, W. D. Primary laparoscopic pull-through for Hirschsprung’s disease in infants and children. J. Pediatr. Surg. 30, 1017–1022 (1995). This article reports a major advance in surgical management of Hirschsprung disease, with the use of minimally invasive surgery.
De la Torre-Mondragón, L. & Ortega-Salgado, J. A. Transanal endorectal pull-through for Hirschsprung’s disease. J. Pediatr. Surg. 33, 1283–1286 (1998).
Langer, J. C., Minkes, R. K., Mazziotti, M. V., Skinner, M. A. & Winthrop, A. L. Transanal one-stage Soave procedure for infants with Hirschsprung’s disease. J. Pediatr. Surg. 34, 148–152 (1999).
Rintala, R. J. & Pakarinen, M. P. Long-term outcomes of Hirschsprung’s disease. Semin. Pediatr. Surg. 21, 336–343 (2012).
Huerta, C. T. et al. Nationwide outcomes of newborns with rectosigmoid versus long-segment Hirschsprung disease. J. Pediatr. Surg. https://doi.org/10.1016/J.JPEDSURG.2023.01.001 (2023).
Kyrklund, K. et al. ERNICA guidelines for the management of rectosigmoid Hirschsprung’s disease. Orphanet J. Rare Dis. 15, 164 (2020).
Chia, S. T., Chen, S. C., Lu, C. L., Sheu, S. M. & Kuo, H. C. Epidemiology of Hirschsprung’s disease in Taiwanese children: a 13-year nationwide population-based study. Pediatr. Neonatol. 57, 201–206 (2016).
Lof Granstrom, A. et al. Maternal risk factors and perinatal characteristics for Hirschsprung disease. Pediatrics 138, e20154608 (2016).
Tilghman, J. M. et al. Molecular genetic anatomy and risk profile of Hirschsprung’s disease. N. Engl. J. Med. 380, 1421–1432 (2019). This study provides insight into the complexity of Hirschsprung disease genetics and heritability.
Anderson, J. E. et al. Epidemiology of Hirschsprung disease in California from 1995 to 2013. Pediatr. Surg. Int. 34, 1299–1303 (2018).
Rajab, A., Freeman, N. V. & Patton, M. A. Hirschsprung’s disease in Oman. J. Pediatr. Surg. 32, 724–727 (1997).
Bradnock, T. J. et al. Hirschsprung’s disease in the UK and Ireland: incidence and anomalies. Arch. Dis. Child. 102, 722–727 (2017).
Taghavi, K. et al. Ethnic variations in the childhood prevalence of Hirschsprung disease in New Zealand. ANZ J. Surg. 89, 1246–1249 (2019).
Xiao, J. et al. Comprehensive characterization of the genetic landscape of familial Hirschsprung’s disease. World J. Pediatr. https://doi.org/10.1007/s12519-023-00686-x (2023).
Goldberg, E. L. An epidemiological study of Hirschsprung’s disease. Int. J. Epidemiol. 13, 479–485 (1984).
Tam, P. K. Hirschsprung’s disease: a bridge for science and surgery. J. Pediatr. Surg. 51, 18–22 (2016).
Gunadi et al. NRG1 variant effects in patients with Hirschsprung disease. BMC Pediatr. 18, 292 (2018).
Fadista, J. et al. Genome-wide association study of Hirschsprung disease detects a novel low-frequency variant at the RET locus. Eur. J. Hum. Genet. 26, 561–569 (2018).
Emison, E. S. et al. Differential contributions of rare and common, coding and noncoding Ret mutations to multifactorial Hirschsprung disease liability. Am. J. Hum. Genet. 87, 60–74 (2010).
Moore, S. W. Chromosomal and related Mendelian syndromes associated with Hirschsprung’s disease. Pediatr. Surg. Int. 28, 1045–1058 (2012).
Chen, Y. et al. The prevalence and clinical presentation of Hirschsprung’s disease in preterm infants: a systematic review and meta-analysis. Pediatr. Surg. Int. 38, 523–532 (2022).
Duess, J. W., Hofmann, A. D. & Puri, P. Prevalence of Hirschsprung’s disease in premature infants: a systematic review. Pediatr. Surg. Int. 30, 791–795 (2014).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Dershowitz, L. B., Li, L., Pasca, A. M. & Kaltschmidt, J. A. Anatomical and functional maturation of the mid-gestation human enteric nervous system. Nat. Commun. 14, 2680 (2023).
Amiel, J. et al. Hirschsprung disease, associated syndromes and genetics: a review. J. Med. Genet. 45, 1–14 (2008).
Friedmacher, F. & Puri, P. Hirschsprung’s disease associated with Down syndrome: a meta-analysis of incidence, functional outcomes and mortality. Pediatr. Surg. Int. 29, 937–946 (2013).
Brosens, E. et al. Genetics of enteric neuropathies. Dev. Biol. 417, 198–208 (2016).
Moore, S. W. The contribution of associated congenital anomalies in understanding Hirschsprung’s disease. Pediatr. Surg. Int. 22, 305–315 (2006).
Pini Prato, A. et al. A prospective observational study of associated anomalies in Hirschsprung’s disease. Orphanet J. Rare Dis. 8, 184 (2013).
Pini Prato, A. et al. Congenital anomalies of the kidney and urinary tract in a cohort of 280 consecutive patients with Hirschsprung disease. Pediatr. Nephrol. 36, 3151–3158 (2021).
Hofmann, A. D., Duess, J. W. & Puri, P. Congenital anomalies of the kidney and urinary tract (CAKUT) associated with Hirschsprung’s disease: a systematic review. Pediatr. Surg. Int. 30, 757–761 (2014).
Nagy, N. & Goldstein, A. M. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94–106 (2017). This article comprehensively reviews the current understanding of the factors involved in early development of the enteric nervous system, and areas in need of investigation.
Wallace, A. S. & Burns, A. J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 319, 367–382 (2005).
Rolle, U., Nemeth, L. & Puri, P. Nitrergic innervation of the normal gut and in motility disorders of childhood. J. Pediatr. Surg. 37, 551–567 (2002).
Burns, A. J. & Thapar, N. Advances in ontogeny of the enteric nervous system. Neurogastroenterol. Motil. 18, 876–887 (2006).
Uesaka, T., Nagashimada, M. & Enomoto, H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci. 35, 9879–9888 (2015).
Uribe, R. A., Hong, S. S. & Bronner, M. E. Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Dev. Biol. 433, 17–32 (2018).
Howard, A. G. A. & Uribe, R. A. Hox proteins as regulators of extracellular matrix interactions during neural crest migration. Differentiation 128, 26–32 (2022).
Fu, M., Lui, V. C. H., Sham, M. H., Cheung, A. N. Y. & Tam, P. K. H. HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev. Dyn. 228, 1–10 (2003).
Ganz, J. Gut feelings: studying enteric nervous system development, function, and disease in the zebrafish model system. Dev. Dyn. 247, 268–278 (2018).
Anderson, R. B. et al. The cell adhesion molecule l1 is required for chain migration of neural crest cells in the developing mouse gut. Gastroenterology 130, 1221–1232 (2006).
Baker, P. A., Ibarra-Garcıá-Padilla, R., Venkatesh, A., Singleton, E. W. & Uribe, R. A. In toto imaging of early enteric nervous system development reveals that gut colonization is tied to proliferation downstream of Ret. Development 149, dev200668 (2022).
Barlow, A., De Graaff, E. & Pachnis, V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40, 905–916 (2003).
Nagy, N. & Goldstein, A. M. Endothelin-3 regulates neural crest cell proliferation and differentiation in the hindgut enteric nervous system. Dev. Biol. 293, 203–217 (2006).
Robertson, K., Mason, I. & Hall, S. Hirschsprung’s disease: genetic mutations in mice and men. Gut 41, 436–441 (1997).
Bondurand, N., Natarajan, D., Barlow, A., Thapar, N. & Pachnis, V. Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development 133, 2075–2086 (2006).
Kapur, R. P. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10Dom/Sox10Dom mouse embryos. Pediatr. Dev. Pathol. 2, 559–569 (1999).
Pattyn, A., Morin, X., Cremer, H., Goridis, C. & Brunet, J. F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370 (1999).
Broch, A. et al. Congenital central hypoventilation syndrome and Hirschsprung disease: a retrospective review of the French National Registry Center on 33 cases. J. Pediatr. Surg. 54, 2325–2330 (2019).
Sasaki, A. et al. Novel PHOX2B mutations in congenital central hypoventilation syndrome. Pediatr. Int. 61, 393–396 (2019).
Lake, J. I. & Heuckeroth, R. O. Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G1–G24 (2013).
Newgreen, D. F. & Hartley, L. Extracellular matrix and adhesive molecules in the early development of the gut and its innervation in normal and spotting lethal rat embryos. Acta Anat. 154, 243–260 (1995).
Soret, R. et al. A collagen VI-dependent pathogenic mechanism for Hirschsprung’s disease. J. Clin. Invest. 125, 4483–4496 (2015).
Moore, S. W. Advances in understanding the association between Down syndrome and Hirschsprung disease (DS-HSCR). Pediatr. Surg. Int. 34, 1127–1137 (2018).
Fu, M. et al. 37/67-laminin receptor facilitates neural crest cell migration during enteric nervous system development. FASEB J. 34, 10931–10947 (2020).
Nagy, N. et al. Collagen 18 and agrin are secreted by neural crest cells to remodel their microenvironment and regulate their migration during enteric nervous system development. Development 145, dev160317 (2018).
Nagy, N. et al. Sonic hedgehog controls enteric nervous system development by patterning the extracellular matrix. Development 143, 264–275 (2016).
Dutt, S., Kléber, M., Matasci, M., Sommer, L. & Zimmermann, D. R. Versican V0 and V1 guide migratory neural crest cells. J. Biol. Chem. 281, 12123–12131 (2006).
Ring, C., Hassell, J. & Halfter, W. Expression pattern of collagen IX and potential role in the segmentation of the peripheral nervous system. Dev. Biol. 180, 41–53 (1996).
Nagy, N. et al. Endothelial cells promote migration and proliferation of enteric neural crest cells via β1 integrin signaling. Dev. Biol. 330, 263–272 (2009).
Akbareian, S. E. et al. Enteric neural crest-derived cells promote their migration by modifying their microenvironment through tenascin-C production. Dev. Biol. 382, 446–456 (2013).
Raghavan, S., Gilmont, R. R. & Bitar, K. N. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials 34, 6649–6658 (2013).
Kapur, R. P. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev. Biol. 227, 146–155 (2000).
Huang, T. et al. Direct interaction of Sox10 with cadherin-19 mediates early sacral neural crest cell migration: implications for enteric nervous system development defects. Gastroenterology 162, 179–192.e11 (2022).
Burns, A. J., Champeval, D. & Le Douarin, N. M. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev. Biol. 219, 30–43 (2000).
Burns, A. J. & Le Douarin, N. M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125, 4335–4347 (1998).
Uesaka, T. et al. Enhanced enteric neurogenesis by Schwann cell precursors in mouse models of Hirschsprung disease. Glia 69, 2575–2590 (2021).
Sribudiani, Y. et al. Identification of variants in RET and IHH pathway members in a large family with history of Hirschsprung disease. Gastroenterology 155, 118–129.e6 (2018).
Karim, A., Tang, C. S. & Tam, P. K. The emerging genetic landscape of Hirschsprung disease and its potential clinical applications. Front. Pediatr. 9, 638093 (2021). This article provides an extensive review of gene mutations and syndromes associated with Hirschsprung disease.
Ke, J., Zhu, Y. & Miao, X. The advances of genetics research on Hirschsprung’s disease. Pediatr. Investig. 2, 189–195 (2018).
Mueller, J. L. & Goldstein, A. M. The science of Hirschsprung disease: what we know and where we are headed. Semin. Pediatr. Surg. 31, 151157 (2022).
Tang, C. S., Karim, A., Zhong, Y., Chung, P. H. & Tam, P. K. Genetics of Hirschsprung’s disease. Pediatr. Surg. Int. 39, 104 (2023).
Kuil, L. E. et al. Size matters: large copy number losses in Hirschsprung disease patients reveal genes involved in enteric nervous system development. PLoS Genet. 17, e1009698 (2021).
Gui, H. et al. Whole exome sequencing coupled with unbiased functional analysis reveals new Hirschsprung disease genes. Genome Biol. 18, 48 (2017). This study identifies for the first time novel genes involved in Hirschsprung disease using whole-genome sequencing, providing novel insights into the development of the enteric nervous system.
Le, T. L. et al. Dysregulation of the NRG1/ERBB pathway causes a developmental disorder with gastrointestinal dysmotility in humans. J. Clin. Invest. 131, e145837 (2021).
Brosens, E., MacKenzie, K. C., Alves, M. M. & Hofstra, R. M. W. Do RET somatic mutations play a role in Hirschsprung disease? Genet. Med. 20, 1477–1478 (2018).
Heuckeroth, R. O. Hirschsprung disease – integrating basic science and clinical medicine to improve outcomes. Nat. Rev. Gastroenterol. Hepatol. 15, 152–167 (2018). This review provides insights into the pathophysiology of Hirschsprung disease, and future research direction.
Tam, P. K. & Garcia-Barcelo, M. Genetic basis of Hirschsprung’s disease. Pediatr. Surg. Int. 25, 543–558 (2009).
Lyonnet, S., Pelet, A., Abel, L. & Bolino, A. A gene for Hirschsprung disease maps to the proximal arm of chromosome 10. Nat. Genet. 4, 346–350 (1993). This study is the first to show the mutation of the RET gene, the most commonly involved gene in Hirschsprung disease.
Emison, E. S. et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434, 857–863 (2005).
Chatterjee, S. et al. Enhancer variants synergistically drive dysfunction of a gene regulatory network in Hirschsprung disease. Cell 167, 355–368.e10 (2016).
Luzon-Toro, B. et al. What is new about the genetic background of Hirschsprung disease? Clin. Genet. 97, 114–124 (2020).
Torroglosa, A. Epigenetic mechanisms in Hirschsprung disease. Int J. Mol. Sci. 20, 3123 (2019).
Strobl-Mazzulla, P. H., Marini, M. & Buzzi, A. Epigenetic landscape and miRNA involvement during neural crest development. Dev. Dyn. 241, 1849–1856 (2012).
Fujiwara, N., Nakazawa-Tanaka, N. & Yamataka, A. Animal models of Hirschsprung’s disease: state of the art in translating experimental research to the bedside. Eur. J. Pediatr. Surg. 29, 361–377 (2019).
Cardinal, T. et al. Male-biased aganglionic megacolon in the TashT mouse model of Hirschsprung disease involves upregulation of p53 protein activity and Ddx3y gene expression. PLoS Genet. 16, e1009008 (2020).
Garcia, S. B., Minto, S. B., De Marques, I. S. & Kannen, V. Myenteric denervation of the gut with benzalkonium chloride: a review of forty years of an experimental model. Can. J. Gastroenterol. Hepatol. 2019, 3562492 (2019).
Fu, M. et al. Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development 137, 631–640 (2010).
Moore, S. W. Total colonic aganglionosis and Hirschsprung’s disease: shades of the same or different? Pediatr. Surg. Int. 25, 659–666 (2009).
Alnajar, H., Murro, D., Alsadi, A. & Jakate, S. Spectrum of clinicopathological deviations in long-segment Hirschsprung disease compared with short-segment Hirschsprung disease: a single-institution study. Int. J. Surg. Pathol. 25, 216–221 (2017).
Lewit, R. A., Kuruvilla, K. P., Fu, M. & Gosain, A. Current understanding of Hirschsprung-associated enterocolitis: pathogenesis, diagnosis and treatment. Semin. Pediatr. Surg. 31, 151162 (2022). This article provides a comprehensive review of our current understanding of Hirschsprung-associated enterocolitis, which is the most severe and frequent complication related to this disease.
Gosain, A. et al. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr. Surg. Int. 33, 517–521 (2017).
Abbo, O. et al. Necrotizing enterocolitis in full term neonates: is there always an underlying cause? J. Neonatal Surg. 2, 29 (2013).
Raboel, E. H. Necrotizing enterocolitis in full-term neonates: is it aganglionosis? Eur. J. Pediatr. Surg. 19, 101–104 (2009).
Beltman, L., Labib, H., Oosterlaan, J., van Heurn, E. & Derikx, J. Risk factors for complications in patients with Hirschsprung disease while awaiting surgery: beware of bowel perforation. J. Pediatr. Surg. https://doi.org/10.1016/J.JPEDSURG.2022.02.022 (2022).
Mc Laughlin, D. & Puri, P. Familial Hirschsprung’s disease: a systematic review. Pediatr. Surg. Int. 31, 695–700 (2015).
Zani, A. & Montalva, L. in Encyclopedia of Gastroenterology 2nd edn (ed. Kuipers, E. J.) 644–651 (Academic, 2020).
Garcia, R. et al. Use of the recto-sigmoid index to diagnose Hirschsprung’s disease. Clin. Pediatr. 46, 59–63 (2007).
Stranzinger, E., DiPietro, M. A., Teitelbaum, D. H. & Strouse, P. J. Imaging of total colonic Hirschsprung disease. Pediatr. Radiol. 38, 1162–1170 (2008).
De Lorijn, F., Kremer, L. C. M., Reitsma, J. B. & Benninga, M. A. Diagnostic tests in Hirschsprung disease: a systematic review. J. Pediatr. Gastroenterol. Nutr. 42, 496–505 (2006).
Doodnath, R. & Puri, P. A systematic review and meta-analysis of Hirschsprung’s disease presenting after childhood. Pediatr. Surg. Int. 26, 1107–1110 (2010).
Jarvi, K., Koivusalo, A., Rintala, R. J. & Pakarinen, M. P. Anorectal manometry with reference to operative rectal biopsy for the diagnosis/exclusion of Hirschprung’s disease in children under 1 year of age. Int. J. Colorectal Dis. 24, 451–454 (2009).
Liang, Y., An, T. & Xin, W. Exploring the value of rectal anal canal pressure measurement in the diagnosis of Hirschsprung’s disease. Heliyon 8, e09619 (2022).
Baaleman, D. F. et al. The not-so-rare absent RAIR: internal anal sphincter achalasia in a review of 1072 children with constipation undergoing high-resolution anorectal manometry. Neurogastroenterol. Motil. 33, e14028 (2021).
Wright, N. J. et al. Mortality from gastrointestinal congenital anomalies at 264 hospitals in 74 low-income, middle-income, and high-income countries: a multicentre, international, prospective cohort study. Lancet 398, 325–339 (2021).
Muise, E. D. & Cowles, R. A. Rectal biopsy for Hirschsprung’s disease: a review of techniques, pathology, and complications. World J. Pediatr. 12, 135–141 (2016).
Friedmacher, F. & Puri, P. Rectal suction biopsy for the diagnosis of Hirschsprung’s disease: a systematic review of diagnostic accuracy and complications. Pediatr. Surg. Int. 31, 821–830 (2015).
Ambartsumyan, L., Smith, C. & Kapur, R. P. Diagnosis of Hirschsprung disease. Pediatr. Dev. Pathol. 23, 8–22 (2020).
Muise, E. D., Hardee, S., Morotti, R. A. & Cowles, R. A. A comparison of suction and full-thickness rectal biopsy in children. J. Surg. Res. 201, 149–155 (2016).
Meier-Ruge, W. A. & Bruder, E. Pathology of chronic constipation in pediatric and adult coloproctology. Pathobiology 72, 1–106 (2005).
Kapur, R. P. Calretinin-immunoreactive mucosal innervation in very short-segment Hirschsprung disease: a potentially misleading observation. Pediatr. Dev. Pathol. 17, 28–35 (2014).
Green, N., Smith, C. A., Bradford, M. C., Ambartsumyan, L. & Kapur, R. P. Rectal suction biopsy versus incisional rectal biopsy in the diagnosis of Hirschsprung disease. Pediatr. Surg. Int. 38, 1989–1996 (2022).
Croffie, J. M. et al. At what age is a suction rectal biopsy less likely to provide adequate tissue for identification of ganglion cells? J. Pediatr. Gastroenterol. Nutr. 44, 198–202 (2007).
Veras, L. V. et al. Guidelines for synoptic reporting of surgery and pathology in Hirschsprung disease. J. Pediatr. Surg. 54, 2017–2023 (2019).
Venugopal, S., Mancer, K. & Shandling, B. The validity of rectal biopsy in relation to morphology and distribution of ganglion cells. J. Pediatr. Surg. 16, 433–437 (1981).
Aldridge, R. T. & Campbell, P. E. Ganglion cell distribution in the normal rectum and anal canal. A basis for the diagnosis of Hirschsprung’s disease by anorectal biopsy. J. Pediatr. Surg. 3, 475–490 (1968).
Weinberg, A. G. The anorectal myenteric plexus: its relation to hypoganglionosis of the colon. Am. J. Clin. Pathol. 54, 637–642 (1970).
Qualman, S. J., Jaffe, R., Bove, K. E. & Monforte-Muñoz, H. Diagnosis of hirschsprung disease using the rectal biopsy: multi-institutional survey. Pediatr. Dev. Pathol. 2, 588–596 (1999).
Yunis, E. J., Dibbins, A. W. & Sherman, F. E. Rectal suction biopsy in the diagnosis of Hirschsprung disease in infants. Arch. Pathol. Lab. Med. 100, 329–333 (1976).
Kakita, Y., Oshiro, K., O’Briain, D. S. & Puri, P. Selective demonstration of mural nerves in ganglionic and aganglionic colon by immunohistochemistry for glucose transporter-1: prominent extrinsic nerve pattern staining in Hirschsprung disease. Arch. Pathol. Lab. Med. 124, 1314–1319 (2000).
Drabent, P., Bonnard, A., Guimiot, F., Peuchmaur, M. & Berrebi, D. PHOX2B immunostaining: a simple and helpful tool for the recognition of ganglionic cells and diagnosis of Hirschsprung disease. Am. J. Surg. Pathol. 44, 1389–1397 (2020).
Logan, S. J. et al. Calretinin staining in anorectal line biopsies accurately distinguished Hirschsprung disease in a retrospective study. Pediatr. Dev. Pathol. 25, 645–655 (2022).
Guinard-Samuel, V., Bonnard, A., Peuchmaur, M. & Berrebi, D. A variant pattern of calretinin immunohistochemistry on rectal suction-biopsies is fully specific of short-segment Hirschsprung’s disease. Pediatr. Surg. Int. 30, 803–808 (2014).
Somme, S. & Langer, J. C. Primary versus staged pull-through for the treatment of Hirschsprung disease. Semin. Pediatr. Surg. 13, 249–255 (2004).
Jarvi K. et al. Bowel function and gastrointestinal quality of life among adults operated for Hirschsprung disease during childhood: a population-based study. Ann Surg. 252, 977–981 (2010). This study evaluated for the first time controlled long-term outcomes and quality of life in adults born with Hirschsprung disease.
Apte, A., McKenna, E. & Levitt, M. A. Image of the month: decision-making in surgery for late onset Hirschsprung disease. Eur. J. Pediatr. Surg. Rep. 8, e99–e101 (2020).
Moore, S. W. Total colonic aganglionosis and Hirschsprung’s disease: a review. Pediatr. Surg. Int. 31, 1–9 (2015).
Smith, C., Ambartsumyan, L. & Kapur, R. P. Surgery, surgical pathology, and postoperative management of patients with Hirschsprung disease. Pediatr. Dev. Pathol. 23, 23–39 (2020).
Teitelbaum, D. H. et al. A decade of experience with the primary pull-through for Hirschsprung disease in the newborn period: a multicenter analysis of outcomes. Ann. Surg. 232, 372–380 (2000).
Kastenberg, Z. J. et al. Perioperative and long-term functional outcomes of neonatal versus delayed primary endorectal pull-through for children with Hirschsprung disease: a pediatric colorectal and pelvic learning consortium study. J. Pediatr. Surg. 56, 1465–1469 (2021).
Westfal, M. L. et al. Optimal timing for Soave primary pull-through in short-segment Hirschsprung disease: a meta-analysis. J. Pediatr. Surg. 57, 719–725 (2022).
Stolwijk, L. J. et al. Neurodevelopmental outcomes after neonatal surgery for major noncardiac anomalies. Pediatrics 137, e20151728 (2016).
Keunen, K., Sperna Weiland, N. H., de Bakker, B. S., de Vries, L. S. & Stevens, M. F. Impact of surgery and anesthesia during early brain development: a perfect storm. Paediatr. Anaesth. 32, 697–705 (2022).
Grabowski, J. et al. The effects of early anesthesia on neurodevelopment: a systematic review. J. Pediatr. Surg. 56, 851–861 (2021).
Zani, A. et al. European Paediatric Surgeons’ Association survey on the management of Hirschsprung disease. Eur. J. Pediatr. Surg. 27, 96–101 (2017).
Bischoff, A., Levitt, M. A. & Peña, A. Total colonic aganglionosis: a surgical challenge. How to avoid complications? Pediatr. Surg. Int. 27, 1047–1052 (2011).
Lamoshi, A., Ham, P. B., Chen, Z., Wilding, G. & Vali, K. Timing of the definitive procedure and ileostomy closure for total colonic aganglionosis HD: systematic review. J. Pediatr. Surg. 55, 2366–2370 (2020).
Wood, R. J. & Garrison, A. P. Total colonic aganglionosis in Hirschsprung disease. Semin. Pediatr. Surg. 31, 151165 (2022).
Langer, J. C. et al. One-stage transanal Soave pullthrough for Hirschsprung disease: a multicenter experience with 141 children. Ann. Surg. 238, 569–576 (2003).
Giuliani, S., Honeyford, K., Chang, C. Y., Bottle, A. & Aylin, P. Outcomes of primary versus multiple-staged repair in Hirschsprung’s disease in England. Eur. J. Pediatr. Surg. 30, 104–110 (2020).
Hutchings, E. E., Townley, O. G., Lindley, R. M. & Murthi, G. V. S. The role of stomas in the initial and long-term management of Hirschsprung disease. J. Pediatr. Surg. 58, 236–240 (2023).
Langer, J. C. Surgical approach to Hirschsprung disease. Semin. Pediatr. Surg. 31, 151156 (2022).
Mottadelli, G. et al. Robotic surgery in Hirschsprung disease: a unicentric experience on 31 procedures. J. Robot. Surg. https://doi.org/10.1007/S11701-022-01488-5 (2022).
Celtik, U., Yavuz, I. & Ergün, O. Transanal endorectal or transabdominal pull-through for Hirschsprung’s disease; which is better? A systematic review and meta-analysis. Pediatr. Surg. Int. 39, 89 (2023).
Stensrud, K. J., Emblem, R. & Bjørnland, K. Anal endosonography and bowel function in patients undergoing different types of endorectal pull-through procedures for Hirschsprung disease. J. Pediatr. Surg. 50, 1341–1346 (2015).
Delgado-Miguel, C. & Camps, J. I. Robotic Soave pull-through procedure for Hirschsprung’s disease in children under 12-months: long-term outcomes. Pediatr. Surg. Int. 38, 51–57 (2022).
Li, W. et al. Surgical management of Hirschsprung’s disease: a comparative study between conventional laparoscopic surgery, transumbilical single-site laparoscopic surgery, and robotic surgery. Front. Surg. 9, 924850 (2022).
Payen, E. et al. Outcome of total colonic aganglionosis involving the small bowel depends on bowel length, liver disease, and enterocolitis. J. Pediatr. Gastroenterol. Nutr. 74, 582–587 (2022).
Pini Prato, A. et al. Skipped aganglionic lengthening transposition (SALT) for short bowel syndrome in patients with total intestinal aganglionosis: technical report and feasibility. Pediatr. Surg. Int. 36, 1507–1510 (2020).
Sauvat, F. et al. Intestinal transplantation for total intestinal aganglionosis: a series of 12 consecutive children. J. Pediatr. Surg. 43, 1833–1838 (2008).
O’Hare, T. J., McDermott, M., O’Sullivan, M., Dicker, P. & Antao, B. A retrospective cohort study of total colonic aganglionosis: is the appendix a reliable diagnostic tool? J. Neonatal Surg. 5, 44 (2016).
Reppucci, M. L. et al. Is the appendix a good organ to diagnose total colonic aganglionosis? Pediatr. Surg. Int. 38, 25–30 (2022).
Lane, V., Levitt, M., Baker, P., Minneci, P. & Deans, K. The appendix and aganglionosis. A note of caution – how the histology can mislead the surgeon in total colonic Hirschsprung disease. Eur. J. Pediatr. Surg. Rep. 3, 3–6 (2015).
Maia, D. M. The reliability of frozen-section diagnosis in the pathologic evaluation of Hirschsprung’s disease. Am. J. Surg. Pathol. 24, 1675–1677 (2000).
Shayan, K., Smith, C. & Langer, J. C. Reliability of intraoperative frozen sections in the management of Hirschsprung’s disease. J. Pediatr. Surg. 39, 1345–1348 (2004).
Langer, J. C. et al. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr. Surg. Int. 33, 523–526 (2017).
Saadai, P. et al. Guidelines for the management of postoperative soiling in children with Hirschsprung disease. Pediatr. Surg. Int. 35, 829–834 (2019).
Ahmad, H. et al. A Hirschsprung pull-through, ‘with a twist’. Eur. J. Pediatr. Surg. Rep. 8, e95–e98 (2020).
Gupta, D. K., Khanna, K. & Sharma, S. Experience with the redo pull-through for Hirschsprung’s disease. J. Indian Assoc. Pediatr. Surg. 24, 45–51 (2019).
Beltman, L. et al. Transition zone pull-through in patients with Hirschsprung disease: is redo surgery beneficial for the long-term outcomes? J. Pediatr. Surg. https://doi.org/10.1016/J.JPEDSURG.2023.02.043 (2023).
Vickery, J. M., Shehata, B. M., Chang, E. P. & Husain, A. N. Reoperation for Hirschsprung disease: two cases of vanishing ganglion cells and review of the literature. Pediatr. Dev. Pathol. 26, 77–85 (2023).
Sun, S., Chen, G., Zheng, S., Dong, K. & Xiao, X. Usefulness of posterior sagittal anorectoplasty for redo pull-through in complicated and recurrent Hirschsprung disease: experience with a single surgical group. J. Pediatr. Surg. 52, 458–462 (2017).
Bokova, E. et al. Reconstructing the anal sphincters to reverse iatrogenic overstretching following a pull-through for Hirschsprung disease. One-year outcomes. J. Pediatr. Surg. 58, 484–489 (2023).
Chantakhow, S., Tepmalai, K., Singhavejsakul, J., Tantraworasin, A. & Khorana, J. Prognostic factors of postoperative Hirschsprung-associated enterocolitis: a cohort study. Pediatr. Surg. Int. 39, 77 (2023).
Roorda, D., Oosterlaan, J., van Heurn, E. & Derikx, J. P. M. Risk factors for enterocolitis in patients with Hirschsprung disease: a retrospective observational study. J. Pediatr. Surg. 56, 1791–1798 (2021).
Menezes, M. & Puri, P. Long-term clinical outcome in patients with Hirschsprung’s disease and associated Down’s syndrome. J. Pediatr. Surg. 40, 810–812 (2005).
Arnaud, A. P. et al. Different fecal microbiota in Hirschsprung’s patients with and without associated enterocolitis. Front. Microbiol. 13, 904758 (2022).
Pierre, J. F. et al. Intestinal dysbiosis and bacterial enteroinvasion in a murine model of Hirschsprung’s disease. J. Pediatr. Surg. 49, 1242–1251 (2014).
Gosain, A. et al. Impaired cellular immunity in the murine neural crest conditional deletion of endothelin receptor-B model of Hirschsprung’s disease. PLoS ONE 10, e0128822 (2015).
Neuvonen, M. I. et al. Bowel function and quality of life after transanal endorectal pull-through for Hirschsprung disease: controlled outcomes up to adulthood. Ann. Surg. 265, 622–629 (2017).
Davidson, J. R. et al. Long-term surgical and patient-reported outcomes of Hirschsprung disease. J. Pediatr. Surg. 56, 1502–1511 (2021).
Davidson, J. R. et al. Comparative cohort study of Duhamel and endorectal pull-through for Hirschsprung’s disease. BJS Open 6, zrab143 (2022).
Stenström, P. et al. Total colonic aganglionosis: multicentre study of surgical treatment and patient-reported outcomes up to adulthood. BJS Open. 4, 943–953 (2020).
Davidson, J. R. et al. Outcomes in Hirschsprung’s disease with coexisting learning disability. Eur. J. Pediatr. 180, 3499–3507 (2021).
Vakkilainen, S., Taskinen, M. & Mäkitie, O. Immunodeficiency in cartilage–hair hypoplasia: pathogenesis, clinical course and management. Scand. J. Immunol. 92, e12913 (2020).
Mäkitie, O., Kaitila, I. & Rintala, R. Hirschsprung disease associated with severe cartilage–hair hypoplasia. J. Pediatr. 138, 929–931 (2001).
Svetanoff, W. J. et al. Psychosocial factors affecting quality of life in patients with anorectal malformation and Hirschsprung disease – a qualitative systematic review. J. Pediatr. Surg. 57, 387–393 (2022).
Davidson, J. R. et al. Sexual function, quality of life, and fertility in women who had surgery for neonatal Hirschsprung’s disease. Br. J. Surg. 108, E79–E80 (2021).
Trinidad, S. et al. Long-term male sexual function and fecal incontinence outcomes for adult patients with Hirschsprung disease or anorectal malformation. J. Pediatr. Surg. https://doi.org/10.1016/J.JPEDSURG.2023.04.006 (2023).
Roberts, K., Brindle, M. & McLuckie, D. Enhanced recovery after surgery in paediatrics: a review of the literature. BJA Educ. 20, 235–241 (2020).
Neuvonen, M. I. et al. Intestinal microbiota in Hirschsprung disease. J. Pediatr. Gastroenterol. Nutr. 67, 594–600 (2018).
Neuvonen, M. et al. Lower urinary tract symptoms and sexual functions after endorectal pull-through for Hirschsprung disease: controlled long-term outcomes. J. Pediatr. Surg. 52, 1296–1301 (2017).
Virtanen, V. B. et al. Thyroid cancer and co-occurring RET mutations in Hirschsprung disease. Endocr. Relat. Cancer 20, 595–602 (2013).
Granström, A. L., Amin, L., Arnell, H. & Wester, T. Increased risk of inflammatory bowel disease in a population-based cohort study of patients with Hirschsprung disease. J. Pediatr. Gastroenterol. Nutr. 66, 398–401 (2018).
Granström, A. L., Ludvigsson, J. F. & Wester, T. Clinical characteristics and validation of diagnosis in individuals with Hirschsprung disease and inflammatory bowel disease. J. Pediatr. Surg. 56, 1799–1802 (2021).
Sutthatarn, P. et al. Hirschsprung-associated inflammatory bowel disease: a multicenter study from the APSA Hirschsprung disease interest group. J. Pediatr. Surg. 58, 856–861 (2023).
Huang, S. G. et al. Machine learning-based quantitative analysis of barium enema and clinical features for early diagnosis of short-segment Hirschsprung disease in neonate. J. Pediatr. Surg. 56, 1711–1717 (2021).
Greenberg, A. et al. Automatic ganglion cell detection for improving the efficiency and accuracy of Hirschprung disease diagnosis. Sci. Rep. 11, 3306 (2021).
Shimojima, N. et al. Visualization of the human enteric nervous system by confocal laser endomicroscopy in Hirschsprung’s disease: an alternative to intraoperative histopathological diagnosis? Neurogastroenterol. Motil. 32, e13805 (2020).
Harada, A. et al. Visualization of the human enteric nervous system by probe confocal laser endomicroscopy: a first real-time observation of Hirschsprung’s disease and allied disorders. BMC Med. Imaging 21, 118 (2021).
Nakazawa-Tanaka, N. et al. Increased enteric neural crest cell differentiation after transplantation into aganglionic mouse gut. Pediatr. Surg. Int. 39, 29 (2022).
Zhang, L. et al. Cotransplantation of neuroepithelial stem cells with interstitial cells of Cajal improves neuronal differentiation in a rat aganglionic model. J. Pediatr. Surg. 52, 1188–1195 (2017).
Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 531, 105–109 (2016). This is the first description of a human pluripotent stem cell-based platform that can be used both for studying human enteric nervous system development and as a potential cell-based treatment for Hirschsprung disease.
Smits, R. M. et al. Common needs in uncommon conditions: a qualitative study to explore the need for care in pediatric patients with rare diseases. Orphanet J. Rare Dis. 17, 153 (2022).
Groenewoud, A. S., Westert, G. P. & Kremer, J. A. M. Value based competition in health care’s ethical drawbacks and the need for a values-driven approach. BMC Health Serv. Res. 19, 256 (2019).
Antonarakis, S. E. et al. Down syndrome. Nat. Rev. Dis. Primers 6, 9 (2020).
Garavelli, L. & Mainardi, P. C. Mowat-Wilson syndrome. Orphanet J. Rare Dis. 2, 42 (2007).
Read, A. P. & Newton, V. E. Waardenburg syndrome. J. Med. Genet. 34, 656–665 (1997).
Pingault, V. et al. Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 31, 391–406 (2010).
Marini, F. et al. Multiple endocrine neoplasia type 2. Orphanet J. Rare Dis. 1, 45 (2006).
Porter, F. D. Smith–Lemli–Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 16, 535–541 (2008).
Christaller, W. A. A., Vos, Y., Gebre-Medhin, S., Hofstra, R. M. W. & Schäfer, M. K. E. L1 syndrome diagnosis complemented with functional analysis of L1CAM variants located to the two N-terminal Ig-like domains. Clin. Genet. 91, 115–120 (2017).
Forsythe, E. & Beales, P. L. Bardet–Biedl syndrome. Eur. J. Hum. Genet. 21, 8–13 (2013).
Trang, H. et al. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J. Rare Dis. 15, 252 (2020).
Vakkilainen, S., Taskinen, M., Klemetti, P., Pukkala, E. & Mäkitie, O. A 30-year prospective follow-up study reveals risk factors for early death in cartilage–hair hypoplasia. Front. Immunol. 10, 1581 (2019).
Robinson, P. N. et al. Shprintzen–Goldberg syndrome: fourteen new patients and a clinical analysis. Am. J. Med. Genet. A 135, 251–262 (2005).
Bedeschi, M. F. et al. Impairment of different protein domains causes variable clinical presentation within Pitt–Hopkins syndrome and suggests intragenic molecular syndromology of TCF4. Eur. J. Med. Genet. 60, 565–571 (2017).
Naiki, M. et al. MBTPS2 mutation causes BRESEK/BRESHECK syndrome. Am. J. Med. Genet. A 158A, 97–102 (2012).
Slavotinek, A. M. et al. Mutation analysis of the MKKS gene in McKusick–Kaufman syndrome and selected Bardet–Biedl syndrome patients. Hum. Genet. 110, 561–567 (2002).
Orphanet. The portal for rare diseases and orphan drugs. orphanet https://www.orpha.net/consor/cgi-bin/Disease.php?lng=EN (2023).
Adam, M. P. et al. (eds) GeneReviews [online] https://www.ncbi.nlm.nih.gov/books/NBK1116/ (2023).
Jensen, A. R. & Frischer, J. S. Surgical history of Hirschsprung disease. Semin. Pediatr. Surg. 31, 151174 (2022).
Raveenthiran, V. Knowledge of ancient Hindu surgeons on Hirschsprung disease: evidence from Sushruta Samhita of circa 1200-600 BC. J. Pediatr. Surg. 46, 2204–2208 (2011).
Goldstein, A. M., Hofstra, R. M. & Burns, A. J. Building a brain in the gut: development of the enteric nervous system. Clin. Genet. 83, 307–316 (2013).
Bondurand, N., Dufour, S. & Pingault, V. News from the endothelin-3/EDNRB signaling pathway: role during enteric nervous system development and involvement in neural crest-associated disorders. Dev. Biol. 444, S156–S169 (2018).
Stavely, R. et al. A distinct transcriptome characterizes neural crest-derived cells at the migratory wavefront during enteric nervous system development. Development 150, dev201090 (2023).
Heuckeroth, R. O. Hirschsprung’s disease, Down syndrome, and missing heritability: too much collagen slows migration. J. Clin. Invest. 125, 4323–4326 (2015).
Ahmad, H. et al. Evaluation and treatment of the post pull-through Hirschsprung patient who is not doing well; update for 2022. Semin. Pediatr. Surg. 31, 151164 (2022).
Langer, J. C. & Birnbaum, E. Preliminary experience with intrasphincteric botulinum toxin for persistent constipation after pull-through for Hirschsprung’s disease. J. Pediatr. Surg. 32, 1059–1062 (1997).
Svetanoff, W. J., Lim-Beutal, I. I. P., Wood, R. J., Levitt, M. A. & Rentea, R. M. The utilization of botulinum toxin for Hirschsprung disease. Semin. Pediatr. Surg. 31, 151161 (2022).
Acknowledgements
L.S.C. is supported by grants from the National Institutes of Health (K08DK133673), the American College of Surgeons, and the American Pediatric Surgical Association. A.G. supported by grants (R01DK125047 and R21AI163503) from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Introduction (L.M. and A.G.); Epidemiology (K.K. and I.de B.); Mechanisms/pathophysiology (L.S.C., L.M. and I.de B.); Diagnosis (R.K., D.B. and L.M.); Management (A.B., L.S.C., J.C.L., L.M. and A.G.); Quality of life (M.P. and K.K.); Outlook (L.S.C. and A.G.); Overview of the Primer (L.M. and A.G.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks A. Burns, R. Hotta, P. Tam, T. Wester and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montalva, L., Cheng, L.S., Kapur, R. et al. Hirschsprung disease. Nat Rev Dis Primers 9, 54 (2023). https://doi.org/10.1038/s41572-023-00465-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-023-00465-y
This article is cited by
-
Translation and validation of the Hirschsprung and anorectal malformation quality of life (HAQL) questionnaire in a Danish Hirschsprung population
Pediatric Surgery International (2024)
-
Clinical characteristics and influence of postoperative Hirschsprung-associated enterocolitis: retrospective study at a tertiary children’s hospital
Pediatric Surgery International (2024)