Abstract
Genetic pain loss includes congenital insensitivity to pain (CIP), hereditary sensory neuropathies and, if autonomic nerves are involved, hereditary sensory and autonomic neuropathy (HSAN). This heterogeneous group of disorders highlights the essential role of nociception in protecting against tissue damage. Patients with genetic pain loss have recurrent injuries, burns and poorly healing wounds as disease hallmarks. CIP and HSAN are caused by pathogenic genetic variants in >20 genes that lead to developmental defects, neurodegeneration or altered neuronal excitability of peripheral damage-sensing neurons. These genetic variants lead to hyperactivity of sodium channels, disturbed haem metabolism, altered clathrin-mediated transport and impaired gene regulatory mechanisms affecting epigenetic marks, long non-coding RNAs and repetitive elements. Therapies for pain loss disorders are mainly symptomatic but the first targeted therapies are being tested. Conversely, chronic pain remains one of the greatest unresolved medical challenges, and the genes and mechanisms associated with pain loss offer new targets for analgesics. Given the progress that has been made, the coming years are promising both in terms of targeted treatments for pain loss disorders and the development of innovative pain medicines based on knowledge of these genetic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cox, J. J., Woods, C. G. & Kurth, I. Peripheral sensory neuropathies–pain loss vs. pain gain. Med. Genet. 32, 233–241 (2020).
Rotthier, A. et al. Genes for hereditary sensory and autonomic neuropathies: a genotype-phenotype correlation. Brain 132, 2699–2711 (2009).
Rotthier, A., Baets, J., Timmerman, V. & Janssens, K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat. Rev. Neurol. 8, 73–85 (2012).
Nicholson, G. A. SPTLC1-Related Hereditary Sensory Neuropathy. GeneReviews [online] https://www.ncbi.nlm.nih.gov/books/NBK1390/ (updated 2 Dec 2021).
Schon, K. R. et al. Congenital Insensitivity to Pain Overview. GeneReviews [online] https://www.ncbi.nlm.nih.gov/books/NBK481553/ (updated 11 Jun 2020).
Haga, N., Kubota, M. & Miwa, Z. Epidemiology of hereditary sensory and autonomic neuropathy type IV and V in Japan. Am. J. Med. Genet. A 161A, 871–874 (2013).
Kurth, I. Hereditary Sensory and Autonomic Neuropathy Type II. GeneReviews [online] https://www.ncbi.nlm.nih.gov/books/NBK49247/ (updated 1 Apr 2021).
Curro, R. et al. RFC1 expansions are a common cause of idiopathic sensory neuropathy. Brain 144, 1542–1550 (2021).
Lafreniere, R. G. et al. Identification of a novel gene (HSN2) causing hereditary sensory and autonomic neuropathy type II through the study of Canadian genetic isolates. Am. J. Hum. Genet. 74, 1064–1073 (2004).
Dong, J., Edelmann, L., Bajwa, A. M., Kornreich, R. & Desnick, R. J. Familial dysautonomia: detection of the IKBKAP IVS20(+6T –> C) and R696P mutations and frequencies among Ashkenazi Jews. Am. J. Med. Genet. 110, 253–257 (2002).
Davidson, G. et al. Frequency of mutations in the genes associated with hereditary sensory and autonomic neuropathy in a UK cohort. J. Neurol. 259, 1673–1685 (2012).
Houlden, H. et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 129, 411–425 (2006).
Dawkins, J. L., Hulme, D. J., Brahmbhatt, S. B., Auer-Grumbach, M. & Nicholson, G. A. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet. 27, 309–312 (2001).
Nicholson, G. A. et al. Hereditary sensory neuropathy type I: haplotype analysis shows founders in southern England and Europe. Am. J. Hum. Genet. 69, 655–659 (2001).
Edvardson, S. et al. Hereditary sensory autonomic neuropathy caused by a mutation in dystonin. Ann. Neurol. 71, 569–572 (2012).
Manganelli, F. et al. Novel mutations in dystonin provide clues to the pathomechanisms of HSAN-VI. Neurology 88, 2132–2140 (2017).
Fortugno, P. et al. Recessive mutations in the neuronal isoforms of DST, encoding dystonin, lead to abnormal actin cytoskeleton organization and HSAN type VI. Hum. Mutat. 40, 106–114 (2019).
Jin, J. Y. et al. Novel compound heterozygous DST variants causing hereditary sensory and autonomic neuropathies VI in twins of a Chinese family. Front. Genet. 11, 492 (2020).
Yoshioka, N. et al. Diverse dystonin gene mutations cause distinct patterns of Dst isoform deficiency and phenotypic heterogeneity in Dystonia musculorum mice. Dis. Model Mech. https://doi.org/10.1242/dmm.041608 (2020).
Young, K. G. & Kothary, R. Dystonin/Bpag1–a link to what? Cell Motil. Cytoskeleton 64, 897–905 (2007).
Young, K. G. & Kothary, R. Dystonin/Bpag1 is a necessary endoplasmic reticulum/nuclear envelope protein in sensory neurons. Exp. Cell Res. 314, 2750–2761 (2008).
Tseng, K. W., Peng, M. L., Wen, Y. C., Liu, K. J. & Chien, C. L. Neuronal degeneration in autonomic nervous system of Dystonia musculorum mice. J. Biomed. Sci. 18, 9 (2011).
Ryan, S. D. et al. Neuronal dystonin isoform 2 is a mediator of endoplasmic reticulum structure and function. Mol. Biol. Cell 23, 553–566 (2012).
Ferrier, A., Boyer, J. G. & Kothary, R. Cellular and molecular biology of neuronal dystonin. Int. Rev. Cell Mol. Biol. 300, 85–120 (2013).
Ferrier, A. et al. Disruption in the autophagic process underlies the sensory neuropathy in Dystonia musculorum mice. Autophagy 11, 1025–1036 (2015).
Groves, R. W. et al. A homozygous nonsense mutation within the dystonin gene coding for the coiled-coil domain of the epithelial isoform of BPAG1 underlies a new subtype of autosomal recessive epidermolysis bullosa simplex. J. Invest. Dermatol. 130, 1551–1557 (2010).
Liu, L. et al. Autosomal recessive epidermolysis bullosa simplex due to loss of BPAG1-e expression. J. Invest. Dermatol. 132, 742–744 (2012).
Brown, A., Bernier, G., Mathieu, M., Rossant, J. & Kothary, R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat. Genet. 10, 301–306 (1995).
Scott, B. L. et al. Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics. Nat. Commun. 9, 419 (2018).
Nahorski, M. S. et al. A novel disorder reveals clathrin heavy chain-22 is essential for human pain and touch development. Brain 138, 2147–2160 (2015).
Nahorski, M. S. et al. Clathrin heavy chain 22 contributes to the control of neuropeptide degradation and secretion during neuronal development. Sci. Rep. 8, 2340 (2018).
Riviere, J. B. et al. KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am. J. Hum. Genet. 89, 219–230 (2011).
Lee, J. R. et al. De novo mutations in the motor domain of KIF1A cause cognitive impairment, spastic paraparesis, axonal neuropathy, and cerebellar atrophy. Hum. Mutat. https://doi.org/10.1002/humu.22709 (2014).
Nemani, T. et al. KIF1A-related disorders in children: a wide spectrum of central and peripheral nervous system involvement. J. Peripher. Nerv. Syst. 25, 117–124 (2020).
Hirokawa, N. & Tanaka, Y. Kinesin superfamily proteins (KIFs): various functions and their relevance for important phenomena in life and diseases. Exp. Cell Res. 334, 16–25 (2015).
Okada, Y., Yamazaki, H., Sekine-Aizawa, Y. & Hirokawa, N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780 (1995).
Sgro, A. E., Bajjalieh, S. M. & Chiu, D. T. Single-axonal organelle analysis method reveals new protein-motor associations. ACS Chem. Neurosci. 4, 277–284 (2013).
Hummel, J. J. A. & Hoogenraad, C. C. Specific KIF1A-adaptor interactions control selective cargo recognition. J. Cell Biol. https://doi.org/10.1083/jcb.202105011 (2021).
Verhoeven, K. et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am. J. Hum. Genet. 72, 722–727 (2003).
Houlden, H. et al. A novel RAB7 mutation associated with ulcero-mutilating neuropathy. Ann. Neurol. 56, 586–590 (2004).
Zhang, K. et al. Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J. Neurosci. 33, 7451–7462 (2013).
Ponomareva, O. Y., Eliceiri, K. W. & Halloran, M. C. Charcot-Marie-Tooth 2b associated Rab7 mutations cause axon growth and guidance defects during vertebrate sensory neuron development. Neural Dev. 11, 2 (2016).
Lowe, H., Toyang, N., Steele, B., Bryant, J. & Ngwa, W. The endocannabinoid system: a potential target for the treatment of various diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22179472 (2021).
Cravatt, B. F. et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl Acad. Sci. USA 98, 9371–9376 (2001).
Habib, A. M. et al. Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br. J. Anaesth. 123, e249–e253 (2019).
Chiang, K. P., Gerber, A. L., Sipe, J. C. & Cravatt, B. F. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 13, 2113–2119 (2004).
Mikaeili, H. et al. CRISPR interference at the FAAH-OUT genomic region reduces FAAH expression. Preprint at bioRxiv https://doi.org/10.1101/633396 (2019).
Cravatt, B. F. et al. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc. Natl Acad. Sci. USA 101, 10821–10826 (2004).
Dincheva, I. et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 6, 6395 (2015).
Lichtman, A. H., Shelton, C. C., Advani, T. & Cravatt, B. F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 109, 319–327 (2004).
Clapper, J. R. et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 13, 1265–1270 (2010).
Klein, C. J. et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 43, 595–600 (2011).
Winkelmann, J. et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum. Mol. Genet. 21, 2205–2210 (2012).
Baets, J. et al. Defects of mutant DNMT1 are linked to a spectrum of neurological disorders. Brain 138, 845–861 (2015).
Chen, Y. C. et al. Transcriptional regulator PRDM12 is essential for human pain perception. Nat. Genet. 47, 803–808 (2015).
Inamadar, A. C. et al. Extending the phenotype of midface toddler excoriation syndrome (MiTES): five new cases in three families with PR domain containing protein 12 (PRDM12) mutations. J. Am. Acad. Dermatol. 81, 1415–1417 (2019).
Landy, M. A., Goyal, M., Casey, K. M., Liu, C. & Lai, H. C. Loss of Prdm12 during development, but not in mature nociceptors, causes defects in pain sensation. Cell Rep. 34, 108913 (2021).
Desiderio, S. et al. Prdm12 directs nociceptive sensory neuron development by regulating the expression of the NGF receptor TrkA. Cell Rep. 26, 3522–3536.e5 (2019).
Bartesaghi, L. et al. PRDM12 is required for initiation of the nociceptive neuron lineage during neurogenesis. Cell Rep. 26, 3484–3492.e4 (2019).
Zhang, S. et al. Clinical features for diagnosis and management of patients with PRDM12 congenital insensitivity to pain. J. Med. Genet. 53, 533–535 (2016).
Habib, A. M. et al. A novel human pain insensitivity disorder caused by a point mutation in ZFHX2. Brain 141, 365–376 (2018).
Durr, A. et al. Atlastin1 mutations are frequent in young-onset autosomal dominant spastic paraplegia. Arch. Neurol. 61, 1867–1872 (2004).
Guelly, C. et al. Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am. J. Hum. Genet. 88, 99–105 (2011).
Zhang, H. & Hu, J. Shaping the endoplasmic reticulum into a social network. Trends Cell Biol. 26, 934–943 (2016).
Leonardis, L., Auer-Grumbach, M., Papic, L. & Zidar, J. The N355K atlastin 1 mutation is associated with hereditary sensory neuropathy and pyramidal tract features. Eur. J. Neurol. 19, 992–998 (2012).
Fischer, D. et al. A novel missense mutation confirms ATL3 as a gene for hereditary sensory neuropathy type 1. Brain 137, e286 (2014).
Kornak, U. et al. Sensory neuropathy with bone destruction due to a mutation in the membrane-shaping atlastin GTPase 3. Brain 137, 683–692 (2014).
Xu, H. et al. ATL3 gene mutation in a Chinese family with hereditary sensory neuropathy type 1F. J. Peripher. Nerv. Syst. 24, 150–155 (2019).
Cintra, V. P. et al. Rare mutations in ATL3, SPTLC2 and SCN9A explaining hereditary sensory neuropathy and congenital insensitivity to pain in a Brazilian cohort. J. Neurol. Sci. 427, 117498 (2021).
Novarino, G. et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science 343, 506–511 (2014).
Nizon, M. et al. ARL6IP1 mutation causes congenital insensitivity to pain, acromutilation and spastic paraplegia. Clin. Genet. 93, 169–172 (2018).
Maddirevula, S. et al. Autozygome and high throughput confirmation of disease genes candidacy. Genet. Med. 21, 736–742 (2019).
Chukhrova, A. L. et al. A new case of infantile-onset hereditary spastic paraplegia with complicated phenotype (SPG61) in a consanguineous Russian family. Eur. J. Neurol. 26, e61–e62 (2019).
Wakil, S. M. et al. Truncating ARL6IP1 variant as the genetic cause of fatal complicated hereditary spastic paraplegia. BMC Med. Genet. 20, 119 (2019).
Cao, Y. et al. Genotype-phenotype study and expansion of ARL6IP1-related complicated hereditary spastic paraplegia. Clin. Genet. 99, 477–480 (2021).
Kurth, I. et al. Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat. Genet. 41, 1179–1181 (2009).
Falcao de Campos, C. et al. Hereditary sensory autonomic neuropathy type II: report of two novel mutations in the FAM134B gene. J. Peripher. Nerv. Syst. 24, 354–358 (2019).
Ilgaz Aydinlar, E., Rolfs, A., Serteser, M. & Parman, Y. Mutation in FAM134B causing hereditary sensory neuropathy with spasticity in a Turkish family. Muscle Nerve 49, 774–775 (2014).
Luo, Z. Y. et al. Late-onset hereditary sensory and autonomic neuropathy type 2B caused by novel compound heterozygous mutations in FAM134B presenting as chronic recurrent ulcers on the soles. Indian. J. Dermatol. Venereol. Leprol. 87, 455 (2021).
Murphy, S. M., Davidson, G. L., Brandner, S., Houlden, H. & Reilly, M. M. Mutation in FAM134B causing severe hereditary sensory neuropathy. J. Neurol. Neurosurg. Psychiatry 83, 119–120 (2012).
Wakil, S. M. et al. Exome sequencing: mutilating sensory neuropathy with spastic paraplegia due to a mutation in FAM134B gene. Case Rep. Genet. 2018, 9468049 (2018).
Yang, Y. S. & Strittmatter, S. M. The reticulons: a family of proteins with diverse functions. Genome Biol. 8, 234 (2007).
Zimmerberg, J. & Kozlov, M. M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9–19 (2006).
Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M. & Rapoport, T. A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573–586 (2006).
Baumann, O. & Walz, B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol. 205, 149–214 (2001).
Hubner, C. A. & Kurth, I. Membrane-shaping disorders: a common pathway in axon degeneration. Brain 137, 3109–3121 (2014).
Hu, J. et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319, 1247–1250 (2008).
Muriel, M. P. et al. Atlastin-1, the dynamin-like GTPase responsible for spastic paraplegia SPG3A, remodels lipid membranes and may form tubules and vesicles in the endoplasmic reticulum. J. Neurochem. 110, 1607–1616 (2009).
Orso, G. et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460, 978–983 (2009).
Behrendt, L., Kurth, I. & Kaether, C. A disease causing ATLASTIN 3 mutation affects multiple endoplasmic reticulum-related pathways. Cell Mol. Life Sci. 76, 1433–1445 (2019).
Krols, M. et al. Sensory neuropathy-causing mutations in ATL3 affect ER-mitochondria contact sites and impair axonal mitochondrial distribution. Hum. Mol. Genet. 28, 615–627 (2019).
Krols, M. et al. Sensory-neuropathy-causing mutations in ATL3 cause aberrant ER membrane tethering. Cell Rep. 23, 2026–2038 (2018).
Yamamoto, Y., Yoshida, A., Miyazaki, N., Iwasaki, K. & Sakisaka, T. Arl6IP1 has the ability to shape the mammalian ER membrane in a reticulon-like fashion. Biochem. J. 458, 69–79 (2014).
Fowler, P. C. & O’Sullivan, N. C. ER-shaping proteins are required for ER and mitochondrial network organization in motor neurons. Hum. Mol. Genet. 25, 2827–2837 (2016).
Dong, R. et al. The inositol 5-phosphatase INPP5K participates in the fine control of ER organization. J. Cell Biol. 217, 3577–3592 (2018).
Nixon-Abell, J. et al. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science https://doi.org/10.1126/science.aaf3928 (2016).
Lee, C. A. & Blackstone, C. ER morphology and endo-lysosomal crosstalk: functions and disease implications. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158544 (2020).
Borgese, N., Francolini, M. & Snapp, E. Endoplasmic reticulum architecture: structures in flux. Curr. Opin. Cell Biol. 18, 358–364 (2006).
Khaminets, A. et al. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354–358 (2015).
Mochida, K. et al. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature 522, 359–362 (2015).
Hubner, C. A. & Dikic, I. ER-phagy and human diseases. Cell Death Differ. 27, 833–842 (2020).
Chen, Q., Teng, J. & Chen, J. ATL3, a cargo receptor for reticulophagy. Autophagy 15, 1465–1466 (2019).
Liang, J. R., Lingeman, E., Ahmed, S. & Corn, J. E. Atlastins remodel the endoplasmic reticulum for selective autophagy. J. Cell Biol. 217, 3354–3367 (2018).
Chen, Q. et al. ATL3 is a tubular ER-phagy receptor for GABARAP-mediated selective autophagy. Curr. Biol. 29, 846–855.e6 (2019).
Fregno, I. et al. ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J. https://doi.org/10.15252/embj.201899259 (2018).
Forrester, A. et al. A selective ER-phagy exerts procollagen quality control via a Calnexin-FAM134B complex. EMBO J. https://doi.org/10.15252/embj.201899847 (2019).
Jiang, X. et al. FAM134B oligomerization drives endoplasmic reticulum membrane scission for ER-phagy. EMBO J. 39, e102608 (2020).
Pleiner, T. et al. WNK1 is an assembly factor for the human ER membrane protein complex. Mol. Cell 81, 2693–2704.e12 (2021).
Shekarabi, M. et al. Mutations in the nervous system-specific HSN2 exon of WNK1 cause hereditary sensory neuropathy type II. J. Clin. Invest. 118, 2496–2505 (2008).
Wang, J. J., Yu, B. & Li, Z. The coexistence of a novel WNK1 variant and a copy number variation causes hereditary sensory and autonomic neuropathy type IIA. BMC Med. Genet. 20, 91 (2019).
Loggia, M. L. et al. Carriers of recessive WNK1/HSN2 mutations for hereditary sensory and autonomic neuropathy type 2 (HSAN2) are more sensitive to thermal stimuli. J. Neurosci. 29, 2162–2166 (2009).
Izadifar, A. et al. Axon morphogenesis and maintenance require an evolutionary conserved safeguard function of Wnk kinases antagonizing Sarm and Axed. Neuron https://doi.org/10.1016/j.neuron.2021.07.006 (2021).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Bradshaw, R. A. Rita Levi-Montalcini (1909-2012). Nature 493, 306 (2013).
Shaikh, S. S. et al. A comprehensive functional analysis of NTRK1 missense mutations causing hereditary sensory and autonomic neuropathy type IV (HSAN IV). Hum. Mutat. 38, 55–63 (2017).
Crowley, C. et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011 (1994).
Smeyne, R. J. et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368, 246–249 (1994).
Einarsdottir, E. et al. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum. Mol. Genet. 13, 799–805 (2004).
Indo, Y. et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet. 13, 485–488 (1996).
Carvalho, O. P. et al. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J. Med. Genet. 48, 131–135 (2011).
Shaikh, S. S., Nahorski, M. S. & Woods, C. G. A third HSAN5 mutation disrupts the nerve growth factor furin cleavage site. Mol. Pain. 14, 1744806918809223 (2018).
Minde, J. et al. A novel NGFB point mutation: a phenotype study of heterozygous patients. J. Neurol. Neurosurg. Psychiatry 80, 188–195 (2009).
Cortese, A. et al. Biallelic expansion of an intronic repeat in RFC1 is a common cause of late-onset ataxia. Nat. Genet. 51, 649–658 (2019).
Rafehi, H. et al. Bioinformatics-based identification of expanded repeats: a non-reference intronic pentamer expansion in RFC1 causes CANVAS. Am. J. Hum. Genet. 105, 151–165 (2019).
Tagliapietra, M. et al. RFC1 AAGGG repeat expansion masquerading as chronic idiopathic axonal polyneuropathy. J. Neurol. https://doi.org/10.1007/s00415-021-10552-3 (2021).
Kumar, K. R. et al. RFC1 expansions can mimic hereditary sensory neuropathy with cough and Sjogren syndrome. Brain 143, e82 (2020).
Beijer, D. et al. RFC1 repeat expansions: a recurrent cause of sensory and autonomic neuropathy with cough and ataxia. Eur. J. Neurol. https://doi.org/10.1111/ene.15310 (2022).
Bennett, D. L., Clark, A. J., Huang, J., Waxman, S. G. & Dib-Hajj, S. D. The role of voltage-gated sodium channels in pain signaling. Physiol. Rev. 99, 1079–1151 (2019).
Körner, J. & Lampert, A. in The Senses: a Comprehensive Reference 2nd edn Vol. 5 (ed. Fritzsch, B.) 120–141 (Academic, 2020).
Cox, J. J. et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 444, 894–898 (2006).
Middleton, S. J. et al. Nav1.7 is required for normal C-low threshold mechanoreceptor function in humans and mice. Brain https://doi.org/10.1093/brain/awab482 (2021).
Goldberg, Y. P. et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin. Genet. 71, 311–319 (2007).
Weiss, J. et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature 472, 186–190 (2011).
McDermott, L. A. et al. Defining the functional role of NaV1.7 in human nociception. Neuron 101, 905–919.e8 (2019).
Nassar, M. A. et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl Acad. Sci. USA 101, 12706–12711 (2004).
Minett, M. S. et al. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat. Commun. 3, 791 (2012).
Minett, M. S. et al. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 6, 301–312 (2014).
Shields, S. D. et al. Insensitivity to pain upon adult-onset deletion of Nav1.7 or its blockade with selective inhibitors. J. Neurosci. 38, 10180–10201 (2018).
Eagles, D. A., Chow, C. Y. & King, G. F. Fifteen years of NaV 1.7 channels as an analgesic target: why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. https://doi.org/10.1111/bph.15327 (2020).
Moreno, A. M. et al. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay9056 (2021).
Kingwell, K. Nav1.7 withholds its pain potential. Nat. Rev. Drug Discov. https://doi.org/10.1038/d41573-41019-00065-41570 (2019).
Pereira, V. et al. Analgesia linked to Nav1.7 loss of function requires micro- and delta-opioid receptors. Wellcome Open Res. 3, 101 (2018).
Minett, M. S. et al. Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nat. Commun. 6, 8967 (2015).
Isensee, J. et al. Synergistic regulation of serotonin and opioid signaling contributes to pain insensitivity in Nav1.7 knockout mice. Sci. Signal. https://doi.org/10.1126/scisignal.aah4874 (2017).
MacDonald, D. I. et al. A central mechanism of analgesia in mice and humans lacking the sodium channel NaV1.7. Neuron 109, 1497–1512.e6 (2021).
Bennett, D. L. & Woods, C. G. Painful and painless channelopathies. Lancet Neurol. 13, 587–599 (2014).
Faber, C. G. et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 71, 26–39 (2012).
Fertleman, C. R. et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52, 767–774 (2006).
Yang, Y. et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 41, 171–174 (2004).
King, M. K., Leipold, E., Goehringer, J. M., Kurth, I. & Challman, T. D. Pain insensitivity: distal S6-segment mutations in NaV1.9 emerge as critical hotspot. Neurogenetics 18, 179–181 (2017).
Phatarakijnirund, V. et al. Congenital insensitivity to pain: fracturing without apparent skeletal pathobiology caused by an autosomal dominant, second mutation in SCN11A encoding voltage-gated sodium channel 1.9. Bone 84, 289–298 (2016).
Leipold, E. et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat. Genet. 45, 1399–1404 (2013).
Cox, J. J. & Wood, J. N. No pain, more gain. Nat. Genet. 45, 1271–1272 (2013).
Woods, C. G., Babiker, M. O., Horrocks, I., Tolmie, J. & Kurth, I. The phenotype of congenital insensitivity to pain due to the NaV1.9 variant p.L811P. Eur. J. Hum. Genet. 23, 561–563 (2015).
Huang, J. et al. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 137, 1627–1642 (2014).
Leipold, E. et al. Cold-aggravated pain in humans caused by a hyperactive NaV1.9 channel mutant. Nat. Commun. 6, 10049 (2015).
Zhang, X. Y. et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am. J. Hum. Genet. 93, 957–966 (2013).
Huang, J. et al. Sodium channel NaV1.9 mutations associated with insensitivity to pain dampen neuronal excitability. J. Clin. Invest. 127, 2805–2814 (2017).
Faber, C. G. et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl Acad. Sci. USA 109, 19444–19449 (2012).
Kaluza, L. et al. Loss-of-function of Nav1.8/D1639N linked to human pain can be rescued by lidocaine. Pflug. Arch. 470, 1787–1801 (2018).
Kist, A. M. et al. SCN10A mutation in a patient with erythromelalgia enhances C-fiber activity dependent slowing. PLoS ONE 11, e0161789 (2016).
Weiss, B. & Stoffel, W. Human and murine serine-palmitoyl-CoA transferase–cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 249, 239–247 (1997).
Bejaoui, K. et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat. Genet. 27, 261–262 (2001).
Rotthier, A. et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am. J. Hum. Genet. 87, 513–522 (2010).
Auer-Grumbach, M. Hereditary sensory neuropathy type I. Orphanet J. Rare Dis. 3, 7 (2008).
Auer-Grumbach, M. et al. Mutations at Ser331 in the HSN type I gene SPTLC1 are associated with a distinct syndromic phenotype. Eur. J. Med. Genet. 56, 266–269 (2013).
Rotthier, A. et al. Characterization of two mutations in the SPTLC1 subunit of serine palmitoyltransferase associated with hereditary sensory and autonomic neuropathy type I. Hum. Mutat. 32, E2211–E2225 (2011).
Penno, A. et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 (2010).
Gantner, M. L. et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N. Engl. J. Med. 381, 1422–1433 (2019).
Mwinyi, J. et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS ONE 12, e0175776 (2017).
Dohrn, M. F. et al. Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur. J. Neurol. 22, 806–e55 (2015).
Othman, A. et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 3, e000073 (2015).
Othman, A. et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes 64, 1035–1045 (2015).
Alecu, I. et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J. Lipid Res. 58, 42–59 (2017).
Wilson, E. R. et al. Hereditary sensory neuropathy type 1-associated deoxysphingolipids cause neurotoxicity, acute calcium handling abnormalities and mitochondrial dysfunction in vitro. Neurobiol. Dis. 117, 1–14 (2018).
Clark, A. J. et al. An iPSC model of hereditary sensory neuropathy-1 reveals L-serine-responsive deficits in neuronal ganglioside composition and axoglial interactions. Cell Rep. Med. 2, 100345 (2021).
Scherer, S. S. The debut of a rational treatment for an inherited neuropathy? J. Clin. Invest. 121, 4624–4627 (2011).
Mohassel, P. et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat. Med. 27, 1197–1204 (2021).
Johnson, J. O. et al. Association of variants in the SPTLC1 gene with juvenile amyotrophic lateral sclerosis. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2021.2598 (2021).
Anderson, S. L. et al. Familial dysautonomia is caused by mutations of the IKAP gene. Am. J. Hum. Genet. 68, 753–758 (2001).
Slaugenhaupt, S. A. et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am. J. Hum. Genet. 68, 598–605 (2001).
Waszak, S. M. et al. Germline elongator mutations in sonic hedgehog medulloblastoma. Nature 580, 396–401 (2020).
Goffena, J. et al. Elongator and codon bias regulate protein levels in mammalian peripheral neurons. Nat. Commun. 9, 889 (2018).
Lefcort, F., Mergy, M., Ohlen, S. B., Ueki, Y. & George, L. Animal and cellular models of familial dysautonomia. Clin. Auton. Res. 27, 235–243 (2017).
Dietrich, P. & Dragatsis, I. Familial dysautonomia: mechanisms and models. Genet. Mol. Biol. 39, 497–514 (2016).
Quigley, J. G. et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell 118, 757–766 (2004).
Chiabrando, D., Fiorito, V., Petrillo, S., Bertino, F. & Tolosano, E. HEME: a neglected player in nociception? Neurosci. Biobehav. Rev. 124, 124–136 (2021).
Rajadhyaksha, A. M. et al. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. Am. J. Hum. Genet. 87, 643–654 (2010).
Chiabrando, D. et al. Mutations in the heme exporter FLVCR1 cause sensory neurodegeneration with loss of pain perception. PLoS Genet. 12, e1006461 (2016).
Bertino, F. et al. Heme and sensory neuropathy: insights from novel mutations in the heme exporter feline leukemia virus subgroup C receptor 1. Pain 160, 2766–2775 (2019).
Grudzinska Pechhacker, M. K. et al. FLVCR1-related disease as a rare cause of retinitis pigmentosa and hereditary sensory autonomic neuropathy. Eur. J. Med. Genet. 63, 104037 (2020).
Koehler, K. et al. Mutations in GMPPA cause a glycosylation disorder characterized by intellectual disability and autonomic dysfunction. Am. J. Hum. Genet. 93, 727–734 (2013).
Gold, W. A. et al. A novel mutation in GMPPA in siblings with apparent intellectual disability, epilepsy, dysmorphism, and autonomic dysfunction. Am. J. Med. Genet. A 173, 2246–2250 (2017).
Benitez, E. O., Morales, J. J., Munoz, L. A., Hubner, C. A. & Mutchinick, O. M. A novel GMPPA mutation in two adult sisters with achalasia, alacrima, short stature, dysmorphism, and intellectual disability. Mol. Syndromol. 9, 110–114 (2018).
Diaz, J., Kane, T. D. & Leon, E. Evidence of GMPPA founder mutation in indigenous Guatemalan population associated with alacrima, achalasia, and mental retardation syndrome. Am. J. Med. Genet. A 182, 425–430 (2020).
Franzka, P. et al. GMPPA defects cause a neuromuscular disorder with α-dystroglycan hyperglycosylation. J. Clin. Invest. https://doi.org/10.1172/JCI139076 (2021).
Zheng, L. et al. Cryo-EM structures of human GMPPA-GMPPB complex reveal how cells maintain GDP-mannose homeostasis. Nat. Struct. Mol. Biol. 28, 1–12 (2021).
Schneeberger, P. E. et al. Biallelic MADD variants cause a phenotypic spectrum ranging from developmental delay to a multisystem disorder. Brain 143, 2437–2453 (2020).
Baumann, M. et al. MPV17 mutations in juvenile- and adult-onset axonal sensorimotor polyneuropathy. Clin. Genet. 95, 182–186 (2019).
Appenzeller, O., Kornfeld, M. & Snyder, R. Acromutilating, paralyzing neuropathy with corneal ulceration in Navajo children. Arch. Neurol. 33, 733–738 (1976).
Johnsen, S. D., Johnson, P. C. & Stein, S. R. Familial sensory autonomic neuropathy with arthropathy in Navajo children. Neurology 43, 1120–1125 (1993).
Karadimas, C. L. et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am. J. Hum. Genet. 79, 544–548 (2006).
Spinazzola, A. et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat. Genet. 38, 570–575 (2006).
Heimer, G. et al. TECPR2 mutations cause a new subtype of familial dysautonomia like hereditary sensory autonomic neuropathy with intellectual disability. Eur. J. Paediatr. Neurol. 20, 69–79 (2016).
Palma, J. A. et al. Expanding the genotypic spectrum of congenital sensory and autonomic neuropathies using whole-exome sequencing. Neurol. Genet. 7, e568 (2021).
Neuser, S. et al. Clinical, neuroimaging, and molecular spectrum of TECPR2-associated hereditary sensory and autonomic neuropathy with intellectual disability. Hum. Mutat. 42, 762–776 (2021).
Covone, A. E. et al. WES in a family trio suggests involvement of TECPR2 in a complex form of progressive motor neuron disease. Clin. Genet. 90, 182–185 (2016).
Oz-Levi, D. et al. Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis. Am. J. Hum. Genet. 91, 1065–1072 (2012).
Fraiberg, M. et al. Lysosomal targeting of autophagosomes by the TECPR domain of TECPR2. Autophagy 17, 3096–3108 (2021).
Tamim-Yecheskel, B. C. et al. A tecpr2 knockout mouse exhibits age-dependent neuroaxonal dystrophy associated with autophagosome accumulation. Autophagy 17, 3082–3095 (2021).
Stadel, D. et al. TECPR2 cooperates with LC3C to regulate COPII-dependent ER export. Mol. Cell 60, 89–104 (2015).
Patwari, P. P., Wolfe, L. F., Sharma, G. D. & Berry-Kravis, E. TECPR2 mutation-associated respiratory dysregulation: more than central apnea. J. Clin. Sleep. Med. 16, 977–982 (2020).
Bouhouche, A., Benomar, A., Bouslam, N., Chkili, T. & Yahyaoui, M. Mutation in the epsilon subunit of the cytosolic chaperonin-containing t-complex peptide-1 (Cct5) gene causes autosomal recessive mutilating sensory neuropathy with spastic paraplegia. J. Med. Genet. 43, 441–443 (2006).
Bouhouche, A. et al. Autosomal recessive mutilating sensory neuropathy with spastic paraplegia maps to chromosome 5p15.31-14.1. Eur. J. Hum. Genet. 14, 249–252 (2006).
Antona, V. et al. A novel CCT5 missense variant associated with early onset motor neuropathy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21207631 (2020).
Makari, G. S., Carroll, J. E. & Burton, E. M. Hereditary sensory neuropathy manifesting as possible child abuse. Pediatrics 93, 842–844 (1994).
van den Bosch, G. E. et al. Pain insensitivity syndrome misinterpreted as inflicted burns. Pediatrics 133, e1381–e1387 (2014).
Marbach, F. et al. Variants in PRKAR1B cause a neurodevelopmental disorder with autism spectrum disorder, apraxia, and insensitivity to pain. Genet. Med. 23, 1465–1473 (2021).
Jinnah, H. A. HPRT1 Disorders. GeneReviews https://www.ncbi.nlm.nih.gov/books/NBK1149/ (updated 6 Aug 2020).
Hepburn, L. et al. Innate immunity. A Spaetzle-like role for nerve growth factor β in vertebrate immunity to Staphylococcus aureus. Science 346, 641–646 (2014).
Li, N. et al. Heterogeneity of clinical features and mutation analysis of NTRK1 in Han Chinese patients with congenital insensitivity to pain with anhidrosis. J. Pain. Res. 12, 453–465 (2019).
Carroll, A. et al. Novel approaches to diagnosis and management of hereditary transthyretin amyloidosis. J. Neurol. Neurosurg. Psychiatry https://doi.org/10.1136/jnnp-2021-327909 (2022).
Obici, L. & Mussinelli, R. Current and emerging therapies for hereditary transthyretin amyloidosis: strides towards a brighter future. Neurotherapeutics 18, 2286–2302 (2021).
Schwartzlow, C. & Kazamel, M. Hereditary sensory and autonomic neuropathies: adding more to the classification. Curr. Neurol. Neurosci. Rep. 19, 52 (2019).
De Jonghe, P. K. in Hereditary Peripheral Neuropathies (eds Kuhlenbäumer G., Stögbauer F., Ringelstein E. B., & Young P.) 41–63 (Springer, 2005).
Lauria, G. et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur. J. Neurol. 17, 903–e49 (2010).
Dyck, P. J. in Peripheral Neuropathy 3rd edn (eds Dyck P. J. et al.) 1065–1093 (Saunders, 1993).
Rolke, R. et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur. J. Pain. 10, 77–88 (2006).
Roberts, R. C. Removing the idiopathic from the chronic sensory neuropathies. Brain 144, 1291–1292 (2021).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Boomsma, D. I. et al. The genome of the Netherlands: design, and project goals. Eur. J. Hum. Genet. 22, 221–227 (2014).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Pinero, J. et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 45, D833–D839 (2017).
Wei, C. H., Allot, A., Leaman, R. & Lu, Z. PubTator central: automated concept annotation for biomedical full text articles. Nucleic Acids Res. 47, W587–W593 (2019).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47, D607–D613 (2019).
Consortium, G. T. The GTEx consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Vaser, R., Adusumalli, S., Leng, S. N., Sikic, M. & Ng, P. C. SIFT missense predictions for genomes. Nat. Protoc. 11, 1–9 (2016).
Jagadeesh, K. A. et al. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 48, 1581–1586 (2016).
Heyne, H. O. et al. Predicting functional effects of missense variants in voltage-gated sodium and calcium channels. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay6848 (2020).
Kopanos, C. et al. VarSome: the human genomic variant search engine. Bioinformatics 35, 1978–1980 (2019).
Holtgrewe, M. et al. VarFish: comprehensive DNA variant analysis for diagnostics and research. Nucleic Acids Res. 48, W162–W169 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Matthijs, G. et al. Guidelines for diagnostic next-generation sequencing. Eur. J. Hum. Genet. 24, 2–5 (2016).
Kobren, S. N. et al. Commonalities across computational workflows for uncovering explanatory variants in undiagnosed cases. Genet. Med. https://doi.org/10.1038/s41436-020-01084-8 (2021).
Bis-Brewer, D. M. et al. Assessing non-Mendelian inheritance in inherited axonopathies. Genet. Med. 22, 2114–2119 (2020).
Kurth, I. et al. Whole exome sequencing in congenital pain insensitivity identifies a novel causative intronic NTRK1-mutation due to uniparental disomy. Am. J. Med. Genet. B Neuropsychiatr. Genet. 171, 875–878 (2016).
Li, L. et al. Novel gross deletion mutations in NTRK1 gene associated with congenital insensitivity to pain with anhidrosis. Front. Pediatr. 9, 638190 (2021).
Marchi, M. et al. Congenital insensitivity to pain: a novel mutation affecting a U12-type intron causes multiple aberrant splicing of SCN9A. Pain https://doi.org/10.1097/j.pain.0000000000002535 (2021).
Geng, X. et al. Novel NTRK1 mutations in Chinese patients with congenital insensitivity to pain with anhidrosis. Mol. Pain. 14, 1744806918781140 (2018).
GATK Team. (How to) Call common and rare germline copy number variants. GATK https://gatk.broadinstitute.org/hc/en-us/articles/360035531152–How-to-Call-common-and-rare-germline-copy-number-variants (2022).
Kraft, F. & Kurth, I. Long-read sequencing to understand genome biology and cell function. Int. J. Biochem. Cell Biol. 126, 105799 (2020).
Axelrod, F. B. Familial dysautonomia: a review of the current pharmacological treatments. Expert. Opin. Pharmacother. 6, 561–567 (2005).
Bar-On, E. et al. Orthopaedic manifestations of familial dysautonomia. A review of one hundred and thirty-six patients. J. Bone Jt. Surg. Am. 82, 1563–1570 (2000).
Kayani, B. et al. Orthopaedic manifestations of congenital indifference to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV). Eur. J. Paediatr. Neurol. 21, 318–326 (2017).
Bar-On, E. et al. Congenital insensitivity to pain. Orthopaedic manifestations. J. Bone Jt. Surg. Br. 84, 252–257 (2002).
Loh, J., Cyr, K. & Martin, R. Ankle fracture in hereditary sensory neuropathy type 1. J. Foot Ankle Surg. 60, 621–625 (2021).
Auer-Grumbach, M. Hereditary sensory and autonomic neuropathies. Handb. Clin. Neurol. 115, 893–906 (2013).
Fruchtman, Y., Perry, Z. H. & Levy, J. Morbidity characteristics of patients with congenital insensitivity to pain with anhidrosis (CIPA). J. Pediatr. Endocrinol. Metab. 26, 325–332 (2013).
Weingarten, T. N. et al. Anesthesia and patients with congenital hyposensitivity to pain. Anesthesiology 105, 338–345 (2006).
Ngai, J., Kreynin, I., Kim, J. T. & Axelrod, F. B. Anesthesia management of familial dysautonomia. Paediatr. Anaesth. 16, 611–620 (2006).
Zlotnik, A. et al. Anesthetic management of patients with congenital insensitivity to pain with anhidrosis: a retrospective analysis of 358 procedures performed under general anesthesia. Anesth. Analg. 121, 1316–1320 (2015).
Ozmete, O., Sener, M., Bali, C., Caliskan, E. & Aribogan, A. Congenital insensitivity to pain: how should anesthesia be managed? Turk. J. Pediatr. 59, 87–89 (2017).
Weingarten, T. N., Sprung, J. & Burgher, A. H. Perioperative management of familial dysautonomia: a systematic review. Eur. J. Anaesthesiol. 24, 309–316 (2007).
Elhennawy, K. et al. Oral manifestations, dental management, and a rare homozygous mutation of the PRDM12 gene in a boy with hereditary sensory and autonomic neuropathy type VIII: a case report and review of the literature. J. Med. Case Rep. 11, 233 (2017).
Axelrod, F. B. & Berlin, D. Pregabalin: a new approach to treatment of the dysautonomic crisis. Pediatrics 124, 743–746 (2009).
Norcliffe-Kaufmann, L., Martinez, J., Axelrod, F. & Kaufmann, H. Hyperdopaminergic crises in familial dysautonomia: a randomized trial of carbidopa. Neurology 80, 1611–1617 (2013).
Norcliffe-Kaufmann, L., Palma, J. A., Martinez, J. & Kaufmann, H. Carbidopa for afferent baroreflex failure in familial dysautonomia: a double-blind randomized crossover clinical trial. Hypertension 76, 724–731 (2020).
Spalink, C. L., Barnes, E., Palma, J. A., Norcliffe-Kaufmann, L. & Kaufmann, H. Intranasal dexmedetomidine for adrenergic crisis in familial dysautonomia. Clin. Auton. Res. 27, 279–282 (2017).
Shirazi, E., Sayyahfar, S., Motamed, M. & Alaghband-Rad, J. A case of congenital insensitivity to pain with anhidrosis comorbid with attention deficit hyperactivity disorder: clinical implications for pathophysiology and treatment. J. Nerv. Ment. Dis. 206, 296–299 (2018).
de Greef, B. T. A. et al. Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: a randomized controlled trial. Brain 142, 263–275 (2019).
Colloca, L. et al. Neuropathic pain. Nat. Rev. Dis. Prim. 3, 17002 (2017).
Di Stefano, G., Di Lionardo, A., Di Pietro, G., Cruccu, G. & Truini, A. Pharmacotherapeutic options for managing neuropathic pain: a systematic review and meta-analysis. Pain. Res. Manag. 2021, 6656863 (2021).
Yozu, A. et al. Hereditary sensory and autonomic neuropathy types 4 and 5: review and proposal of a new rehabilitation method. Neurosci. Res. 104, 105–111 (2016).
Missaoui, B. & Thoumie, P. Balance training in ataxic neuropathies. Effects on balance and gait parameters. Gait Posture 38, 471–476 (2013).
Garofalo, K. et al. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 121, 4735–4745 (2011).
Fridman, V. et al. Randomized trial of L-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 92, e359–e370 (2019).
Auranen, M. et al. Clinical and metabolic consequences of L-serine supplementation in hereditary sensory and autonomic neuropathy type 1C. Cold Spring Harb. Mol. Case Stud. https://doi.org/10.1101/mcs.a002212 (2017).
Bode, H. et al. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum. Mol. Genet. 25, 853–865 (2016).
Yang, H., Brown, R. H. Jr, Wang, D., Strauss, K. A. & Gao, G. AAV-mediated gene therapy for glycosphingolipid biosynthesis deficiencies. Trends Mol. Med. 27, 520–523 (2021).
Morini, E. et al. ELP1 splicing correction reverses proprioceptive sensory loss in familial dysautonomia. Am. J. Hum. Genet. 104, 638–650 (2019).
Palma, J. A. et al. Current treatments in familial dysautonomia. Expert. Opin. Pharmacother. 15, 2653–2671 (2014).
Yannai, S., Zonszain, J., Donyo, M. & Ast, G. Combinatorial treatment increases IKAP levels in human cells generated from familial dysautonomia patients. PLoS ONE 14, e0211602 (2019).
Sinha, R. et al. Antisense oligonucleotides correct the familial dysautonomia splicing defect in IKBKAP transgenic mice. Nucleic Acids Res. 46, 4833–4844 (2018).
Bonne, G. The Treatabolome, an emerging concept. J. Neuromuscul. Dis. 8, 337–339 (2021).
Aarestrup, F. M. et al. Towards a European health research and innovation cloud (HRIC). Genome Med. 12, 18 (2020).
Jennings, M. J., Lochmuller, A., Atalaia, A. & Horvath, R. Targeted therapies for hereditary peripheral neuropathies: systematic review and steps towards a ‘treatabolome’. J. Neuromuscul. Dis. 8, 383–400 (2021).
Kugathasan, U. et al. Development of MRC centre MRI calf muscle fat fraction protocol as a sensitive outcome measure in hereditary sensory neuropathy type 1. J. Neurol. Neurosurg. Psychiatry 90, 895–906 (2019).
Schrenk-Siemens, K. et al. PIEZO2 is required for mechanotransduction in human stem cell-derived touch receptors. Nat. Neurosci. 18, 10–16 (2015).
Middleton, S. J. et al. Studying human nociceptors: from fundamentals to clinic. Brain 144, 1312–1335 (2021).
Chambers, S. M. et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 30, 715–720 (2012).
Nickolls, A. R. et al. Transcriptional programming of human mechanosensory neuron subtypes from pluripotent stem cells. Cell Rep. 30, 932–946.e7 (2020).
Namer, B. et al. Pain relief in a neuropathy patient by lacosamide: proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine 39, 401–408 (2019).
Lampert, A. et al. Human sensory neurons derived from pluripotent stem cells for disease modelling and personalized medicine. Neurobiol. Pain. 8, 100055 (2020).
Zeidler, M. et al. NOCICEPTRA: gene and microRNA signatures and their trajectories characterizing human iPSC-derived nociceptor maturation. Adv. Sci. 8, e2102354 (2021).
Eberhardt, E. et al. Pattern of functional TTX-resistant sodium channels reveals a developmental stage of human iPSC- and ESC-derived nociceptors. Stem Cell Rep. 5, 305–313 (2015).
Clark, A. J. et al. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain 140, 898–913 (2017).
Pereira, J. D. et al. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat. Commun. 12, 4744 (2021).
Sharma, A., Sances, S., Workman, M. J. & Svendsen, C. N. Multi-lineage human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 26, 309–329 (2020).
Tavares-Ferreira, D. et al. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 14, eabj8186 (2022).
Haring, M. et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 21, 869–880 (2018).
Kupari, J. et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 12, 1510 (2021).
Nguyen, M. Q., von Buchholtz, L. J., Reker, A. N., Ryba, N. J. & Davidson, S. Single-nucleus transcriptomic analysis of human dorsal root ganglion neurons. eLife https://doi.org/10.7554/eLife.71752 (2021).
Sathyamurthy, A. et al. Massively parallel single nucleus transcriptional profiling defines spinal cord neurons and their activity during behavior. Cell Rep. 22, 2216–2225 (2018).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Vega-Loza, A., Van, C., Moreno, A. M. & Aleman, F. Gene therapies to reduce chronic pain: are we there yet? Pain. Manag. 10, 209–212 (2020).
Mullard, A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat. Rev. Drug Discov. 20, 804–805 (2021).
Just, S. & Buning, H. Key to delivery: the (epi-)genome editing vector toolbox. Methods Mol. Biol. 1767, 147–166 (2018).
Tracey, I. & Mantyh, P. W. The cerebral signature for pain perception and its modulation. Neuron 55, 377–391 (2007).
Ossipov, M. H., Morimura, K. & Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 8, 143–151 (2014).
Schrenk-Siemens, K. et al. HESC-derived sensory neurons reveal an unexpected role for PIEZO2 in nociceptor mechanotransduction. Preprint at bioRxiv https://doi.org/10.1101/741660 (2019).
Richter, T. et al. Rare disease terminology and definitions–a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health 18, 906–914 (2015).
Nahin, R. L. Estimates of pain prevalence and severity in adults: United States, 2012. J. Pain. 16, 769–780 (2015).
Stoicea, N. et al. Current perspectives on the opioid crisis in the US healthcare system: a comprehensive literature review. Medicine 98, e15425 (2019).
Sexton, J. E., Cox, J. J., Zhao, J. & Wood, J. N. The genetics of pain: implications for therapeutics. Annu. Rev. Pharmacol. Toxicol. 58, 123–142 (2018).
Miller, R. E., Block, J. A. & Malfait, A. M. What is new in pain modification in osteoarthritis? Rheumatology 57, iv99–iv107 (2018).
Brown, M. T. et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J. Pain. 13, 790–798 (2012).
Acknowledgements
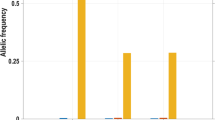
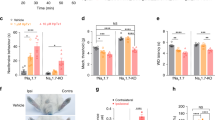
The authors thank the patients and their relatives who have supported their research activity for years. The authors express their gratitude to the family who was available for the experience report during the writing of the article. The European Network on Inherited Sensory Neuropathies and Insensitivity to Pain (ENISNIP) is supported by the Deutsche Forschungsgemeinschaft (DFG) (I.K. and J.S.; KU 1587/6-1, SE 1839/2-1), the Ministry of Education, Youth and Sports (MEYS), Czech Republic (P.L.), the Austrian Science Fund (FWF) (M.A.-G.), the Swiss National Science Foundation (SNSF) (T.H.), the Scientific and Technological Research Council of Turkey (TUBITAK) (A.Ç. and Y.P.), under the frame of the European Joint Programme on Rare Diseases (EJP RD). In addition, the ENISNIP project has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the EJP RD COFUND-EJP no. 825575. The study was supported by a grant from the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University (A.La. and A.Li. and I.K.; IZKF TN1-1/IA 532001, IZKF TN1-2/IA 532002). A.La. has a research contract with Hoffmann-La Roche and Grünenthal and receives consulting fees from Grünenthal, which do not affect the work presented here. J.J.C. is supported by the Medical Research Council (MR/R011737/1) and the Wellcome Trust (200183/Z/15/Z). J.N.W. is supported by the Wellcome trust (200183/Z/15/Z) and Versus Arthritis (21950). The research of C.G.W. is supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. This work was supported by the Association Belge contre les Maladies Neuromusculaires (ABMM). J.B. is supported by a Senior Clinical Researcher mandate of the Research Fund – Flanders (FWO) under grant agreement number 1805021N. J.V.L. is supported by a DOC-PR04 PhD fellowship from the University of Antwerp. Several authors of this publication are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD) and of the European Reference Network for Rare Neurological Diseases (ERN-RND). V.T. and J.B. are partners in the Solve-RD EU project (Horizon 2020 under grant agreement no. 779257). J.B., V.T. and J.V.L. are members of the µNEURO Research Centre of Excellence of the University of Antwerp. The authors thank A. Clark, Nuffield Department of Clinical Neurosciences, Oxford, UK, for providing Fig. 5d and A. Neureiter, Institute of Physiology, Uniklinik RWTH Aachen, Germany, for providing Fig. 5a and Fig. 5b.
Author information
Authors and Affiliations
Contributions
Introduction (I.K., A.Li., M.E., M.M.R., V.T., J.J.C., C.G.W. and D.L.B.); Epidemiology (I.K., A.Ç. and M.M.R.); Mechanisms/pathophysiology (I.K., A.Li., A.Ç., K.E., M.E., T.H., J.S., V.T., J.J.C., C.G.W., C.A.H., J.V.L., J.B., J.N.W., A.La., D.L.B. and M.F.D.); Diagnosis, screening and prevention (I.K., P.L., A.Ç., K.E., Y.P., M.M.R., J.J.C., C.G.W., C.J.R., J.B. and M.F.D.); Management (I.K., M.A.-G., M.E., Y.P., M.M.R. and C.J.R.); Quality of life (I.K., M.M.R. and C.G.W.); Outlook (I.K., P.L., T.H., J.S., C.G.W., J.V.L., A.Li. and D.L.B.); Overview of Primer (I.K.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks Angelo Schenone and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Informed consent
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 4.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lischka, A., Lassuthova, P., Çakar, A. et al. Genetic pain loss disorders. Nat Rev Dis Primers 8, 41 (2022). https://doi.org/10.1038/s41572-022-00365-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-022-00365-7
This article is cited by
-
Transient binocular vision loss and pain insensitivity in Klippel–Feil syndrome: a case report
Journal of Medical Case Reports (2024)
-
Identification of founder and novel mutations that cause congenital insensitivity to pain (CIP) in palestinian patients
BMC Medical Genomics (2023)