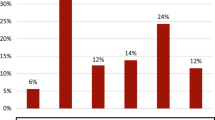
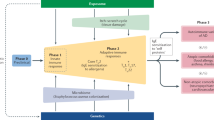
Abstract
Contact dermatitis (CD) is among the most common inflammatory dermatological conditions and includes allergic CD, photoallergic CD, irritant CD, photoirritant CD (also called phototoxic CD) and protein CD. Occupational CD can be of any type and is the most prevalent occupational skin disease. Each CD type is characterized by different immunological mechanisms and/or requisite exposures. Clinical manifestations of CD vary widely and multiple subtypes may occur simultaneously. The diagnosis relies on clinical presentation, thorough exposure assessment and evaluation with techniques such as patch testing and skin-prick testing. Management is based on patient education, avoidance strategies of specific substances, and topical treatments; in severe or recalcitrant cases, which can negatively affect the quality of life of patients, systemic medications may be needed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Alinaghi, F., Bennike, N. H., Egeberg, A., Thyssen, J. P. & Johansen, J. D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermatitis 80, 77–85 (2019). This review and meta-analysis provides extensive information on contact allergy prevalence in the general population, including data from 28 studies between 2007 and 2017, and over 20,000 individuals from multiple continents who underwent patch testing.
Diepgen, T. L. et al. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 174, 319–329 (2016).
Bains, S. N., Nash, P. & Fonacier, L. Irritant contact dermatitis. Clin. Rev. Allergy Immunol. 56, 99–109 (2019).
DeKoven, J. G. et al. North American Contact dermatitis group patch test results: 2015-2016. Dermatitis 29, 297–309 (2018).
Zug, K. A. et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis 25, 345–355 (2014).
Goldenberg, A. et al. Pediatric contact dermatitis registry inaugural case data. Dermatitis 27, 293–302 (2016).
Ortiz Salvador, J. M. et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a Tertiary Hospital. Actas Dermosifiliogr. 108, 571–578 (2017).
Hinton, A. N. & Goldminz, A. M. Feeling the burn: phototoxicity and photoallergy. Dermatol. Clin. 38, 165–175 (2020).
Pongpairoj, K. et al. Proposed ICDRG classification of the clinical presentation of contact allergy. Dermatitis 27, 248–258 (2016).
Pesonen, M., Koskela, K. & Aalto-Korte, K. Contact urticaria and protein contact dermatitis in the Finnish register of occupational diseases in a period of 12 years. Contact Dermatitis 83, 1–7 (2020).
Pesonen, M. et al. Patch test results of the European baseline series among patients with occupational contact dermatitis across Europe-analyses of the European Surveillance System on Contact Allergy network, 2002-2010. Contact Dermatitis 72, 154–163 (2015).
Wiszniewska, M. & Walusiak-Skorupa, J. Recent trends in occupational contact dermatitis. Curr. Allergy Asthma Rep. 15, 43 (2015).
Meyer, J. D., Chen, Y., Holt, D. L., Beck, M. H. & Cherry, N. M. Occupational contact dermatitis in the UK: a surveillance report from EPIDERM and OPRA. Occup. Med. 50, 265–273 (2000).
Turner, S. et al. The incidence of occupational skin disease as reported to The Health and Occupation Reporting (THOR) network between 2002 and 2005. Br. J. Dermatol. 157, 713–722 (2007).
Bhatia, R. & Sharma, V. K. Occupational dermatoses: An Asian perspective. Indian J. Dermatol. Venereol. Leprol. 83, 525–535 (2017).
Cahill, J. L. et al. Occupational skin disease in Victoria, Australia. Australas. J. Dermatol. 57, 108–114 (2016).
Melo, M., Villarinho, A. & Leite, I. D. C. Sociodemographic and clinical profile of patients with occupational contact dermatitis seen at a work-related dermatology service, 2000 - 2014. Bras. Dermatol. 94, 147–156 (2019).
Peiser, M. et al. Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Cell. Mol. Life Sci. 69, 763–781 (2012).
Nassau, S. & Fonacier, L. Allergic contact dermatitis. Med. Clin. North Am. 104, 61–76 (2020).
Cashman, M. W., Reutemann, P. A. & Ehrlich, A. Contact dermatitis in the United States: epidemiology, economic impact, and workplace prevention. Dermatol. Clin. 30, 87–98 (2012).
Agner, T. Noninvasive measuring methods for the investigation of irritant patch test reactions. A study of patients with hand eczema, atopic dermatitis and controls. Acta Derm. Venereol. Suppl. 173, 1–26 (1992).
Thyssen, J. P., McFadden, J. P. & Kimber, I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy 69, 28–36 (2014).
Milam, E. C., Jacob, S. E. & Cohen, D. E. Contact dermatitis in the patient with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 7, 18–26 (2019).
Uehara, M. & Sawai, T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch. Dermatol. 125, 366–368 (1989).
Teo, Y., McFadden, J. P., White, I. R., Lynch, M. & Banerjee, P. Allergic contact dermatitis in atopic individuals: results of a 30-year retrospective study. Contact Dermatitis 81, 409–416 (2019).
Hegewald, J., Uter, W., Pfahlberg, A., Geier, J. & Schnuch, A. A multifactorial analysis of concurrent patch-test reactions to nickel, cobalt, and chromate. Allergy 60, 372–378 (2005).
Malajian, D. & Belsito, D. V. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis. J. Am. Acad. Dermatol. 69, 232–237 (2013).
Ross-Hansen, K. et al. Filaggrin is a predominant member of the denaturation-resistant nickel-binding proteome of human epidermis. J. Invest. Dermatol. 134, 1164–1166 (2014).
Hamann, C. R. et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 77, 70–78 (2017).
Vester, L., Thyssen, J. P., Menné, T. & Johansen, J. D. Occupational food-related hand dermatoses seen over a 10-year period. Contact Dermatitis 66, 264–270 (2012).
Barbaud, A., Poreaux, C., Penven, E. & Waton, J. Occupational protein contact dermatitis. Eur. J. Dermatol. 25, 527–534 (2015).
Lertphanichkul, C. & Scheinman, P. L. Occupational allergic contact dermatitis (OACD) in a cohort of 458 consecutive dermatitis patients; A case series of 17 patients with OACD. J. Am. Acad. Dermatol. 84, 798–804 (2020).
Johnston, G. A. et al. British Association of Dermatologists’ guidelines for the management of contact dermatitis 2017. Br. J. Dermatol. 176, 317–329 (2017).
Goldminz, A. M. & Scheinman, P. L. Comparison of nickel sulfate 2.5% and nickel sulfate 5% for detecting nickel contact allergy. Dermatitis 29, 321–323 (2018).
Ahlström, M., Thyssen, J. & Wennervalft, M. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis 81, 227–241 (2019).
Thyssen, J. P., Linneberg, A., Menné, T., Nielsen, N. H. & Johansen, J. D. The association between hand eczema and nickel allergy has weakened among young women in the general population following the Danish nickel regulation: results from two cross-sectional studies. Contact Dermatitis 61, 342–348 (2009).
Uter, W. et al. ESSCA results with nickel, cobalt and chromium, 2009-2012. Contact Dermatitis 75, 117–121 (2016).
Ahlström, M. G., Thyssen, J. P., Menné, T. & Johansen, J. D. Prevalence of nickel allergy in Europe following the EU Nickel Directive - a review. Contact Dermatitis 77, 193–200 (2017). This review investigates the prevalence of nickel allergy in Europe following the highly effective EU Nickel Directive, an important public health initiative that has successfully reduced rates of nickel contact allergy among the European population.
Bregnbak, D. et al. Chromium allergy and dermatitis: Prevalence and main findings. Contact Dermatitis 73, 261–280 (2015).
Warshaw, E. M. et al. Epidemiology of nickel sensitivity: Retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J. Am. Acad. Dermatol. 80, 701–713 (2019).
Cheng, T. Y., Tseng, Y. H., Sun, C. C. & Chu, C. Y. Contact sensitization to metals in Taiwan. Contact Dermatitis 59, 353–360 (2008).
Goon, A. T. & Goh, C. L. Metal allergy in Singapore. Contact Dermatitis 52, 130–132 (2005).
Dou, X., Zhao, Y., Ni, C., Zhu, X. & Liu, L. Prevalence of contact allergy at a dermatology clinic in China from 1990-2009. Dermatitis 22, 324–331 (2011).
Uter, W. et al. European Surveillance System on Contact Allergies (ESSCA): results with the European baseline series, 2013/14. J. Eur. Acad. Dermatol. Venereol. 31, 1516–1525 (2017).
Kirchlechner, S., Hübner, A. & Uter, W. Survey of sensitizing components of oxidative hair dyes (retail and professional products) in Germany. J. Dtsch. Dermatol. Ges. 14, 707–715 (2016).
Uter, W. et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J. Eur. Acad. Dermatol. Venereol. 34, 333–339 (2020). This survey study assessed changes in isothiazolinone contact allergy following regulations implemented for isothiazolinones in skin-care products, another important example of an effective public health initiative in the area of contact dermatitis.
Rolls, S. et al. Recommendation to include hydroxyethyl (meth)acrylate in the British baseline patch test series. Br. J. Dermatol. 181, 811–817 (2019).
Toholka, R. et al. The first Australian Baseline Series: Recommendations for patch testing in suspected contact dermatitis. Australas. J. Dermatol. 56, 107–115 (2015).
Lee, H. Y., Stieger, M., Yawalkar, N. & Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013, 916497 (2013).
Nosbaum, A., Vocanson, M., Rozieres, A., Hennino, A. & Nicolas, J. F. Allergic and irritant contact dermatitis. EJD 19, 325–332 (2009).
Vocanson, M., Hennino, A., Rozieres, A., Poyet, G. & Nicolas, J. F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy 64, 1699–1714 (2009).
Kaplan, D. H., Igyarto, B. Z. & Gaspari, A. A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 12, 114–124 (2012).
Honda, T., Egawa, G., Grabbe, S. & Kabashima, K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J. Invest. Dermatol. 133, 303–315 (2013).
Martin S. F., Rustemeyer T., Thyssen J. P. Recent advances in understanding and managing contact dermatitis. F1000Res. 2018;https://doi.org/10.12688/f1000research.13499.1
Basketter, D. A., Kan-King-Yu, D., Dierkes, P. & Jowsey, I. R. Does irritation potency contribute to the skin sensitization potency of contact allergens? Cutan. Ocul. Toxicol. 26, 279–286 (2007).
Lepoittevin, J. & Leblond, I. Hapten-peptide T cell receptor interactions: molecular basis for the recognition of haptens by T lymphocytes. EJD 7, 151–154 (1997).
Karlberg, A. T., Bergstrom, M. A., Borje, A., Luthman, K. & Nilsson, J. L. Allergic contact dermatitis–formation, structural requirements, and reactivity of skin sensitizers. Chem. Res. Toxicol. 21, 53–69 (2008).
Barbaud, A. Mechanism and diagnosis of protein contact dermatitis. Curr. Opin. Allergy Clin. Immunol. 20, 117–121 (2020).
Hannuksela, M. Protein Contact Dermatitis 345–348 (Springer, 2006).
Fartasch, M. Ultrastructure of the epidermal barrier after irritation. Microsc. Res. Tech. 37, 193–199 (1997).
Angelova-Fischer, I. Irritants and skin barrier function. Curr. Probl. Dermatol. 49, 80–89 (2016).
Eskes, C. et al. Regulatory assessment of in vitro skin corrosion and irritation data within the European framework: Workshop recommendations. Regul. Toxicol. Pharmacol. 62, 393–403 (2012).
Patel, S. Danger-associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr. Allergy Asthma Rep. 18, 63 (2018).
Gibbs, S. In vitro irritation models and immune reactions. Skin. Pharmacol. Physiol. 22, 103–113 (2009).
Kumari, V., Babina, M., Hazzan, T. & Worm, M. Thymic stromal lymphopoietin induction by skin irritation is independent of tumour necrosis factor-alpha, but supported by interleukin-1. Br. J. Dermatol. 172, 951–960 (2015).
Kendall, A. C., Pilkington, S. M., Sassano, G., Rhodes, L. E. & Nicolaou, A. N-Acyl ethanolamide and eicosanoid involvement in irritant dermatitis. Br. J. Dermatol. 175, 163–171 (2016).
Clemmensen, A. et al. Genome-wide expression analysis of human in vivo irritated epidermis: differential profiles induced by sodium lauryl sulfate and nonanoic acid. J. Invest. Dermatol. 130, 2201–2210 (2010).
Fulzele, S. V., Babu, R. J., Ahaghotu, E. & Singh, M. Estimation of proinflammatory biomarkers of skin irritation by dermal microdialysis following exposure with irritant chemicals. Toxicology 237, 77–88 (2007).
Calhoun, K. N., Luckett-Chastain, L. R., Frempah, B. & Gallucci, R. M. Associations Between Immune Phenotype and Inflammation in Murine Models of Irritant Contact Dermatitis. Toxicol. Sci. 168, 179–189 (2019).
Willis, C. M., Stephens, C. J. & Wilkinson, J. D. Differential patterns of epidermal leukocyte infiltration in patch test reactions to structurally unrelated chemical irritants. J. Invest. Dermatol. 101, 364–370 (1993).
Landsteiner, K. & Jacobs, J. Studies on the sensitization of animals with simple chemical compounds. Ii. J. Exp. Med. 64, 625–639 (1936).
Esser, P. R. & Martin, S. F. Pathomechanisms of contact sensitization. Curr. Allergy Asthma Rep. 17, 83 (2017).
Aptula, A. O. & Roberts, D. W. Mechanistic applicability domains for nonanimal-based prediction of toxicological end points: general principles and application to reactive toxicity. Chem. Res. Toxicol. 19, 1097–1105 (2006).
Karlberg, A. T. et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis 69, 323–334 (2013).
Schmidt, M. & Goebeler, M. Immunology of metal allergies. J. Dtsch. Dermatol. Ges. 13, 653–660 (2015).
Fitzpatrick, J. M., Roberts, D. W. & Patlewicz, G. What determines skin sensitization potency: Myths, maybes and realities. The 500 molecular weight cut-off: An updated analysis. J. Appl. Toxicol. 37, 105–116 (2017).
Fitzpatrick, J. M., Roberts, D. W. & Patlewicz, G. Is skin penetration a determining factor in skin sensitization potential and potency? Refuting the notion of a LogKow threshold for skin sensitization. J. Appl. Toxicol. 37, 117–127 (2017).
Paramasivan, P. et al. Repeated low-dose skin exposure is an effective sensitizing stimulus, a factor to be taken into account in predicting sensitization risk. Br. J. Dermatol. 162, 594–597 (2010).
van Och, F. M., Vandebriel, R. J., De Jong, W. H. & van Loveren, H. Effect of prolonged exposure to low antigen concentration for sensitization. Toxicology 184, 23–30 (2003).
Sekiguchi, K. et al. Enhancement of mouse contact hypersensitivity appears with a short chain triacylglycerol but not with a long chain one. Toxicology 412, 48–54 (2019).
Pickard, C. et al. The cutaneous biochemical redox barrier: a component of the innate immune defenses against sensitization by highly reactive environmental xenobiotics. J. Immunol. 183, 7576–7584 (2009).
Aleksic, M. et al. Mass spectrometric identification of covalent adducts of the skin allergen 2,4-dinitro-1-chlorobenzene and model skin proteins. Toxicol. Vitr. 22, 1169–1176 (2008).
Doi, T., Mizukawa, Y., Shimoda, Y., Yamazaki, Y. & Shiohara, T. Importance of water content of the stratum corneum in mouse models for contact hypersensitivity. J. Invest. Dermatol. 137, 151–158 (2017).
Friedmann, P. S., Sanchez-Elsner, T. & Schnuch, A. Genetic factors in susceptibility to contact sensitivity. Contact Dermatitis.72, 263–274 (2015).
Schnuch, A., Westphal, G., Mossner, R., Uter, W. & Reich, K. Genetic factors in contact allergy–review and future goals. Contact Dermatitis 64, 2–23 (2011).
Gomez de Aguero, M. et al. Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8+ T cells and activating Foxp3+ regulatory T cells. J. Clin. Invest. 122, 1700–1711 (2012).
Cavani, A. et al. Human CD25+regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 171, 5760–5768 (2003).
Luckey, U. et al. Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance. J. Allergy Clin. Immunol. 130, 781–797.e711 (2012).
Vocanson, M. et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J. Invest. Dermatol. 126, 815–820 (2006).
Rozieres, A. et al. CD8+ T cells mediate skin allergy to amoxicillin in a mouse model. Allergy 65, 996–1003 (2010).
Watanabe, H. et al. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J. Immunol. 180, 5826–5832 (2008).
Riemann, H. et al. IL-12 breaks dinitrothiocyanobenzene (DNTB)-mediated tolerance and converts the tolerogen DNTB into an immunogen. J. Immunol. 175, 5866–5874 (2005).
Kimber, I., Dearman, R. J., Basketter, D. A., Ryan, C. A. & Gerberick, G. F. The local lymph node assay: past, present and future. Contact Dermatitis 47, 315–328 (2002).
Wang, Y. & Dai, S. Structural basis of metal hypersensitivity. Immunol. Res. 55, 83–90 (2013).
Bonefeld, C. M. et al. Immunological, chemical and clinical aspects of exposure to mixtures of contact allergens. Contact Dermatitis 77, 133–142 (2017).
Saint-Mezard, P. et al. Psychological stress exerts an adjuvant effect on skin dendritic cell functions in vivo. J. Immunol. 171, 4073–4080 (2003).
Martin, S. F. et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 205, 2151–2162 (2008).
Esser, P. R. et al. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS ONE 7, e41340 (2012).
Migdal, C. et al. Sensitization effect of thimerosal is mediated in vitro via reactive oxygen species and calcium signaling. Toxicology 274, 1–9 (2010).
Muto, J. et al. Hyaluronan digestion controls DC migration from the skin. J. Clin. Invest. 124, 1309–1319 (2014).
Galbiati, V., Papale, A., Galli, C. L., Marinovich, M. & Corsini, E. Role of ROS and HMGB1 in contact allergen-induced IL-18 production in human keratinocytes. J. Invest. Dermatol. 134, 2719–2727 (2014).
Klekotka, P. A., Yang, L. & Yokoyama, W. M. Contrasting roles of the IL-1 and IL-18 receptors in MyD88-dependent contact hypersensitivity. J. Invest. Dermatol. 130, 184–191 (2010).
Yasukawa, S. et al. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat. Commun. 5, 3755 (2014).
Enk A. H. in Immune Mechanisms in Allergic Cotact Dermatitis. 76-80 (Springer, 2005).
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Antonopoulos, C. et al. IL-18 is a key proximal mediator of contact hypersensitivity and allergen-induced Langerhans cell migration in murine epidermis. J. Leukoc. Biol. 83, 361–367 (2008).
Weber, F. C. et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J. Exp. Med. 207, 2609–2619 (2010).
Kostarnoy, A. V. et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc. Natl Acad. Sci. USA 114, E2758–E2765 (2017).
Luis, A. et al. Oxidative stress-dependent activation of the eIF2alpha-ATF4 unfolded protein response branch by skin sensitizer 1-fluoro-2,4-dinitrobenzene modulates dendritic-like cell maturation and inflammatory status in a biphasic manner [corrected]. Free Radic. Biol. Med. 77, 217–229 (2014).
Schmidt, M. et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 11, 814–819 (2010).
Adam, C. et al. Allergy-inducing chromium compounds trigger potent innate immune stimulation Via ROS-dependent inflammasome Activation. J. Invest. Dermatol. 137, 367–376 (2017).
Li, X. & Zhong, F. Nickel induces interleukin-1beta secretion via the NLRP3-ASC-caspase-1 pathway. Inflammation 37, 457–466 (2014).
El Ali, Z. et al. Allergic skin inflammation induced by chemical sensitizers is controlled by the transcription factor Nrf2. Toxicol. Sci. 134, 39–48 (2013).
Helou, D. G., Martin, S. F., Pallardy, M., Chollet-Martin, S. & Kerdine-Römer, S. Nrf2 involvement in chemical-induced skin innate immunity. Front. Immunol. 10, 1004 (2019).
Eaton, L. H., Roberts, R. A., Kimber, I., Dearman, R. J. & Metryka, A. Skin sensitization induced Langerhans’ cell mobilization: variable requirements for tumour necrosis factor-alpha. Immunology 144, 139–148 (2015).
Peng, B. et al. Thimerosal induces skin pseudo-allergic reaction via Mas-related G-protein coupled receptor B2. J. Dermatol. Sci. 95, 99–106 (2019).
Meixiong, J. et al. Activation of mast-cell-expressed Mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50, 1163–1171.e5 (2019).
Dudeck, A. et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34, 973–984 (2011).
Dudeck, J. et al. Mast-cell-derived TNF amplifies CD8+ dendritic cell functionality and CD8+ T cell priming. Cell Rep. 13, 399–411 (2015).
Biedermann, T. et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 192, 1441–1452 (2000).
Weber, F. C. et al. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J. Exp. Med. 212, 15–22 (2015).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Clausen, B. E. & Stoitzner, P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front. Immunol. 6, 534 (2015).
Bacci, S., Alard, P., Dai, R., Nakamura, T. & Streilein, J. W. High and low doses of haptens dictate whether dermal or epidermal antigen-presenting cells promote contact hypersensitivity. Eur. J. Immunol. 27, 442–448 (1997).
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Honda, T. et al. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J. Allergy Clin. Immunol. 125, 1154–1156.e2 (2010).
Noordegraaf, M., Flacher, V., Stoitzner, P. & Clausen, B. E. Functional redundancy of Langerhans cells and Langerin+ dermal dendritic cells in contact hypersensitivity. J. Invest. Dermatol. 130, 2752–2759 (2010).
Cho, Y., Kwon, D. & Kang, S. J. The Cooperative Role of CD326+ and CD11b+ dendritic cell subsets for a hapten-induced Th2 differentiation. J. Immunol. 199, 3137–3146 (2017).
Le Borgne, M. et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24, 191–201 (2006).
Micosse, C. et al. Human “TH9” cells are a subpopulation of PPAR-γ+ TH2 cells. Sci. Immunol. 4, eaat5943 (2019).
Gibson, A. et al. In Vitro Priming of Naive T-cells with p-Phenylenediamine and Bandrowski’s Base. Chem. Res. Toxicol. 28, 2069–2077 (2015).
Bechara, R., Antonios, D., Azouri, H. & Pallardy, M. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and Jak-STAT pathways. J. Invest. Dermatol. 137, 2140–2148 (2017).
Connor, L. M. et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J. Exp. Med. 214, 125–142 (2017).
Larson, R. P. et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J. Immunol. 184, 2974–2984 (2010).
Dhingra, N. et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J. Allergy Clin. Immunol. 134, 362–372 (2014). This study identified differential up-regulation of specific T cell axes in response to varied contact allergens, with important implications for both understanding disease mechanisms and treatment approaches.
Nicolai, S. et al. Human T cell response to CD1a and contact dermatitis allergens in botanical extracts and commercial skin care products. Sci. Immunol. 5, eaax5430 (2020).
Betts, R. J. et al. Contact sensitizers trigger human CD1-autoreactive T-cell responses. Eur. J. Immunol. 47, 1171–1180 (2017).
Nieuwenhuis, E. E. et al. CD1d and CD1d-restricted iNKT-cells play a pivotal role in contact hypersensitivity. Exp. Dermatol. 14, 250–258 (2005).
Goubier, A. et al. Invariant NKT cells suppress CD8+ T-cell-mediated allergic contact dermatitis independently of regulatory CD4+ T cells. J. Invest. Dermatol. 133, 980–987 (2013).
Shimizuhira, C. et al. Natural killer T cells are essential for the development of contact hypersensitivity in BALB/c mice. J. Invest. Dermatol. 134, 2709–2718 (2014).
Kim, J. H. et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 17, 1159–1166 (2016). These investigators used both mouse models and human tissues to study the CD1a recognition of urushiol, the subsequent inflammatory responses and the effects of CD1a blocking antibodies, with evidence supporting CD1a as a potential therapeutic target in cCD.
Wang, K. et al. TLR4 supports the expansion of FasL+CD5+CD1dhi regulatory B cells, which decreases in contact hypersensitivity. Mol. Immunol. 87, 188–199 (2017).
Vocanson, M. et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J. Allergy Clin. Immunol. 126, 280–289, 289.e1-7 (2010).
Ring, S., Enk, A. H. & Mahnke, K. Regulatory T cells from IL-10-deficient mice fail to suppress contact hypersensitivity reactions due to lack of adenosine production. J. Invest. Dermatol. 131, 1494–1502 (2011).
Mahnke, K. et al. Down-regulation of CD62L shedding in T cells by CD39+ regulatory T cells leads to defective sensitization in contact hypersensitivity reactions. J. Invest. Dermatol. 137, 106–114 (2017).
El Beidaq, A. et al. In vivo expansion of endogenous regulatory T cell populations induces long-term suppression of contact hypersensitivity. J. Immunol. 197, 1567–1576 (2016).
Gorbachev, A. V. & Fairchild, R. L. CD4+CD25+ regulatory T cells utilize FasL as a mechanism to restrict DC priming functions in cutaneous immune responses. Eur. J. Immunol. 40, 2006–2015 (2010).
Ring, S., Karakhanova, S., Johnson, T., Enk, A. H. & Mahnke, K. Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8+ T cells. J. Allergy Clin. Immunol. 125, 237–246.e1-7 (2010).
Kish, D. D., Gorbachev, A. V. & Fairchild, R. L. CD8+ T cells produce IL-2, which is required for CD4+CD25+ T cell regulation of effector CD8+T cell development for contact hypersensitivity responses. J. Leukoc. Biol. 78, 725–735 (2005).
Silva-Vilches, C. et al. Production of extracellular adenosine by CD73+ dendritic cells is crucial for induction of tolerance in contact hypersensitivity reactions. J. Invest. Dermatol. 139, 541–551 (2019).
Luckey, U. et al. T cell killing by tolerogenic dendritic cells protects mice from allergy. J. Clin. Invest. 121, 3860–3871 (2011).
Nish, S. A. et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. eLife 3, e01949 (2014).
Gamradt, P. et al. Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J. Allergy Clin. Immunol. 143, 2147–2157 (2019).
Dyring-Andersen, B. et al. CD4+ T cells producing interleukin (IL)-17, IL-22 and interferon-gamma are major effector T cells in nickel allergy. Contact Dermatitis 68, 339–347 (2013).
Gober, M. D., Fishelevich, R., Zhao, Y., Unutmaz, D. & Gaspari, A. A. Human natural killer T cells infiltrate into the skin at elicitation sites of allergic contact dermatitis. J. Invest. Dermatol. 128, 1460–1469 (2008).
Carbone, T. et al. CD56highCD16-CD62L- NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J. Immunol. 184, 1102–1110 (2010).
Rafei-Shamsabadi, D. A. et al. Lack of type 2 innate lymphoid cells promotes a type I-driven enhanced immune response in contact hypersensitivity. J. Invest. Dermatol. 138, 1962–1972 (2018).
Simon, D., Aeberhard, C., Erdemoglu, Y. & Simon, H. U. Th17 cells and tissue remodeling in atopic and contact dermatitis. Allergy 69, 125–131 (2014).
Kawano, M. et al. NKG2D+ IFN-γ+ CD8+ T cells are responsible for palladium allergy. PLoS One 9, e86810 (2014).
Kish, D. D. et al. Neutrophil cathepsin G regulates dendritic cell production of IL-12 during development of CD4 T cell responses to antigens in the skin. J. Immunol. 202, 1045–1056 (2019).
Takeshita, K., Yamasaki, T., Akira, S., Gantner, F. & Bacon, K. B. Essential role of MHC II-independent CD4+ T cells, IL-4 and STAT6 in contact hypersensitivity induced by fluorescein isothiocyanate in the mouse. Int. Immunol. 16, 685–695 (2004).
Gautam, S. C., Matriano, J. A., Chikkala, N. F., Edinger, M. G. & Tubbs, R. R. L3T4 (CD4+) cells that mediate contact sensitivity to trinitrochlorobenzene express I-A determinants. Cell Immunol. 135, 27–41 (1991).
Kish, D. D., Volokh, N., Baldwin, W. M. 3rd & Fairchild, R. L. Hapten application to the skin induces an inflammatory program directing hapten-primed effector CD8 T cell interaction with hapten-presenting endothelial cells. J. Immunol. 186, 2117–2126 (2011).
Trautmann, A. et al. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J. Clin. Invest. 106, 25–35 (2000).
Akiba, H. et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+T cytotoxic 1 cells inducing keratinocyte apoptosis. J. Immunol. 168, 3079–3087 (2002).
He, D. et al. and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 183, 1463–1470 (2009).
Chong, S. Z. et al. CD8 T cells regulate allergic contact dermatitis by modulating CCR2-dependent TNF/iNOS-expressing Ly6C+CD11b+monocytic cells. J. Invest. Dermatol. 134, 666–676 (2014).
Kish, D. D., Gorbachev, A. V., Parameswaran, N., Gupta, N. & Fairchild, R. L. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J. Immunol. 189, 2191–2202 (2012).
Engeman, T., Gorbachev, A. V., Kish, D. D. & Fairchild, R. L. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J. Leukoc. Biol. 76, 941–949 (2004).
Jiang, X. et al. Dermal gammadelta T cells do not freely re-circulate out of skin and produce IL-17 to promote neutrophil infiltration during primary contact hypersensitivity. PLoS One 12, e0169397 (2017).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Dey, N., Szczepanik, M., Lau, K., Majewska-Szczepanik, M. & Askenase, P. W. Stimulatory lipids accumulate in the mouse liver within 30 min of contact sensitization to facilitate the activation of Naive iNKT cells in a CD1d-dependent fashion. Scand. J. Immunol. 74, 52–61 (2011).
Campos, R. A. et al. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 198, 1785–1796 (2003).
Natsuaki, Y. et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 15, 1064–1069 (2014).
Liu, Z. et al. Visualization of T cell-regulated monocyte clusters mediating keratinocyte death in acquired cutaneous immunity. J. Invest. Dermatol. 138, 1328–1337 (2018).
Kehren, J. et al. Cytotoxicity is mandatory for CD8+ T cell-mediated contact hypersensitivity. J. Exp. Med. 189, 779–786 (1999).
Pennino, D. et al. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J. Immunol. 184, 4880–4888 (2010).
Balato, A. et al. CD1d-dependent, iNKT-cell cytotoxicity against keratinocytes in allergic contact dermatitis. Exp. Dermatol. 21, 915–920 (2012).
Suzuki, K., Meguro, K., Nakagomi, D. & Nakajima, H. Roles of alternatively activated M2 macrophages in allergic contact dermatitis. Allergol. Int. 66, 392–397 (2017).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Zhang, L. H., Shin, J. H., Haggadone, M. D. & Sunwoo, J. B. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 213, 2249–2257 (2016).
Stary, V. et al. A discrete subset of epigenetically primed human NK cells mediates antigen-specific immune responses. Sci. Immunol. 5, eaba6232 (2020).
Rouzaire, P. et al. Natural killer cells and T cells induce different types of skin reactions during recall responses to haptens. Eur. J. Immunol. 42, 80–88 (2012).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11, 1127–1135 (2010).
van den Boorn, J. G. et al. Inflammasome-dependent induction of adaptive NK cell memory. Immunity 44, 1406–1421 (2016).
Tomura, M. et al. Activated regulatory T cells are the major T cell type emigrating from the skin during a cutaneous immune response in mice. J. Clin. Invest. 120, 883–893 (2010).
Nakashima, H. et al. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J. Immunol. 184, 4637–4645 (2010).
Hitzler, M. et al. Human Langerhans cells control Th cells via programmed death-ligand 1 in response to bacterial stimuli and nickel-induced contact allergy. PLoS One 7, e46776 (2012).
Ritprajak, P., Hashiguchi, M., Tsushima, F., Chalermsarp, N. & Azuma, M. Keratinocyte-associated B7-H1 directly regulates cutaneous effector CD8+T cell responses. J. Immunol. 184, 4918–4925 (2010).
Hirano, T. PD-L1 on mast cells suppresses effector CD8+ T-cell activation in the skin in murine contact hypersensitivity. J. Allergy Clin. Immunol., https://doi.org/10.1016/j.jaci.2020.12.654 (2021).
Ikebuchi, R. et al. Functional phenotypic diversity of regulatory T cells remaining in inflamed skin. Front. Immunol. 10, 1098 (2019).
Braun, A. et al. Integrin αE(CD103) Is Involved in Regulatory T-Cell Function in Allergic Contact Hypersensitivity. J. Invest. Dermatol. 135, 2982–2991 (2015).
Lehtimäki, S. et al. The temporal and spatial dynamics of Foxp3+Treg cell-mediated suppression during contact hypersensitivity responses in a murine model. J. Invest. Dermatol. 132, 2744–2751 (2012).
Christensen, A. D., Skov, S., Kvist, P. H. & Haase, C. Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells. Clin. Exp. Immunol. 179, 485–499 (2015).
Ikebuchi, R. et al. A rare subset of skin-tropic regulatory T cells expressing Il10/Gzmb inhibits the cutaneous immune response. Sci. Rep. 6, 35002 (2016).
Ring, S., Oliver, S. J., Cronstein, B. N., Enk, A. H. & Mahnke, K. CD4+CD25+ regulatory T cells suppress contact hypersensitivity reactions through a CD39, adenosine-dependent mechanism. J. Allergy Clin. Immunol. 123, 1287–1296.e2 (2009).
Ring, S. et al. Regulatory T cells prevent neutrophilic infiltration of skin during contact hypersensitivity reactions by strengthening the endothelial barrier. J. Invest. Dermatol., https://doi.org/10.1016/j.jid.2021.01.027 (2021).
Campbell, C. & Rudensky, A. Roles of regulatory T cells in tissue pathophysiology and metabolism. Cell Metab. 31, 18–25 (2020).
Gaide, O. et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 21, 647–653 (2015). Using studies of both mouse models and human tissue, this group identified the generation of both central memory T cells in the lymph nodes and resident memory T cells in the skin in response to cutaneous immunization.
Mbitikon-Kobo, F. M. et al. Characterization of a CD44/CD122int memory CD8 T cell subset generated under sterile inflammatory conditions. J. Immunol. 182, 3846–3854 (2009).
Schmidt, J. D. et al. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+ T cells. Contact Dermatitis 76, 218–227 (2017).
Adachi, T. et al. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 21, 1272–1279 (2015).
Hirai, T. et al. Competition for active TGFβ cytokine allows for selective retention of antigen-specific tissue- resident memory T cells in the epidermal niche. Immunity 54, 84–98 (2021).
Jensen, C. D., Johansen, J. D., Menné, T. & Andersen, K. E. Increased retest reactivity by both patch and use test with methyldibromoglutaronitrile in sensitized individuals. Acta Derm. Venereol. 86, 8–12 (2006).
Nahhas, A. F., Oberlin, D. M., Braunberger, T. L. & Lim, H. W. Recent developments in the diagnosis and management of photosensitive disorders. Am. J. Clin. Dermatol. 19, 707–731 (2018).
Greenspoon, J., Ahluwalia, R., Juma, N. & Rosen, C. F. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis 24, 29–32 (2013).
Maverakis, E. et al. Light, including ultraviolet. J. Autoimmun. 34, J247–257 (2010).
Zaheer, M. R. et al. Molecular mechanisms of drug photodegradation and photosensitization. Curr. Pharm. Des. 22, 768–782 (2016).
Nathan, C. & Ding, A. SnapShot: reactive oxygen intermediates (ROI). Cell 140, 951–951.e2 (2010).
de Jager, T. L., Cockrell, A. E., Du & Plessis, S. S. Ultraviolet light induced generation of reactive oxygen species. Adv. Exp. Med. Biol. 996, 15–23 (2017).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–462 (2014).
Nathan, C. & Cunningham-Bussel, A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 (2013).
Pathak, M. A. & Fitzpatrick, T. B. The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J. Photochem. Photobiol. B. 14, 3–22 (1992).
Devleeschouwer, V., Roelandts, R., Garmyn, M. & Goossens, A. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis 58, 159–166 (2008).
Asensio, T., Sanchís, M. E., Sánchez, P., Vega, J. M. & García, J. C. Photocontact dermatitis because of oral dexketoprofen. Contact Dermatitis 58, 59–60 (2008).
Tokura, Y. Drug photoallergy. J. Cutan. Immunol. Allergy 1, 48–57 (2018).
Abeyama, K. et al. A role for NF-kappaB-dependent gene transactivation in sunburn. J. Clin. Invest. 105, 1751–1759 (2000).
Hasegawa, T., Nakashima, M. & Suzuki, Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem. Biophys. Res. Commun. 477, 329–335 (2016).
Hotamisligil, G. S. & Davis, R. J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 8, a006072 (2016).
Leighton, S., Kok, L. F., Halliday, G. M. & Byrne, S. N. Inhibition of UV-induced uric acid production using allopurinol prevents suppression of the contact hypersensitivity response. Exp. Dermatol. 22, 189–194 (2013).
Garg, A. D. et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 31, 1062–1079 (2012).
Kurita, M. et al. Induction of keratinocyte apoptosis by photosensitizing chemicals plus UVA. J. Dermatol. Sci. 45, 105–112 (2007).
Dos Santos, A. F. et al. Distinct photo-oxidation-induced cell death pathways lead to selective killing of human breast cancer cells. Cell Death Dis. 11, 1070 (2020).
Vassileva, S. G., Mateev, G. & Parish, L. C. Antimicrobial photosensitive reactions. Arch. Intern. Med. 158, 1993–2000 (1998).
Calnan, C. D. & Wells, G. C. Suspender dermatitis and nickel sensitivity. Br. Med. J. 1, 1265–1268 (1956).
Williams, J., Cahill, J. & Nixon, R. Occupational autoeczematization or atopic eczema precipitated by occupational contact dermatitis? Contact Dermatitis 56, 21–26 (2007).
Häusermann, P., Harr, T. & Bircher, A. J. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 51, 297–310 (2004).
Veien, N. K. Systemic contact dermatitis. Int. J. Dermatol. 50, 1445–1456 (2011).
Cressey, B. D. & Scheinman, P. L. Periocular dermatitis from systemic exposure to nickel in a palatal expander and dental braces. Dermatitis 23, 179 (2012).
Ham, J. E., Siegel, P. D. & Maibach, H. Undeclared formaldehyde levels in patient consumer products: formaldehyde test kit utility. Cutan. Ocul. Toxicol. 38, 112–117 (2019).
Nikle, A., Ericson, M. & Warshaw, E. Formaldehyde release from personal care products: chromotropic acid method analysis. Dermatitis 30, 67–73 (2019).
Herman, A. et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle® Libre, a newly introduced glucose sensor. Contact Dermatitis 77, 367–373 (2017).
Friis, U. F., Menné, T., Flyvholm, M. A., Bonde, J. P. & Johansen, J. D. Difficulties in using Material Safety Data Sheets to analyse occupational exposures to contact allergens. Contact Dermatitis 72, 147–153 (2015).
Bruze, M., Frick, M. & Persson, L. Patch testing with thin-layer chromatograms. Contact Dermatitis 48, 278–279 (2003).
Lammintausta, K. et al. An epidemic of furniture-related dermatitis: searching for a cause. Br. J. Dermatol. 162, 108–116 (2010).
Erfani, B., Midander, K., Lidén, C. & Julander, A. Development, validation and testing of a skin sampling method for assessment of metal exposure. Contact Dermatitis 77, 17–24 (2017).
Johansen, J. D. et al. European Society of Contact Dermatitis guideline for diagnostic patch testing - recommendations on best practice. Contact Dermatitis 73, 195–221 (2015). The European Society of Contact Dermatitis guidelines represent a comprehensive set of recommendations for patch testing in clinical practice.
Bruze, M., Isaksson, M., Gruvberger, B. & Frick-Engfeldt, M. Recommendation of appropriate amounts of petrolatum preparation to be applied at patch testing. Contact Dermatitis 56, 281–285 (2007).
van Amerongen, C. C. A., Ofenloch, R., Dittmar, D. & Schuttelaar, M. L. A. New positive patch test reactions on day 7 — the additional value of the day 7 patch test reading. Contact Dermatitis 81, 280–287 (2019).
Davis, M. D. et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J. Am. Acad. Dermatol. 59, 225–233 (2008).
Santiago, F., Gonçalo, M., Vieira, R., Coelho, S. & Figueiredo, A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis 62, 47–53 (2010).
Gonçalo, M. et al. Photopatch testing: recommendations for a European photopatch test baseline series. Contact Dermatitis.68, 239–243 (2013).
DeGroot A. C. Patch testing: test concentrations and vehicles for 4900 chemicals (Elsevier, 2018).
Friis, U. F., Menné, T., Flyvholm, M. A., Bonde, J. P. & Johansen, J. D. Occupational allergic contact dermatitis diagnosed by a systematic stepwise exposure assessment of allergens in the work environment. Contact Dermatitis 69, 153–163 (2013).
Hjorth, N. & Roed-Petersen, J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis 2, 28–42 (1976).
Vester, L., Thyssen, J. P., Menné, T. & Johansen, J. D. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermatitis 67, 328–333 (2012).
Friis, U. F. et al. Occupational irritant contact dermatitis diagnosed by analysis of contact irritants and allergens in the work environment. Contact Dermatitis 71, 364–370 (2014).
Agner, T. et al. Classification of hand eczema. J. Eur. Acad. Dermatol. Venereol. 29, 2417–2422 (2015).
Diepgen, T. L. et al. Hand eczema classification: a cross-sectional, multicentre study of the aetiology and morphology of hand eczema. Br. J. Dermatol. 160, 353–358 (2009).
Mathias, C. G. Contact dermatitis and workers’ compensation: criteria for establishing occupational causation and aggravation. J. Am. Acad. Dermatol. 20, 842–848 (1989). The ‘Mathias Critera’ continue to serve as the basis for the evaluation of occupational CD.
Johansen, J. D. et al. The repeated open application test: suggestions for a scale of evaluation. Contact Dermatitis 39, 95–96 (1998).
Fischer, L. A., Voelund, A., Andersen, K. E., Menné, T. & Johansen, J. D. The dose-response relationship between the patch test and ROAT and the potential use for regulatory purposes. Contact Dermatitis 61, 201–208 (2009).
Goldminz, A. M., Wald, M. S. & Scheinman, P. L. Positive occluded patch test in the face of negative repeat open application test. Dermatitis 29, 162–163 (2018).
Hindsén, M., Bruze, M. & Christensen, O. B. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J. Am. Acad. Dermatol. 44, 616–623 (2001).
Bregnhøj, A., Menné, T., Johansen, J. D. & Søsted, H. Prevention of hand eczema among Danish hairdressing apprentices: an intervention study. Occup. Env. Med. 69, 310–316 (2012).
Held, E., Mygind, K., Wolff, C., Gyntelberg, F. & Agner, T. Prevention of work related skin problems: an intervention study in wet work employees. Occup. Env. Med. 59, 556–561 (2002).
Schwensen, J. F., Bregnbak, D. & Johansen, J. D. Recent trends in epidemiology, sensitization and legal requirements of selected relevant contact allergens. Expert Rev. Clin. Immunol. 12, 289–300 (2016).
Hald, M., Agner, T., Blands, J. & Johansen, J. D. Delay in medical attention to hand eczema: a follow-up study. Brit. J. Dermatol. 161, 1294–1300 (2009).
Silvestri, D. L. & Barmettler, S. Pruritus ani as a manifestation of systemic contact dermatitis: resolution with dietary nickel restriction. Dermatitis 22, 50–55 (2011).
Thyssen, J. P. & Menné, T. Metal allergy–a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem. Res. Toxicol. 23, 309–318 (2010).
Jacob, S. E. & Castanedo-Tardan, M. P. Pharmacotherapy for allergic contact dermatitis. Expert Opin. Pharmacother. 8, 2757–2774 (2007).
Wollenberg, A. et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J. Eur. Acad. Dermatol. Venereol. 32, 657–682 (2018).
Saripalli, Y. V., Gadzia, J. E. & Belsito, D. V. Tacrolimus ointment 0.1% in the treatment of nickel-induced allergic contact dermatitis. J. Am. Acad. Dermatol. 49, 477–482 (2003).
Mose, K. F. et al. Anti-inflammatory potency testing of topical corticosteroids and calcineurin inhibitors in human volunteers sensitized to diphenylcyclopropenone. Br. J. Clin. Pharmacol. 84, 1719–1728 (2018).
Lejding, T. et al. Skin application of glutathione and iron sulfate can inhibit elicitation of allergic contact dermatitis from hexavalent chromium. Contact Dermatitis 82, 45–53 (2020).
Kemény, L., Varga, E. & Novak, Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert Rev. Clin. Immunol. 15, 1205–1214 (2019).
Schempp, C. M., Müller, H., Czech, W., Schöpf, E. & Simon, J. C. Treatment of chronic palmoplantar eczema with local bath-PUVA therapy. J. Am. Acad. Dermatol. 36, 733–737 (1997).
Sezer, E. & Etikan, I. Local narrowband UVB phototherapy vs. local PUVA in the treatment of chronic hand eczema. Photodermatol. Photoimmunol. Photomed. 23, 10–14 (2007).
Sung, C. T., McGowan, M. A., Machler, B. C. & Jacob, S. E. Systemic treatments for allergic contact dermatitis. Dermatitis 30, 46–53 (2019).
Cinats, A., Heck, E. & Robertson, L. Janus kinase inhibitors: a review of their emerging applications in dermatology. Skin. Ther. Lett. 23, 5–9 (2018).
Mowad, C. M. et al. Allergic contact dermatitis: patient management and education. J. Am. Acad. Dermatol. 74, 1043–1054 (2016).
Scheinman, P. L. The foul side of fragrance-free products: what every clinician should know about managing patients with fragrance allergy. J. Am. Acad. Dermatol. 41, 1020–1024 (1999).
Rubin, C. B. & Brod, B. Natural does not mean safe — the dirt on clean beauty products. 155, 1344–1345 (2019).
Lönnroth, E. C., Wellendorf, H. & Ruyter, E. Permeability of different types of medical protective gloves to acrylic monomers. Eur. J. Oral. Sci. 111, 440–446 (2003).
Scheman, A. et al. Alternatives for Allergens in the 2018 American Contact dermatitis society core series: report by the American Contact Alternatives Group. Dermatitis 30, 87–105 (2019).
Dizdarevic, A. et al. Intervention study to evaluate the importance of information given to patients with contact allergy: a randomized, investigator-blinded clinical trial. Br. J. Dermatol. 184, 43–49 (2020).
Dou, X., Liu, L. L. & Zhu, X. J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis 48, 126–129 (2003).
Stuckert, J. & Nedorost, S. Low-cobalt diet for dyshidrotic eczema patients. Contact Dermatitis 59, 361–365 (2008).
Mislankar, M. & Zirwas, M. J. Low-nickel diet scoring system for systemic nickel allergy. Dermatitis 24, 190–195 (2013).
Jensen, C. S., Menné, T. & Johansen, J. D. Systemic contact dermatitis after oral exposure to nickel: a review with a modified meta-analysis. Contact Dermatitis 54, 79–86 (2006).
Salam, T. N. & Fowler, J. F. Jr. Balsam-related systemic contact dermatitis. J. Am. Acad. Dermatol. 45, 377–381 (2001).
Scheman, A., Cha, C., Jacob, S. E. & Nedorost, S. Food avoidance diets for systemic, lip, and oral contact allergy: an american contact alternatives group article. Dermatitis 23, 248–257 (2012).
Tammaro, A., De Marco, G., Persechino, S., Narcisi, A. & Camplone, G. Allergy to nickel: first results on patients administered with an oral hyposensitization therapy. Int. J. Immunopathol. Pharmacol. 22, 837–840 (2009).
Christensen, J. D. Disulfiram treatment of three patients with nickel dermatitis. Contact Dermatitis 8, 105–108 (1982).
Kaaber, K., Menné, T., Veien, N. & Hougaard, P. Treatment of nickel dermatitis with Antabuse; a double blind study. Contact Dermatitis 9, 297–299 (1983).
Peddawad, D., Nagendra, S., Chatterjee, R., Faldu, H. & Chheda, A. Fulminant encephalopathy with unusual brain imaging in disulfiram toxicity. Neurology 90, 518–519 (2018).
Davis, M. D., Mowad, C. M. & Scheinman, P. Orthopedic prostheses: is there any point in patch testing? Dermatitis 15, 210–212 (2004).
Schalock, P. C. & Thyssen, J. P. Metal hypersensitivity reactions to implants: opinions and practices of patch testing dermatologists. Dermatitis 24, 313–320 (2013).
Resor, C. D. & et al. Systemic allergic contact dermatitis due to a GORE CARDIOFORM septal occluder device. A case report and literature review. J. Am. Coll. Cardiol. Case Rep. 2, 1867–1871 (2020).
Aquino, M. & Mucci, T. Systemic contact dermatitis and allergy to biomedical devices. Curr. Allergy Asthma Rep. 13, 518–527 (2013).
Bibas, N., Lassere, J., Paul, C., Aquilina, C. & Giordano-Labadie, F. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis 24, 35–36 (2013).
Lindsey, R. W. & Harper, A. Unusual complications related to spinal implants. JBJS Case Connect. 8, e10 (2018).
Jia, Z., Tu, J., Wang, K., Jiang, G. & Wang, W. Allergic reaction following implantation of a nitinol alloy inferior vena cava filter. J. Vasc. Interv. Radiol. 26, 1375–1377 (2015).
Pigatto, P. D. et al. Systemic allergic contact dermatitis associated with allergy to intraoral metals.Dermatol. Online J. 20, 13030/qt74632201 (2014).
Andrews, I. D. & Scheinman, P. Systemic hypersensitivity reaction (without cutaneous manifestations) to an implantable cardioverter-defibrillator. Dermatitis 22, 161–164 (2011).
Sharma, V. et al. Surgical explantation of atrial septal closure devices for refractory nickel allergy symptoms. J. Thorac. Cardiovasc. Surg. 160, 502–509.e1 (2020).
Schalock, P. C. et al. Hypersensitivity reactions to metallic implants - diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis 66, 4–19 (2012).
Schalock, P. C. et al. Patch testing for evaluation of hypersensitivity to implanted metal devices: a perspective from the American Contact Dermatitis Society. Dermatitis 27, 241–247 (2016).
Lai, D. W., Saver, J. L., Araujo, J. A., Reidl, M. & Tobis, J. Pericarditis associated with nickel hypersensitivity to the Amplatzer occluder device: a case report. Catheter. Cardiovasc. Interv. 66, 424–426 (2005).
Kimyon, R. S. & Warshaw, E. M. Airborne allergic contact dermatitis: management and responsible allergens on the American Contact Dermatitis Society core series. Dermatitis 30, 106–115 (2019).
Gruye, L. E., Yanovsky, R. L. & Goldminz, A. M. Preventing relapses of airborne allergic contact dermatitis to isothiazolinones in wall paint by painting over with an isothiazolinone-free paint. Contact Dermatitis 82, 130–131 (2020).
Walls, A. C. & Silvestri, D. L. Prevention of airborne propolis-induced allergic contact dermatitis with barrier cream. Dermatitis 23, 128–129 (2012).
Dogra, S., Parsad, D. & Handa, S. Narrowband ultraviolet B in airborne contact dermatitis: a ray of hope! Br. J. Dermatol. 150, 373–374 (2004).
Verma, K. K., Sethuraman, G. & Kalavani, M. Weekly azathioprine pulse versus daily azathioprine in the treatment of Parthenium dermatitis: A non-inferiority randomized controlled study. Indian J. Dermatol. Venereol. Leprol. 81, 251–256 (2015).
De, D., Sarangal, R. & Handa, S. The comparative efficacy and safety of azathioprine vs methotrexate as steroid-sparing agent in the treatment of airborne-contact dermatitis due to Parthenium. Indian J. Dermatol. Venereol. Leprol. 79, 240–241 (2013).
Lakshmi, C., Srinivas, C. R. & Jayaraman, A. Ciclosporin in parthenium dermatitis–a report of 2 cases. Contact Dermatitis 59, 245–248 (2008).
Snyder, M., Turrentine, J. E. & Cruz, P. D. Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin. Rev. Allergy Immunol. 56, 32–40 (2019).
Yu, J. et al. Occupational dermatitis to facial personal protective equipment in healthcare workers: a systematic review. J. Am. Acad. Dermatol. 84, 486–494 (2020).
Weisshaar, E. et al. Multicentre study ‘rehabilitation of occupational skin diseases -optimization and quality assurance of inpatient management (ROQ)’-results from 12-month follow-up. Contact Dermatitis 68, 169–174 (2013).
van Gils, R. F. et al. Process evaluation of an integrated, multidisciplinary intervention programme for hand eczema. Contact Dermatitis 66, 254–263 (2012).
van Gils, R. F. et al. The effectiveness of integrated care for patients with hand eczema: results of a randomized, controlled trial. Contact Dermatitis 66, 197–204 (2012).
Gomez, P., Kudla, I. & Wozniak, G. The impact of a multidisciplinary team and a dedicated return-to-work coordinator for workers with work-related skin disease. Dermatitis 22, 308–309 (2011).
Kurt, O. K. & Basaran, N. Occupational exposure to metals and solvents: allergy and airway diseases. Curr. Allergy Asthma Rep. 20, 38 (2020).
Lazarov, A., Rabin, B., Fraidlin, N. & Abraham, D. Medical and psychosocial outcome of patients with occupational contact dermatitis in Israel. J. Eur. Acad. Dermatol. Venereol. 20, 1061–1065 (2006).
Ng, W. T. & Koh, D. Occupational contact dermatitis in manual cloud seeding operations. Singap. Med. J. 52, e85–87 (2011).
Yan, Y. et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol. Ther. 33, e13310 (2020).
Holness, D. L. Occupational skin allergies: testing and treatment (the case of occupational allergic contact dermatitis). Curr. Allergy Asthma Rep. 14, 410 (2014).
Holness, D. L. Occupational dermatosis. Curr. Allergy Asthma Rep. 19, 42 (2019).
Mercader, P., de la Cuadra-Oyanguren, J., Rodríguez-Serna, M., Pitarch-Bort, G. & Fortea-Baixauli, J. M. Treatment of protein contact dermatitis with topical tacrolimus. Acta Derm. Venereol. 85, 555–556 (2005).
Karimkhani, C. et al. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. 153, 406–412 (2017).
Lau, M. Y., Burgess, J. A., Nixon, R., Dharmage, S. C. & Matheson, M. C. A review of the impact of occupational contact dermatitis on quality of life. J. Allergy 2011, 964509 (2011).
Holness, D. L. et al. Hand and upper extremity function in workers with hand dermatitis. Dermatitis 24, 131–136 (2013).
Anderson, R. T. & Rajagopalan, R. Development and validation of a quality of life instrument for cutaneous diseases. J. Am. Acad. Dermatol. 37, 41–50 (1997).
Boonchai, W., Charoenpipatsin, N., Winayanuwattikun, W., Phaitoonwattanakij, S. & Sukakul, T. Assessment of the quality of life (QoL) of patients with dermatitis and the impact of patch testing on QoL: A study of 519 patients diagnosed with dermatitis. Contact Dermatitis 83, 182–188 (2020).
Ramirez, F., Chren, M. M. & Botto, N. A review of the impact of patch testing on quality of life in allergic contact dermatitis. J. Am. Acad. Dermatol. 76, 1000–1004 (2017).
Bhatia, R., Sharma, V. K., Ramam, M., Sethuraman, G. & Yadav, C. P. Clinical profile and quality of life of patients with occupational contact dermatitis from New Delhi, India. Contact Dermatitis 73, 172–181 (2015).
Nagpal, N., Gordon-Elliott, J. & Lipner, S. Comparison of quality of life and illness perception among patients with acne, eczema, and psoriasis. Dermatol. Online J. 25, 13030/qt3fk3f989 (2019).
Kadyk, D. L., McCarter, K., Achen, F. & Belsito, D. V. Quality of life in patients with allergic contact dermatitis. J. Am. Acad. Dermatol. 49, 1037–1048 (2003).
Anderson, R. T. & Rajagopalan, R. Effects of allergic dermatosis on health-related quality of life. Curr. Allergy Asthma Rep. 1, 309–315 (2001).
Botto, N. et al. Validating a quality-of-life instrument for allergic contact dermatitis. Dermatitis 30, 300–305 (2019). This is the first quality of life instrument validated for use in allergic CD.
Raffi, J., Elaine Allen, I. & Botto, N. Validating responsiveness of a quality-of-life instrument for allergic contact dermatitis. Dermatitis 31, 209–214 (2020).
Boehm, D. et al. Anxiety, depression and impaired health-related quality of life in patients with occupational hand eczema. Contact Dermatitis 67, 184–192 (2012).
Rajagopalan, R. et al. An economic evaluation of patch testing in the diagnosis and management of allergic contact dermatitis. Am. J. Contact Dermatitis 9, 149–154 (1998).
Thomson, K. F., Wilkinson, S. M., Sommer, S. & Pollock, B. Eczema: quality of life by body site and the effect of patch testing. Br. J. Dermatol. 146, 627–630 (2002).
Woo, P. N., Hay, I. C. & Ormerod, A. D. An audit of the value of patch testing and its effect on quality of life. Contact Dermatitis.48, 244–247 (2003).
Korkmaz, P. & Boyvat, A. Effect of patch testing on the course of allergic contact dermatitis and prognostic factors that influence outcomes. Dermatitis 30, 135–141 (2019).
De Groot, A. C. New contact allergens: 2008 to 2015. Dermatitis 26, 199–215 (2015).
Wilkinson, M. et al. The European baseline series and recommended additions: 2019. Contact Dermatitis 80, 1–4 (2019).
Uter, W., Lessmann, H., Geier, J. & Schnuch, A. Is the irritant benzalkonium chloride a contact allergen? A contribution to the ongoing debate from a clinical perspective. Contact Dermatitis 58, 359–363 (2008).
Basketter, D. A., Marriott, M., Gilmour, N. J. & White, I. R. Strong irritants masquerading as skin allergens: The case of benzalkonium chloride. Contact Dermatitis 50, 213–217 (2004).
Schnuch, A., Lessmann, H., Geier, J. & Uter, W. Is cocamidopropyl betaine a contact allergen? Analysis of network data and short review of the literature. Contact Dermatitis 64, 203–211 (2011).
Rietschel R. L., Fowler J. F. & Fisher A. A. in Contact Dermatitis 7th edn Ch. “Rubber” (eds Fowler, J. F. & Zirwas, M. J.) (Contact Dermatitis Institute, 2019).
Geier, J., Uter, W., Pirker, C. & Frosch, P. J. Patch testing with the irritant sodium lauryl sulfate (SLS) is useful in interpreting weak reactions to contact allergens as allergic or irritant. Contact Dermatitis 48, 99–107 (2003).
Löffler, H., Becker, D., Brasch, J. & Geier, J. Simultaneous sodium lauryl sulphate testing improves the diagnostic validity of allergic patch tests. Results from a prospective multicentre study of the German Contact Dermatitis Research Group (Deutsche Kontaktallergie-Gruppe, DKG). Br. J. Dermatol. 152, 709–719 (2005).
Goh, C. L., Wong, W. K. & Ng, S. K. Comparison between 1-day and 2-day occlusion times in patch testing. Contact Dermatitis.31, 48–50 (1994).
Rudzki, E., Zakrzewski, Z., Prokopczyk, G. & Kozłowska, A. Patch tests with potassium dichromate removed after 24 and 48 hours. Contact Dermatitis 2, 309–310 (1976).
Brasch, J. et al. Reproducibility of patch tests. A multicenter study of synchronous left-versus right-sided patch tests by the German Contact Dermatitis Research Group. J. Am. Acad. Dermatol. 31, 584–591 (1994).
Machácková, J. & Seda, O. Reproducibility of patch tests. J. Am. Acad. Dermatol. 25, 732–733 (1991).
Ale, S. I. & Maibach, H. I. 24-Hour versus 48-hour occlusion in patch testing. Exog. Dermatol. 2, 270–276 (2003).
Higgins, C. L., Palmer, A. M., Cahill, J. L. & Nixon, R. L. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis 75, 213–222 (2016).
Halbert, A. R., Gebauer, K. A. & Wall, L. M. Prognosis of occupational chromate dermatitis. Contact Dermatitis 27, 214–219 (1992).
Nichol, K., Copes, R., Kersey, K., Eriksson, J. & Holness, D. L. Screening for hand dermatitis in healthcare workers: comparing workplace screening with dermatologist photo screening. Contact Dermatitis 80, 374–381 (2019).
Fisker, M. H., Ebbehøj, N. E., Jungersted, J. M. & Agner, T. What do patients with occupational hand eczema know about skin care? Contact Dermatitis 69, 93–98 (2013).
Graversgaard, C., Agner, T., Jemec, G. B. E., Thomsen, S. F. & Ibler, K. S. A long-term follow-up study of the Hand Eczema Trial (HET): a randomized clinical trial of a secondary preventive programme introduced to Danish healthcare workers. Contact Dermatitis 78, 329–334 (2018).
Clemmensen, K. K. B., Randbøll, I., Ryborg, M. F., Ebbehøj, N. E. & Agner, T. Evidence-based training as primary prevention of hand eczema in a population of hospital cleaning workers. Contact Dermatitis 72, 47–54 (2015).
Olesen, C. M., Agner, T., Ebbehøj, N. E. & Carøe, T. K. Factors influencing prognosis for occupational hand eczema: new trends. Br. J. Dermatol. 181, 1280–1286 (2019).
Keegel, T. et al. Incidence and prevalence rates for occupational contact dermatitis in an Australian suburban area. Contact Dermatitis 52, 254–259 (2005).
Nurmohamed, S., Bodley, T., Thompson, A. & Holness, D. L. Health care utilization characteristics in patch test patients. Dermatitis 25, 268–272 (2014).
Koppes, S. A. et al. Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis 77, 1–16 (2017).
Wei, Z. et al. Two-dimensional cellular and three-dimensional bio-printed skin models to screen topical-use compounds for irritation potential. Front. Bioeng. Biotechnol. 8, 109 (2020).
Malmberg, P., Guttenberg, T., Ericson, M. & Hagvall, L. Imaging mass spectrometry for novel insights into contact allergy - a proof-of-concept study on nickel. Contact Dermatitis 78, 109–116 (2018).
Hamilton, J. D., Ungar, B. & Guttman-Yassky, E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy 7, 1043–1058 (2015).
Machler, B. C. et al. Dupilumab use in allergic contact dermatitis. J. Am. Acad. Dermatol. 80, 280–281.e281 (2019).
Maloney, N. J. et al. Dupilumab in dermatology: potential for uses beyond atopic dermatitis. J. Drugs Dermatol. 18, S1545961619P1053X (2019).
Puza, C. J. & Atwater, A. R. Positive patch test reaction in a patient taking dupilumab. Dermatitis 29, 89–89 (2018).
Ruge, I. F., Skov, L., Zachariae, C. & Thyssen, J. P. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis 83, 137–139 (2020).
Joshi, S. R. & Khan, D. A. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis 29, 282–284 (2018).
Goldminz, A. M. & Scheinman, P. L. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol. Ther. 31, 8–10 (2018).
Raffi, J. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J. Am. Acad. Dermatol. 82, 132–138 (2020).
Tuckermann, J. P. et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Invest. 117, 1381–1390 (2007).
Suwanpradid, J. et al. Arginase1 deficiency in monocytes/macrophages upregulates inducible nitric oxide synthase to promote cutaneous contact hypersensitivity. J. Immunol. 199, 1827–1834 (2017).
Smith, J. S., Rajagopal, S. & Atwater, A. R. Chemokine signaling in allergic contact dermatitis: toward targeted therapies. Dermatitis 29, 179–186 (2018). For many years, allergen avoidance, topical corticosteroids, phototherapy and traditional, systemic immunosuppressants have been the mainstay for the treatment of CD; targeted therapies represent a new and exciting approach to benefit patients with recalcitrant disease.
Alonso, M. D. et al. Occupational protein contact dermatitis from lettuce. Contact Dermatitis 29, 109–110 (1993).
Hafner, J., Riess, C. E. & Wüthrich, B. Protein contact dermatitis from paprika and curry in a cook. Contact Dermatitis 26, 51–52 (1992).
Iliev, D. & Wüthrich, B. Occupational protein contact dermatitis with type I allergy to different kinds of meat and vegetables. Int. Arch. Occup. Env. Health 71, 289–292 (1998).
Kumar, A. & Freeman, S. Protein contact dermatitis in food workers. Case report of a meat sorter and summary of seven other cases. Australas. J. Dermatol. 40, 138–140 (1999).
Laurière, M. et al. Hydrolysed wheat proteins present in cosmetics can induce immediate hypersensitivities. Contact Dermatitis.54, 283–289 (2006).
Crippa, M., Sala, E. & Alessio, L. Occupational protein contact dermatitis from milk proteins. Contact Dermatitis 51, 42 (2004).
Doyen, V. et al. Protein contact dermatitis and food allergy to mare milk. Ann. Allergy Asthma Immunol. 110, 390–391 (2013).
Kanerva, L., Vanhanen, M. & Tupasela, O. Occupational contact urticaria from cellulase enzyme. Contact Dermatitis 38, 176–177 (1998).
Nettis, E., Colanardi, M. C. & Ferrannini, A. Type I latex allergy in health care workers with latex-induced contact urticaria syndrome: a follow-up study. Allergy 59, 718–723 (2004).
Crisi, G. & Belsito, D. V. Contact urticaria from latex in a patient with immediate hypersensitivity to banana, avocado and peach. Contact Dermatitis 28, 247–248 (1993).
Uter, W., Gefeller, O., Mahler, V. & Geier, J. Trends and current spectrum of contact allergy in Central Europe: results of the information network of departments of dermatology (IVDK) 2007-2018. Br. J. Dermatol. 183, 857–865 (2020).
Tagka, A. et al. Prevalence of contact dermatitis in the Greek population: a retrospective observational study. Contact Dermatitis 81, 460–462 (2019).
Ochi, H., Cheng, S. W., Leow, Y. H. & Goon, A. T. Contact allergy trends in Singapore - a retrospective study of patch test data from 2009 to 2013. Contact Dermatitis 76, 49–50 (2017).
Süß, H. et al. Contact urticaria: frequency, elicitors and cofactors in three cohorts (Information Network of Departments of Dermatology; Network of Anaphylaxis; and Department of Dermatology, University Hospital Erlangen, Germany). Contact Dermatitis 81, 341–353 (2019).
Amaro, C. & Goossens, A. Immunological occupational contact urticaria and contact dermatitis from proteins: a review. Contact Dermatitis 58, 67–75 (2008).
Saluja, S. S., Davis, C. L., Chong, T. A. & Powell, D. L. Contact urticaria to nickel: a series of 11 patients who were prick test positive and patch test negative to nickel sulfate 2.5% and 5.0. Dermatitis 27, 282–287 (2016).
Verhulst, L. & Goossens, A. Cosmetic components causing contact urticaria: a review and update. Contact Dermatitis 75, 333–344 (2016).
Bhatia, R., Alikhan, A. & Maibach, H. I. Contact urticaria: present scenario. Indian J. Dermatol. 54, 264–268 (2009).
Author information
Authors and Affiliations
Contributions
Introduction (A.M.G.); Epidemiology (K.D. and R.L.N.); Mechanisms/pathophysiology (M.V. and J.M.); Diagnosis, screening and prevention (J.P.T. and J.D.J.); Management (P.L.S.); Quality of life (N.C.B.); Outlook (K.D. and R.L.N.); Overview of Primer (A.M.G.).
Corresponding author
Ethics declarations
Competing interests
P.L.S. is a member of the American Academy of Dermatology and the American Contact Dermatitis Society and has held leadership and committee positions for both of these organizations. P.L.S. is a consultant for Ella-Ola brands. N.C.B. is a member of the American Academy of Dermatology and the American Contact Dermatitis Society and has held leadership and committee positions for both of these organizations. A.M.G. is a member of the American Academy of Dermatology and the American Contact Dermatitis Society and has held leadership and/or committee positions for both of these organizations. A.M.G. is an investigator with research grants from Regeneron and The Human Skin Disease Research Center. A.M.G.’s spouse owns stock in Johnson and Johnson. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks J. English, M. Gonçalo, S. Martin, M. Pallardy, and T. Rustemeyer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Allergen
-
A substance, usually a protein, that can stimulate the immune system and induce sensitization.
- Irritant
-
A substance that has irritant properties that does not rely on the process of sensitization or immunological memory.
- Hapten
-
A chemical that reacts with skin self-proteins through a process termed haptenization; haptens bind to self-proteins and generate hapten–self-protein complexes (or haptenated proteins) that will be processed in hapten–self-peptide complexes (or neo-antigens). These neo-antigens will then be presented in MHC to T cells.
- Sensitization
-
The initial phase of contact allergy where the immune system is primed to the allergen after exposure, with subsequent immune memory that can recognize the allergen with re-exposure.
- Elicitation
-
In a previously sensitized individual, re-exposure to an allergen can cause mobilization of the immune system (immunological memory).
- Prehaptens
-
Haptens that need to be activated by exogenous triggers (such as UV light, air or others) before they can react with self-proteins.
- Prohaptens
-
Haptens that need to be activated by endogenous mechanisms (via enzymes or other biological processes) before they can react with self-proteins.
Rights and permissions
About this article
Cite this article
Scheinman, P.L., Vocanson, M., Thyssen, J.P. et al. Contact dermatitis. Nat Rev Dis Primers 7, 38 (2021). https://doi.org/10.1038/s41572-021-00271-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00271-4
This article is cited by
-
Orchestrierung der Entzündung bei Kontaktallergie durch angeborene Immun- und zelluläre Stressantworten
Allergo Journal (2024)
-
Occupational Hand Dermatitis
Current Allergy and Asthma Reports (2023)
-
Management von Kontaktekzemen
Allergo Journal (2023)
-
Nanomaterials for antigen-specific immune tolerance therapy
Drug Delivery and Translational Research (2023)
-
Management of contact dermatitis
Allergo Journal International (2023)