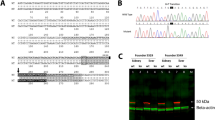
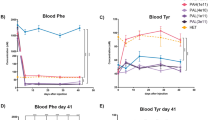
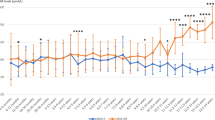
Abstract
Phenylketonuria (PKU; also known as phenylalanine hydroxylase (PAH) deficiency) is an autosomal recessive disorder of phenylalanine metabolism, in which especially high phenylalanine concentrations cause brain dysfunction. If untreated, this brain dysfunction results in severe intellectual disability, epilepsy and behavioural problems. The prevalence varies worldwide, with an average of about 1:10,000 newborns. Early diagnosis is based on newborn screening, and if treatment is started early and continued, intelligence is within normal limits with, on average, some suboptimal neurocognitive function. Dietary restriction of phenylalanine has been the mainstay of treatment for over 60 years and has been highly successful, although outcomes are still suboptimal and patients can find the treatment difficult to adhere to. Pharmacological treatments are available, such as tetrahydrobiopterin, which is effective in only a minority of patients (usually those with milder PKU), and pegylated phenylalanine ammonia lyase, which requires daily subcutaneous injections and causes adverse immune responses. Given the drawbacks of these approaches, other treatments are in development, such as mRNA and gene therapy. Even though PAH deficiency is the most common defect of amino acid metabolism in humans, brain dysfunction in individuals with PKU is still not well understood and further research is needed to facilitate development of pathophysiology-driven treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anikster, Y. et al. Biallelic mutations in DNAJC12 cause hyperphenylalaninemia, dystonia, and intellectual disability. Am. J. Hum. Genet. 100, 257–266 (2017).
Blau, N. Sapropterin dihydrochloride for the treatment of hyperphenylalaninemias. Expert. Opin. Drug. Metab. Toxicol. 9, 1207–1218 (2013).
Dhondt, J.-L. Lessons from 30 years of selective screening for tetrahydrobiopterin deficiency. J. Inherit. Metab. Dis. 33, S219–S223 (2010).
Straniero, L. et al. DNAJC12 and dopa-responsive nonprogressive parkinsonism. Ann. Neurol. 82, 640–646 (2017).
Blau, N., van Spronsen, F. J. & Levy, H. L. Phenylketonuria. Lancet 376, 1417–1427 (2010).
van Spronsen, F. J. et al. Phenylalanine tolerance can already reliably be assessed at the age of 2 years in patients with PKU. J. Inherit. Metab. Dis. 32, 27–31 (2009).
Güttler, F. & Hansen, G. Different phenotypes for phenylalanine hydroxylase deficiency. Ann. Clin. Biochem. 14, 124–134 (1977).
van Spronsen, F. J. et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 5, 743–756 (2017).
Følling, I. A. Über Ausscheidung von Phenylbrenztraubensäure in den Harn als Stoffwechselanomalie in Verbindung mit Imbezillität. Hoppe-Seyler’s Z. für Physiologische Chem. 227, 169–181 (1934).
Penrose, L. S. Inheritance of phenylpyruvic amentia (phenylketonuria). Lancet 226, 192–194 (1935).
Cowie, V. A. An atypical case of phenylketonuria. Lancet 1, 272 (1951).
Jervis, G. A. Phenylpyruvic oligophrenia deficiency of phenylalanine-oxidizing system. Proc. Soc. Exp. Biol. Med. 82, 514–515 (1953).
Jervis, G. A. Studies on phenylpyruvic oligophrenia; the position of the metabolic error. J. Biol. Chem. 169, 651–656 (1947).
Bickel, H., Gerrard, J. & Hickmans, E. M. Influence of phenylalanine intake on phenylketonuria. Lancet 265, 812–813 (1953).
Wortis, J. & Giancotti, A. M. A new simple test paper for mass detection of phenylketonuria. Am. J. Public Health Nations Health 49, 463–464 (1959).
Dent, C. Discussion of Armstrong MD: Relation of biochemical abnormality to development of mental defect in phenylketonuria in etiological factors in mental retardation. In Report of 23rd Ross Conference, Columbus, Ohio (1957).
Guthrie, R. & Susi, A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 32, 338–343 (1963).
Kaufman, S. The phenylalanine hydroxylating system from mammalian liver. Adv. Enzymol. Relat. Areas Mol. Biol. 35, 245–319 (1971).
Bartholome, K. Letter: A new molecular defect in phenylketonuria. Lancet 2, 1580 (1974).
Hoskins, J. A. et al. Enzymatic control of phenylalanine intake in phenylketonuria. Lancet 1, 392–394 (1980).
Woo, S. L., Lidsky, A. S., Güttler, F., Chandra, T. & Robson, K. J. Cloned human phenylalanine hydroxylase gene allows prenatal diagnosis and carrier detection of classical phenylketonuria. Nature 306, 151–155 (1983).
Konecki, D. S., Wang, Y., Trefz, F. K., Lichter-Konecki, U. & Woo, S. L. Structural characterization of the 5′ regions of the human phenylalanine hydroxylase gene. Biochemistry 31, 8363–8368 (1992).
Standing, S. J. & Taylor, R. P. Phenylalanine: application of a simple HPLC technique to its measurement in dried blood spots. Ann. Clin. Biochem. 29, 668–670 (1992).
Kure, S. et al. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J. Pediatr. 135, 375–378 (1999).
Longo, N. et al. Single-dose, subcutaneous recombinant phenylalanine ammonia lyase conjugated with polyethylene glycol in adult patients with phenylketonuria: an open-label, multicentre, phase 1 dose-escalation trial. Lancet 384, 37–44 (2014).
Walter, J. H. et al. How practical are recommendations for dietary control in phenylketonuria? Lancet 360, 55–57 (2002).
Scriver, C. R. The PAH gene, phenylketonuria, and a paradigm shift. Hum. Mutat. 28, 831–845 (2007).
Hillert, A. et al. The genetic landscape and epidemiology of phenylketonuria. Am. J. Hum. Genet. 107, 234–250 (2020).
Desviat, L. R. et al. Genetic and phenotypic aspects of phenylalanine hydroxylase deficiency in Spain: molecular survey by regions. Eur. J. Hum. Genet. 7, 386–392 (1999).
Ozalp, I., Coskun, T., Tokol, S., Demircin, G. & Mönch, E. Inherited metabolic disorders in Turkey. J. Inherit. Metab. Dis. 13, 732–738 (1990).
Senemar, S., Ganjekarimi, A., Senemar, S., Tarami, B. & Bazrgar, M. The prevalence and clinical study of galactosemia disease in a pilot screening program of neonates, southern Iran. Iran. J. Public. Health 40, 99–104 (2011).
Gundorova, P. et al. Molecular-genetic causes for the high frequency of phenylketonuria in the population from the North Caucasus. PLoS ONE 13, e0201489 (2018).
Sutivijit, Y., Banpavichit, A. & Wiwanitkit, V. Prevalence of neonatal hypothyroidism and phenylketonuria in Southern Thailand: a 10-year report. Indian. J. Endocrinol. Metab. 15, 115–117 (2011).
Okano, Y., Kudo, S., Nishi, Y., Sakaguchi, T. & Aso, K. Molecular characterization of phenylketonuria and tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency in Japan. J. Hum. Genet. 56, 306–312 (2011).
Borrajo, G. J. C. Newborn screening in Latin America at the beginning of the 21st century. J. Inherit. Metab. Dis. 30, 466–481 (2007).
Hitzeroth, H. W., Niehaus, C. E. & Brill, D. C. Phenylketonuria in South Africa. A report on the status quo. S Afr. Med. J. 85, 33–36 (1995).
Hardelid, P. et al. The birth prevalence of PKU in populations of European, South Asian and sub-Saharan African ancestry living in South East England. Ann. Hum. Genet. 72, 65–71 (2008).
Opladen, T., Hoffmann, G. F., Kühn, A. A. & Blau, N. Pitfalls in phenylalanine loading test in the diagnosis of dopa-responsive dystonia. Mol. Genet. Metab. 108, 195–197 (2013).
van Spronsen, F. J. et al. Heterogeneous clinical spectrum of DNAJC12-deficient hyperphenylalaninemia: from attention deficit to severe dystonia and intellectual disability. J. Med. Genet 55, 249–253 (2018).
Garbade, S. F. et al. Allelic phenotype values: a model for genotype-based phenotype prediction in phenylketonuria. Genet. Med. 21, 580–590 (2019).
Eisensmith, R. C. et al. Multiple origins for phenylketonuria in Europe. Am. J. Hum. Genet. 51, 1355–1365 (1992).
Waters, P. J., Parniak, M. A., Nowacki, P. & Scriver, C. R. In vitro expression analysis of mutations in phenylalanine hydroxylase: linking genotype to phenotype and structure to function. Hum. Mutat. 11, 4–17 (1998).
Shen, N. et al. Co-expression of phenylalanine hydroxylase variants and effects of interallelic complementation on in vitro enzyme activity and genotype–phenotype correlation. Mol. Genet. Metab. 117, 328–335 (2016).
Himmelreich, N. et al. Relationship between genotype, phenylalanine hydroxylase expression and in vitro activity and metabolic phenotype in phenylketonuria. Mol. Genet. Metab. 125, 86–95 (2018).
Blau, N. & Erlandsen, H. The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol. Genet. Metab. 82, 101–111 (2004).
Shedlovsky, A., McDonald, J. D., Symula, D. & Dove, W. F. Mouse models of human phenylketonuria. Genetics 134, 1205–1210 (1993).
Gersting, S. W. et al. Pahenu1 is a mouse model for tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency and promotes analysis of the pharmacological chaperone mechanism in vivo. Hum. Mol. Genet. 19, 2039–2049 (2010).
Levy, H. L. et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 370, 504–510 (2007).
McDonald, J. D., Bode, V. C., Dove, W. F. & Shedlovsky, A. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc. Natl Acad. Sci. USA 87, 1965–1967 (1990).
Saugstad, L. F. Birthweights in children with phenylketonuria and in their siblings. Lancet 1, 809–813 (1972).
Verkerk, P. H., van Spronsen, F. J., Smit, G. P. & Sengers, R. C. Impaired prenatal and postnatal growth in Dutch patients with phenylketonuria. The National PKU Steering Committee. Arch. Dis. Child. 71, 114–118 (1994).
Ilgaz, F. et al. Long-term growth in phenylketonuria: a systematic review and meta-analysis. Nutrients 11, 2070 (2019).
Ayling, J. E., Helfand, G. D. & Pirson, W. D. Phenylalanine hydroxylase from human kidney. Enzyme 20, 6–19 (1975).
Huttenlocher, P. R. The neuropathology of phenylketonuria: human and animal studies. Eur. J. Pediatr. 159 (Suppl 2), 102–106 (2000).
Bauman, M. L. & Kemper, T. L. Morphologic and histoanatomic observations of the brain in untreated human phenylketonuria. Acta Neuropathol. 58, 55–63 (1982).
Horling, K. et al. Hippocampal synaptic connectivity in phenylketonuria. Hum. Mol. Genet. 24, 1007–1018 (2015).
Surtees, R. & Blau, N. The neurochemistry of phenylketonuria. Eur. J. Pediatr. 159 (Suppl 2), 109–113 (2000).
Jervis, G. A. Studies on phenylpyruvic oligophrenia; phenylpyruvic acid content on blood. Proc. Soc. Exp. Biol. Med. 81, 715–720 (1952).
Batshaw, M. L., Valle, D. & Bessman, S. P. Unsuccessful treatment of phenylketonuria with tyrosine. J. Pediatr. 99, 159–160 (1981).
Azen, C. G. et al. Intellectual development in 12-year-old children treated for phenylketonuria. Am. J. Dis. Child. 145, 35–39 (1991).
van Vliet, D. et al. Can untreated PKU patients escape from intellectual disability? A systematic review. Orphanet J. Rare Dis. 13, 149 (2018).
Hörster, F. et al. Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr. Res. 59, 544–548 (2006).
Hartwig, C. et al. Elevated phenylalanine levels interfere with neurite outgrowth stimulated by the neuronal cell adhesion molecule L1 in vitro. FEBS Lett. 580, 3489–3492 (2006).
Andolina, D. et al. 5-Hydroxytryptophan during critical postnatal period improves cognitive performances and promotes dendritic spine maturation in genetic mouse model of phenylketonuria. Int. J. Neuropsychopharmacol. 14, 479–489 (2011).
Schlegel, G., Scholz, R., Ullrich, K., Santer, R. & Rune, G. M. Phenylketonuria: direct and indirect effects of phenylalanine. Exp. Neurol. 281, 28–36 (2016).
Christ, S. E. et al. Morphometric analysis of gray matter integrity in individuals with early-treated phenylketonuria. Mol. Genet. Metab. 118, 3–8 (2016).
Pilotto, A. et al. Cerebrospinal fluid biogenic amines depletion and brain atrophy in adult patients with phenylketonuria. J. Inherit. Metab. Dis. 42, 398–406 (2019).
Dyer, C. A. et al. Evidence for central nervous system glial cell plasticity in phenylketonuria. J. Neuropathol. Exp. Neurol. 55, 795–814 (1996).
Schoemans, R. et al. Oligodendrocyte development and myelinogenesis are not impaired by high concentrations of phenylalanine or its metabolites. J. Inherit. Metab. Dis. 33, 113–120 (2010).
Shefer, S. et al. Is there a relationship between 3-hydroxy-3-methylglutaryl coenzyme a reductase activity and forebrain pathology in the PKU mouse? J. Neurosci. Res. 61, 549–563 (2000).
Qin, M. & Smith, C. B. Regionally selective decreases in cerebral glucose metabolism in a mouse model of phenylketonuria. J. Inherit. Metab. Dis. 30, 318–325 (2007).
Winn, S. R., Scherer, T., Thöny, B. & Harding, C. O. High dose sapropterin dihydrochloride therapy improves monoamine neurotransmitter turnover in murine phenylketonuria (PKU). Mol. Genet. Metab. 117, 5–11 (2016).
Cabib, S., Pascucci, T., Ventura, R., Romano, V. & Puglisi-Allegra, S. The behavioral profile of severe mental retardation in a genetic mouse model of phenylketonuria. Behav. Genet. 33, 301–310 (2003).
Zagreda, L., Goodman, J., Druin, D. P., McDonald, D. & Diamond, A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J. Neurosci. 19, 6175–6182 (1999).
Hasselbalch, S. et al. Cerebral glucose metabolism is decreased in white matter changes in patients with phenylketonuria. Pediatr. Res. 40, 21–24 (1996).
Ficicioglu, C. et al. A pilot study of fluorodeoxyglucose positron emission tomography findings in patients with phenylketonuria before and during sapropterin supplementation. J. Clin. Neurol. 9, 151–156 (2013).
Wasserstein, M. P., Snyderman, S. E., Sansaricq, C. & Buchsbaum, M. S. Cerebral glucose metabolism in adults with early treated classic phenylketonuria. Mol. Genet. Metab. 87, 272–277 (2006).
Miller, A. L., Hawkins, R. A. & Veech, R. L. Phenylketonuria: phenylalanine inhibits brain pyruvate kinase in vivo. Science 179, 904–906 (1973).
Choi, T. B. & Pardridge, W. M. Phenylalanine transport at the human blood–brain barrier. Studies with isolated human brain capillaries. J. Biol. Chem. 261, 6536–6541 (1986).
Kanai, Y. et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 273, 23629–23632 (1998).
de Groot, M. J., Hoeksma, M., Blau, N., Reijngoud, D. J. & van Spronsen, F. J. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol. Genet. Metab. 99 (Suppl 1), 86–89 (2010).
Hoeksma, M. et al. Phenylketonuria: high plasma phenylalanine decreases cerebral protein synthesis. Mol. Genet. Metab. 96, 177–182 (2009).
de Groot, M. J. et al. Phenylketonuria: reduced tyrosine brain influx relates to reduced cerebral protein synthesis. Orphanet J. Rare Dis. 8, 133 (2013).
van Vliet, D. et al. Large neutral amino acid supplementation exerts its effect through three synergistic mechanisms: proof of principle in phenylketonuria mice. PLoS One 10, e0143833 (2015).
Pietz, J. et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J. Clin. Invest. 103, 1169–1178 (1999).
Koch, R., Moseley, K. D., Yano, S., Nelson, M. & Moats, R. A. Large neutral amino acid therapy and phenylketonuria: a promising approach to treatment. Mol. Genet. Metab. 79, 110–113 (2003).
Schindeler, S. et al. The effects of large neutral amino acid supplements in PKU: an MRS and neuropsychological study. Mol. Genet. Metab. 91, 48–54 (2007).
van Vliet, D. et al. Therapeutic brain modulation with targeted large neutral amino acid supplements in the Pah-enu2 phenylketonuria mouse model. Am. J. Clin. Nutr. 104, 1292–1300 (2016).
O’Kane, R. L. & Hawkins, R. A. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood–brain barrier. Am. J. Physiol. Endocrinol. Metab. 285, E1167–E1173 (2003).
Hawkins, R. A., O’Kane, R. L., Simpson, I. A. & Viña, J. R. Structure of the blood–brain barrier and its role in the transport of amino acids. J. Nutr. 136, 218S–226SS (2006).
Wegrzyn, A. In silico patient: systems medicine approach to inborn errors of metabolism. Thesis, Univ. Groningen (2020).
Pare, C. M., Sandler, M. & Stacey, R. S. 5-Hydroxytryptamine deficiency in phenylketonuria. Lancet 272, 551–553 (1957).
McKean, C. M. The effects of high phenylalanine concentrations on serotonin and catecholamine metabolism in the human brain. Brain Res. 47, 469–476 (1972).
Harding, C. O. et al. Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU). J. Inherit. Metab. Dis. 37, 735–743 (2014).
Puglisi-Allegra, S. et al. Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria. Neuroreport 11, 1361–1364 (2000).
Pascucci, T., Ventura, R., Puglisi-Allegra, S. & Cabib, S. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport 13, 2561–2564 (2002).
Pascucci, T. et al. 5-Hydroxytryptophan rescues serotonin response to stress in prefrontal cortex of hyperphenylalaninaemic mice. Int. J. Neuropsychopharmacol. 12, 1067–1079 (2009).
Pascucci, T. et al. In vivo catecholaminergic metabolism in the medial prefrontal cortex of ENU2 mice: an investigation of the cortical dopamine deficit in phenylketonuria. J. Inherit. Metab. Dis. 35, 1001–1009 (2012).
van Vliet, D. et al. Large neutral amino acid supplementation as an alternative to the phenylalanine-restricted diet in adults with phenylketonuria: evidence from adult Pah-enu2 mice. J. Nutr. Biochem. 53, 20–27 (2018).
Christ, S. E., Huijbregts, S. C. J., de Sonneville, L. M. J. & White, D. A. Executive function in early-treated phenylketonuria: profile and underlying mechanisms. Mol. Genet. Metab. 99 (Suppl 1), 22–32 (2010).
Adler-Abramovich, L. et al. Phenylalanine assembly into toxic fibrils suggests amyloid etiology in phenylketonuria. Nat. Chem. Biol. 8, 701–706 (2012).
Dobrowolski, S. F. et al. Altered DNA methylation in PAH deficient phenylketonuria. Mol. Genet. Metab. 115, 72–77 (2015).
Dobrowolski, S. F. et al. DNA methylation in the pathophysiology of hyperphenylalaninemia in the PAH(enu2) mouse model of phenylketonuria. Mol. Genet. Metab. 119, 1–7 (2016).
Ercal, N., Aykin-Burns, N., Gurer-Orhan, H. & McDonald, J. D. Oxidative stress in a phenylketonuria animal model. Free Radic. Biol. Med. 32, 906–911 (2002).
van der Goot, E. et al. Hippocampal microglia modifications in C57Bl/6 Pahenu2 and BTBR Pahenu2 phenylketonuria (PKU) mice depend on the genetic background, irrespective of disturbed sleep patterns. Neurobiol. Learn. Mem. 160, 139–143 (2019).
Thompson, A. J. et al. Neurological deterioration in young adults with phenylketonuria. Lancet 336, 602–605 (1990).
Rubin, S. et al. Sight-threatening phenylketonuric encephalopathy in a young adult, reversed by diet. JIMD Rep. 10, 83–85 (2013).
Jaulent, P. et al. Neurological manifestations in adults with phenylketonuria: new cases and review of the literature. J. Neurol. 267, 531–542 (2020).
Rosini, F., Rufa, A., Monti, L., Tirelli, L. & Federico, A. Adult-onset phenylketonuria revealed by acute reversible dementia, prosopagnosia and parkinsonism. J. Neurol. 261, 2446–2448 (2014).
Arnold, G. L., Vladutiu, C. J., Orlowski, C. C., Blakely, E. M. & DeLuca, J. Prevalence of stimulant use for attentional dysfunction in children with phenylketonuria. J. Inherit. Metab. Dis. 27, 137–143 (2004).
Bilder, D. A. et al. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev. Neuropsychol. 41, 245–260 (2016).
Jahja, R. et al. Long-term follow-up of cognition and mental health in adult phenylketonuria: a PKU-COBESO study. Behav. Genet. 47, 486–497 (2017).
van Wegberg, A. M. J. et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 12, 162 (2017).
Anjema, K. et al. The neonatal tetrahydrobiopterin loading test in phenylketonuria: what is the predictive value? Orphanet J. Rare Dis. 11, 10 (2016).
Evers, R. A. F. et al. The first European guidelines on phenylketonuria: usefulness and implications for BH4 responsiveness testing. J. Inherit. Metab. Dis. 43, 244–250 (2020).
Blau, N. & van Spronsen, F. J. in Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases (eds Blau, N., Duran, M., Gibson, K. M. & Vici, C. D.) 3–21 (Springer, 2014).
Kornguth, S., Gilbert-Barness, E., Langer, E. & Hegstrand, L. Golgi-Kopsch silver study of the brain of a patient with untreated phenylketonuria, seizures, and cortical blindness. Am. J. Med. Genet. 44, 443–448 (1992).
Leuzzi, V. et al. Subclinical visual impairment in phenylketonuria. A neurophysiological study (VEP-P) with clinical, biochemical, and neuroradiological (MRI) correlations. J. Inherit. Metab. Dis. 21, 351–364 (1998).
MacDonald, A. et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J. Rare Dis. 15, 171 (2020).
Knox, W. E. An evaluation of the treatment of phenylketonuria with diets low in phenylalanine. Pediatrics 26, 1–11 (1960).
Rouse, B. M. Phenylalanine deficiency syndrome. J. Pediatr. 69, 246–249 (1966).
Hanley, W. B., Linsao, L., Davidson, W. & Moes, C. A. Malnutrition with early treatment of phenylketonuria. Pediatr. Res. 4, 318–327 (1970).
Pode-Shakked, B. et al. Man made disease: clinical manifestations of low phenylalanine levels in an inadequately treated phenylketonuria patient and mouse study. Mol. Genet. Metab. 110 (Suppl), 66–70 (2013).
Daly, A., Evans, S., Pinto, A., Ashmore, C. & MacDonald, A. Protein substitutes in PKU; their historical evolution. Nutrients 13, 484 (2021).
Sawin, E. A. et al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G590–G601 (2015).
Pinto, A. et al. Nutritional status in patients with phenylketonuria using glycomacropeptide as their major protein source. Eur. J. Clin. Nutr. 71, 1230–1234 (2017).
Gropper, S. S., Gropper, D. M. & Acosta, P. B. Plasma amino acid response to ingestion of L-amino acids and whole protein. J. Pediatr. Gastroenterol. Nutr. 16, 143–150 (1993).
MacDonald, A., Ashmore, C., Daly, A., Pinto, A. & Evans, S. An observational study evaluating the introduction of a prolonged-release protein substitute to the dietary management of children with phenylketonuria. Nutrients 12, 2686 (2020).
Keil, S. et al. Long-term follow-up and outcome of phenylketonuria patients on sapropterin: a retrospective study. Pediatrics 131, e1881–e1888 (2013).
Muntau, A. C. et al. Efficacy, safety and population pharmacokinetics of sapropterin in PKU patients <4 years: results from the SPARK open-label, multicentre, randomized phase IIIb trial. Orphanet J. Rare Dis. 12, 47 (2017).
Burton, B. K. et al. Pegvaliase for the treatment of phenylketonuria: results of the phase 2 dose-finding studies with long-term follow-up. Mol. Genet. Metab. 130, 239–246 (2020).
Levy, H. L., Sarkissian, C. N. & Scriver, C. R. Phenylalanine ammonia lyase (PAL): from discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metab. 124, 223–229 (2018).
Sarkissian, C. N. et al. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc. Natl Acad. Sci. USA 96, 2339–2344 (1999).
Gámez, A. et al. Development of pegylated forms of recombinant Rhodosporidium toruloides phenylalanine ammonia-lyase for the treatment of classical phenylketonuria. Mol. Ther. 11, 986–989 (2005).
Gupta, S. et al. Association of immune response with efficacy and safety outcomes in adults with phenylketonuria administered pegvaliase in phase 3 clinical trials. EBioMedicine 37, 366–373 (2018).
Longo, N. et al. Evidence- and consensus-based recommendations for the use of pegvaliase in adults with phenylketonuria. Genet. Med. 21, 1851–1867 (2019).
Chang, T. M., Bourget, L. & Lister, C. A new theory of enterorecirculation of amino acids and its use for depleting unwanted amino acids using oral enzyme-artificial cells, as in removing phenylalanine in phenylketonuria. Artif. Cell Blood Substit. Immobil. Biotechnol. 23, 1–21 (1995).
Fisch, R. O., Jenness, R., Doeden, D. & Anderson, J. A. The effect of excess L-phenylalamine on mothers and on their breast-fed infants. J. Pediatr. 71, 176–180 (1967).
Oseid, B. Breast-feeding and infant health. Semin. Perinatol. 3, 249–254 (1979).
McCabe, E. R. & McCabe, L. Issues in the dietary management of phenylketonuria: breast-feeding and trace-metal nutriture. Ann. N. Y. Acad. Sci. 477, 215–222 (1986).
Rocha, J. C. & MacDonald, A. Dietary intervention in the management of phenylketonuria: current perspectives. Pediatr. Health Med. Ther. 7, 155–163 (2016).
van Rijn, M. et al. A different approach to breast-feeding of the infant with phenylketonuria. Eur. J. Pediatr. 162, 323–326 (2003).
Weinmann, A. et al. Tetrahydrobiopterin is present in high quantity in human milk and has a vasorelaxing effect on newborn rat mesenteric arteries. Pediatr. Res. 69, 325–329 (2011).
Bekhof, J. et al. Influence of knowledge of the disease on metabolic control in phenylketonuria. Eur. J. Pediatr. 162, 440–442 (2003).
Ozel, H. G. et al. Does maternal knowledge impact blood phenylalanine concentration in Turkish children with phenylketonuria? J. Inherit. Metab. Dis. 31 (Suppl 2), 213–217 (2008).
Macdonald, A. et al. Does maternal knowledge and parent education affect blood phenylalanine control in phenylketonuria? J. Hum. Nutr. Diet. 21, 351–358 (2008).
Durham-Shearer, S. J., Judd, P. A., Whelan, K. & Thomas, J. E. Knowledge, compliance and serum phenylalanine concentrations in adolescents and adults with phenylketonuria and the effect of a patient-focused educational resource. J. Hum. Nutr. Diet. 21, 474–485 (2008).
Crone, M. R. et al. Behavioural factors related to metabolic control in patients with phenylketonuria. J. Inherit. Metab. Dis. 28, 627–637 (2005).
Bilginsoy, C., Waitzman, N., Leonard, C. O. & Ernst, S. L. Living with phenylketonuria: perspectives of patients and their families. J. Inherit. Metab. Dis. 28, 639–649 (2005).
Rudolf, I. et al. Assessment of a mobile app by adolescents and young adults with cystic fibrosis: pilot evaluation. JMIR Mhealth Uhealth 7, e12442 (2019).
Jahja, R., Huijbregts, S. C. J., de Sonneville, L. M. J., van der Meere, J. J. & van Spronsen, F. J. Neurocognitive evidence for revision of treatment targets and guidelines for phenylketonuria. J. Pediatr. 164, 895–899.e2 (2014).
Feldmann, R. et al. Neurocognitive functioning in adults with phenylketonuria: report of a 10-year follow-up. Mol. Genet. Metab. 126, 246–249 (2019).
Leuzzi, V., Chiarotti, F., Nardecchia, F., van Vliet, D. & van Spronsen, F. J. Predictability and inconsistencies of cognitive outcome in patients with phenylketonuria and personalised therapy: the challenge for the future guidelines. J. Med. Genet. 57, 145–150 (2020).
van Vliet, D. et al. Untreated PKU patients without intellectual disability: what do they teach us? Nutrients 11, 2572 (2019).
Burlina, A. P. et al. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: a systematic review. J. Inherit. Metab. Dis. 42, 209–219 (2019).
Klimek, A. et al. Everyday life, dietary practices, and health conditions of adult PKU patients: a multicenter, cross-sectional study. Ann. Nutr. Metab. 76, 251–258 (2020).
Burgard, P. et al. Issues with European guidelines for phenylketonuria. Lancet Diabetes Endocrinol. 5, 681–683 (2017).
van Spronsen, F. J. et al. Issues with European guidelines for phenylketonuria – Authors’ reply. Lancet Diabetes Endocrinol. 5, 683–684 (2017).
De Felice, S., Romani, C., Geberhiwot, T., MacDonald, A. & Palermo, L. Language processing and executive functions in early treated adults with phenylketonuria (PKU). Cogn. Neuropsychol. 35, 148–170 (2018).
Palermo, L. et al. Cognitive outcomes in early-treated adults with phenylketonuria (PKU): A comprehensive picture across domains. Neuropsychology 31, 255–267 (2017).
Palermo, L. et al. Emotional health in early-treated adults with phenylketonuria (PKU): Relationship with cognitive abilities and blood phenylalanine. J. Clin. Exp. Neuropsychol. 42, 142–159 (2020).
Romani, C., MacDonald, A., De Felice, S. & Palermo, L. Speed of processing and executive functions in adults with phenylketonuria: quick in finding the word, but not the ladybird. Cogn. Neuropsychol. 35, 171–198 (2018).
Romani, C. et al. Adult cognitive outcomes in phenylketonuria: explaining causes of variability beyond average Phe levels. Orphanet J. Rare Dis. 14, 273 (2019).
Romani, C. et al. Cognitive outcomes and relationships with phenylalanine in phenylketonuria: a comparison between Italian and English adult samples. Nutrients 12, 3033 (2020).
Romani, C. et al. The impact of phenylalanine levels on cognitive outcomes in adults with phenylketonuria: effects across tasks and developmental stages. Neuropsychology 31, 242–254 (2017).
Manti, F. et al. Psychiatric disorders in adolescent and young adult patients with phenylketonuria. Mol. Genet. Metab. 117, 12–18 (2016).
Nardecchia, F., Manti, F., De Leo, S., Carducci, C. & Leuzzi, V. Clinical characterization of tremor in patients with phenylketonuria. Mol. Genet. Metab. 128, 53–56 (2019).
Nardecchia, F. et al. Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: a longitudinal study. Mol. Genet. Metab. 115, 84–90 (2015).
Scala, I. et al. Large neutral amino acids (LNAAs) supplementation improves neuropsychological performances in adult patients with phenylketonuria. Nutrients 12, 1092 (2020).
Weglage, J. et al. Neurocognitive functioning in adults with phenylketonuria: results of a long term study. Mol. Genet. Metab. 110 (Suppl), 44–48 (2013).
Bartus, A. et al. The influence of blood phenylalanine levels on neurocognitive function in adult PKU patients. Metab. Brain Dis. 33, 1609–1615 (2018).
Christ, S. E. et al. Executive function in phenylketonuria (PKU): insights from the Behavior Rating Inventory of Executive Function (BRIEF) and a large sample of individuals with PKU. Neuropsychology 34, 456–466 (2020).
Hellewell, S. C., Welton, T., Eisenhuth, K., Tchan, M. C. & Grieve, S. M. Diffusion kurtosis imaging detects subclinical white matter abnormalities in phenylketonuria. Neuroimage Clin. 29, 102555 (2021).
Jahja, R. et al. Cognitive profile and mental health in adult phenylketonuria: a PKU-COBESO study. Neuropsychology 31, 437–447 (2017).
Sundermann, B. et al. Approaching altered inhibitory control in phenylketonuria: a functional MRI study with a Go-NoGo task in young female adults. Eur. J. Neurosci. 52, 3951–3962 (2020).
Bessman, S. P. PKU–some skepticism. N. Engl. J. Med. 278, 1176–1177 (1968).
Bessman, S. P. Historical perspective: tyrosine and maternal phenylketonuria, welcome news. Am. J. Clin. Nutr. 67, 357–358 (1998).
Bessman, S. P. The justification theory: the essential nature of the non-essential amino acids. Nutr. Rev. 37, 209–220 (1979).
Cabalska, B. et al. Termination of dietary treatment in phenylketonuria. Eur. J. Pediatr. 126, 253–262 (1977).
Poustie, V. J. & Wildgoose, J. Dietary interventions for phenylketonuria. Cochrane Database Syst. Rev. 1, CD001304 (2010).
Waisbren, S. E., Schnell, R. R. & Levy, H. L. Diet termination in children with phenylketonuria: a review of psychological assessments used to determine outcome. J. Inherit. Metab. Dis. 3, 149–153 (1980).
Diamond, A. Phenylalanine levels of 6-10mg/dl may not be as benign as once thought. Acta Paediatr. 407, 89–91 (1994).
Waisbren, S. E. et al. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol. Genet. Metab. 92, 63–70 (2007).
Lammardo, A. M. et al. Main issues in micronutrient supplementation in phenylketonuria. Mol. Genet. Metab. 110 (Suppl), 1–5 (2013).
Mabry, C. C., Denniston, J. C., Nelson, T. L. & Son, C. D. Maternal phenylketonuria. A cause of mental retardation in children without the metabolic defect. N. Engl. J. Med. 269, 1404–1408 (1963).
Lenke, R. R. & Levy, H. L. Maternal phenylketonuria and hyperphenylalaninemia. An international survey of the outcome of untreated and treated pregnancies. N. Engl. J. Med. 303, 1202–1208 (1980).
Drogari, E., Smith, I., Beasley, M. & Lloyd, J. K. Timing of strict diet in relation to fetal damage in maternal phenylketonuria. An international collaborative study by the MRC/DHSS Phenylketonuria Register. Lancet 2, 927–930 (1987).
Maillot, F., Lilburn, M., Baudin, J., Morley, D. W. & Lee, P. J. Factors influencing outcomes in the offspring of mothers with phenylketonuria during pregnancy: the importance of variation in maternal blood phenylalanine. Am. J. Clin. Nutr. 88, 700–705 (2008).
Koch, R. et al. The maternal phenylketonuria international study: 1984–2002. Pediatrics 112, 1523–1529 (2003).
Grange, D. K. et al. Sapropterin dihydrochloride use in pregnant women with phenylketonuria: an interim report of the PKU MOMS sub-registry. Mol. Genet. Metab. 112, 9–16 (2014).
Feillet, F. et al. Use of sapropterin dihydrochloride in maternal phenylketonuria. A European experience of eight cases. J. Inherit. Metab. Dis. 37, 753–762 (2014).
Teissier, R. et al. Maternal phenylketonuria: low phenylalaninemia might increase the risk of intra uterine growth retardation. J. Inherit. Metab. Dis. 35, 993–999 (2012).
Dhondt, J. L., Loeber, J., Elvers, L. H. & Paux, E. Preparation of the first European working standard for phenylalanine determination in dried blood spots. J. Med. Screen. 5, 63–66 (1998).
Gregory, C. O., Yu, C. & Singh, R. H. Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: comparison of methods. Genet. Med. 9, 761–765 (2007).
Groselj, U. et al. Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring–implications for clinical management of patients with hyperphenylalaninemia. Clin. Biochem. 48, 14–18 (2015).
Holub, M. et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin. Chim. Acta 373, 27–31 (2006).
Lawson, A. J., Bernstone, L. & Hall, S. K. Newborn screening blood spot analysis in the UK: influence of spot size, punch location and haematocrit. J. Med. Screen. 23, 7–16 (2016).
van Vliet, K. et al. Dried blood spot versus venous blood sampling for phenylalanine and tyrosine. Orphanet J. Rare Dis. 15, 82 (2020).
Koch, R. et al. Long-term beneficial effects of the phenylalanine-restricted diet in late-diagnosed individuals with phenylketonuria. Mol. Genet. Metab. 67, 148–155 (1999).
Singh, R. H. et al. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet. Med. 16, 121–131 (2014).
Vockley, J. et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 16, 188–200 (2014).
Bosch, A. M. et al. The course of life and quality of life of early and continuously treated Dutch patients with phenylketonuria. J. Inherit. Metab. Dis. 30, 29–34 (2007).
Simon, E. et al. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU). Health Qual. Life Outcomes 6, 25 (2008).
Thimm, E., Schmidt, L. E., Heldt, K. & Spiekerkoetter, U. Health-related quality of life in children and adolescents with phenylketonuria: unimpaired HRQoL in patients but feared school failure in parents. J. Inherit. Metab. Dis. 36, 767–772 (2013).
Demirdas, S. et al. Evaluation of quality of life in PKU before and after introducing tetrahydrobiopterin (BH4); a prospective multi-center cohort study. Mol. Genet. Metab. 110 (Suppl), 49–56 (2013).
Cazzorla, C. et al. Quality of life (QoL) assessment in a cohort of patients with phenylketonuria. BMC Public. Health 14, 1243 (2014).
Bosch, A. M. et al. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet J. Rare Dis. 10, 80 (2015).
Feldmann, R., Wolfgart, E., Weglage, J. & Rutsch, F. Sapropterin treatment does not enhance the health-related quality of life of patients with phenylketonuria and their parents. Acta Paediatr. 106, 953–959 (2017).
Huijbregts, S. C. J. et al. The impact of metabolic control and tetrahydrobiopterin treatment on health related quality of life of patients with early-treated phenylketonuria: a PKU-COBESO study. Mol. Genet. Metab. 125, 96–103 (2018).
Cotugno, G. et al. Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr. 100, 1144–1149 (2011).
Vieira Neto, E. et al. Quality of life and adherence to treatment in early-treated Brazilian phenylketonuria pediatric patients. Braz. J. Med. Biol. Res. 51, e6709 (2017).
Bik-Multanowski, M. et al. Quality of life in noncompliant adults with phenylketonuria after resumption of the diet. J. Inherit. Metab. Dis. 31 (Suppl 2), 415–418 (2008).
ten Hoedt, A. E. et al. High phenylalanine levels directly affect mood and sustained attention in adults with phenylketonuria: a randomised, double-blind, placebo-controlled, crossover trial. J. Inherit. Metab. Dis. 34, 165–171 (2011).
Ziesch, B. et al. Tetrahydrobiopterin (BH4) in PKU: effect on dietary treatment, metabolic control, and quality of life. J. Inherit. Metab. Dis. 35, 983–992 (2012).
Harding, C. O. et al. Pegvaliase for the treatment of phenylketonuria: a pivotal, double-blind randomized discontinuation phase 3 clinical trial. Mol. Genet. Metab. 124, 20–26 (2018).
Regnault, A. et al. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: the phenylketonuria – quality of life (PKU-QOL) questionnaires. Orphanet J. Rare Dis. 10, 59 (2015).
Mapi: Merck-Serono. Phenylketonuria impact and treatment Quality Of Life Questionnaire (PKU-QOL). https://eprovide.mapi-trust.org/instruments/phenylketonuria-impact-and-treatment-quality-of-life-questionnaire
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24, 1519–1525 (2018).
Ahmed, S. S. et al. Sustained correction of a murine model of phenylketonuria following a single intravenous administration of AAVHSC15-PAH. Mol. Ther. Methods Clin. Dev. 17, 568–580 (2020).
Oh, H.-J., Park, E.-S., Kang, S., Jo, I. & Jung, S.-C. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr. Res. 56, 278–284 (2004).
Mochizuki, S. et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 11, 1081–1086 (2004).
Harding, C. O. et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 13, 457–462 (2006).
Ding, Z., Georgiev, P. & Thöny, B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 13, 587–593 (2006).
Yagi, H. et al. Complete restoration of phenylalanine oxidation in phenylketonuria mouse by a self-complementary adeno-associated virus vector. J. Gene Med. 13, 114–122 (2011).
Thöny, B., Ding, Z., Rebuffat, A. & Viecelli, H. M. Phenotypic reversion of fair hair upon gene therapy of the phenylketonuria mice. Hum. Gene Ther. 25, 573–574 (2014).
Rebuffat, A., Harding, C. O., Ding, Z. & Thöny, B. Comparison of adeno-associated virus pseudotype 1, 2, and 8 vectors administered by intramuscular injection in the treatment of murine phenylketonuria. Hum. Gene Ther. 21, 463–477 (2010).
Wagemaker, G. Lentiviral hematopoietic stem cell gene therapy in inherited metabolic disorders. Hum. Gene Ther. 25, 862–865 (2014).
An, D. et al. Systemic messenger RNA therapy as a treatment for methylmalonic acidemia. Cell Rep. 21, 3548–3558 (2017).
Truong, B. et al. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl Acad. Sci. USA 116, 21150–21159 (2019).
Cao, J. et al. mRNA therapy improves metabolic and behavioral abnormalities in a murine model of citrin deficiency. Mol. Ther. 27, 1242–1251 (2019).
Jiang, L. et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria. Nat. Med. 24, 1899–1909 (2018).
Zhu, X. et al. Systemic mRNA therapy for the treatment of Fabry disease: preclinical studies in wild-type mice, Fabry mouse model, and wild-type non-human primates. Am. J. Hum. Genet. 104, 625–637 (2019).
Balakrishnan, B. et al. Novel mRNA-based therapy reduces toxic galactose metabolites and overcomes galactose sensitivity in a mouse model of classic galactosemia. Mol. Ther. 28, 304–312 (2020).
Pascucci, T. et al. A new therapy prevents intellectual disability in mouse with phenylketonuria. Mol. Genet. Metab. 124, 39–49 (2018).
Rossi, L. et al. Erythrocyte-mediated delivery of phenylalanine ammonia lyase for the treatment of phenylketonuria in BTBR-Pah(enu2) mice. J. Control. Rel. 194, 37–44 (2014).
Smith, N., Longo, N., Levert, K., Hyland, K. & Blau, N. Exploratory study of the effect of one week of orally administered CNSA-001 (sepiapterin) on CNS levels of tetrahydrobiopterin, dihydrobiopterin and monoamine neurotransmitter metabolites in healthy volunteers. Mol. Genet. Metab. Rep. 21, 100500 (2019).
Smith, N., Longo, N., Levert, K., Hyland, K. & Blau, N. Phase I clinical evaluation of CNSA-001 (sepiapterin), a novel pharmacological treatment for phenylketonuria and tetrahydrobiopterin deficiencies, in healthy volunteers. Mol. Genet. Metab. 126, 406–412 (2019).
Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 36, 857–864 (2018).
Vockley, J., Sacharow, S., Searle, S., Kurtz, C. & Querbes, W. A phase 1/2a oral placebo-controlled study of SYNB1618 in healthy adult volunteers and subjects with phenylketonuria [abstract O-027]. J. Inherit. Metab. Dis. 42 (Suppl. 1), 13 (2019).
Lane, J. D., Schöne, B., Langenbeck, U. & Neuhoff, V. Characterization of experimental phenylketonuria augmentation of hyperphenylalaninemia with α-methylphenylalanine and p-chlorophenylalanine. Biochim. Biophys. Acta 627, 144–156 (1980).
Haefele, M. J., White, G. & McDonald, J. D. Characterization of the mouse phenylalanine hydroxylase mutation Pah(enu3). Mol. Genet. Metab. 72, 27–30 (2001).
Richards, D. Y. et al. A novel Pah-exon1 deleted murine model of phenylalanine hydroxylase (PAH) deficiency. Mol. Genet. Metab. 131, 306–315 (2020).
Koppes, E. A. et al. A porcine model of phenylketonuria generated by CRISPR/Cas9 genome editing. JCI Insight 5, e141523 (2020).
Kaiser, R. A. et al. Development of a porcine model of phenylketonuria with a humanized R408W mutation for gene editing. PLoS ONE 16, e0245831 (2021).
Douglas, T. D., Ramakrishnan, U., Kable, J. A. & Singh, R. H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Health Qual. Life Outcomes 11, 218 (2013).
Acknowledgements
The authors thank D. Abeln for giving his thoughts from the perspective of a parent.
Author information
Authors and Affiliations
Contributions
Introduction (F.J.v.S., N.B., C.H., A.B., N.L. and A.M.B.); Epidemiology (N.B.); Mechanisms/pathophysiology (C.H. and F.J.v.S.); Diagnosis, screening and prevention (N.B. and A.B.); Management (F.J.v.S.); Quality of life (F.J.v.S. and A.M.B.); Outlook (F.J.v.S. and N.L.).
Corresponding author
Ethics declarations
Competing interests
F.J.v.S. has been a member of scientific advisory boards for defects in amino acid metabolism of APR, Agios, Arla Food International, BioMarin, Eurocept Int, Lucana, Moderna TX, Nutricia, Rivium, Homoly and Nestlé-Codexis; his institute has received research grants from Alexion, Biomarin, Codexis, Nutricia, SoBi and Vitaflo; his institute has received grants from patient organizations ESPKU, Metakids, NPKUA, Stofwisselkracht, Stichting PKU research and the Tyrosinemia Foundation; and his institute has received honoraria as consultant and speaker from APR, Pluvia, Biomarin, MendeliKABS and Nutricia. N.B. has received honoraria and/or consulting fees from BioMarin, Pharmaceuticals, Censa, Nestlé Pharmaceuticals and Homology Medicines. C.H. has received consulting fees, speaker fees, and travel and research support from BioMarin, Cydan Development Inc., Dimension Therapeutics, Horizon Pharma, Pfizer, Rubius Therapeutics, StrideBio and Synlogic. A.B. has received advisory board honoraria, speaker fees and travel support from Biomarin Pharmaceuticals, Nutricia, Cambrooke, PIAM, APR, Sanofi Genzyme and Takeda. N.L. has received consulting fees from Aeglea, BioMarin, Censa Pharmaceuticals, Dimension Therapeutics, Genzyme/Sanofi, Hemoshear, Horizon, Lumos Pharma, Moderna, Mitobridge, Pfizer, Retrophin and Stealth Therapeutics, and has conducted contracted research for Aeglea, BioMarin, Genzyme/Sanofi, Horizon, Lumos Pharma, Protalix, Retrophin, Shire, Stealth Therapeutics and Ultragenyx. A.M.B. has received a speaker fee from Nutricia and has been a member of advisory boards for Biomarin.
Additional information
Peer review information
Nature Reviews Disease Primers thanks T. Coskun, L. Desviat, H. Levy, A. MacDonald and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NIH toolbox: https://www.healthmeasures.net/explore-measurement-systems/nih-toolbox/intro-to-nih-toolbox
Rights and permissions
About this article
Cite this article
van Spronsen, F.J., Blau, N., Harding, C. et al. Phenylketonuria. Nat Rev Dis Primers 7, 36 (2021). https://doi.org/10.1038/s41572-021-00267-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00267-0
This article is cited by
-
Phenylketonuria from the perspectives of patients in Türkiye
Orphanet Journal of Rare Diseases (2024)
-
mRNA biotherapeutics landscape for rare genetic disorders
Journal of Biosciences (2024)
-
Health-related quality of life in a european sample of adults with early-treated classical PKU
Orphanet Journal of Rare Diseases (2023)
-
Allelic phenotype prediction of phenylketonuria based on the machine learning method
Human Genomics (2023)
-
The self-assembly of l-histidine might be the cause of histidinemia
Scientific Reports (2023)