Abstract
Acute heart failure (AHF) is a syndrome defined as the new onset (de novo heart failure (HF)) or worsening (acutely decompensated heart failure (ADHF)) of symptoms and signs of HF, mostly related to systemic congestion. In the presence of an underlying structural or functional cardiac dysfunction (whether chronic in ADHF or undiagnosed in de novo HF), one or more precipitating factors can induce AHF, although sometimes de novo HF can result directly from the onset of a new cardiac dysfunction, most frequently an acute coronary syndrome. Despite leading to similar clinical presentations, the underlying cardiac disease and precipitating factors may vary greatly and, therefore, the pathophysiology of AHF is highly heterogeneous. Left ventricular diastolic or systolic dysfunction results in increased preload and afterload, which in turn lead to pulmonary congestion. Fluid retention and redistribution result in systemic congestion, eventually causing organ dysfunction due to hypoperfusion. Current treatment of AHF is mostly symptomatic, centred on decongestive drugs, at best tailored according to the initial haemodynamic status with little regard to the underlying pathophysiological particularities. As a consequence, AHF is still associated with high mortality and hospital readmission rates. There is an unmet need for increased individualization of in-hospital management, including treatments targeting the causative factors, and continuation of treatment after hospital discharge to improve long-term outcomes.
Similar content being viewed by others
Introduction
Heart failure (HF) is a chronic and progressive clinical syndrome induced by structural or functional cardiac abnormalities displaying either reduced (in HF with reduced ejection fraction (HFrEF)) or preserved (in HF with preserved ejection fraction (HFpEF)) left ventricular ejection fraction (LVEF)1. Cardiac dysfunction leads to elevated cardiac filling pressures at rest and during stress1. HF symptoms include dyspnoea (shortness of breath) and fatigue, often accompanied by typical physical signs, such as pulmonary rales (abnormal crackling sounds), peripheral oedema or distended jugular veins1. The substantial reduction in short-term mortality in patients with several cardiac conditions (particularly acute coronary syndromes and congenital heart disease) and the relevant improvement in long-term survival in patients with HFrEF (as a result of widespread use of effective disease-modifying oral therapies and devices), combined with several demographic changes, such as extended life expectancy, have sharply increased the number of patients living with HF2. In developed countries, HF has become a substantial public health problem, affecting 2% of the adult population, and the number of hospital admissions related to HF has tripled since the 1990s2.
Acute HF (AHF) is defined as new or worsening of symptoms and signs of HF and is the most frequent cause of unplanned hospital admission in patients of >65 years of age3. From a clinical perspective, we distinguish de novo HF — in which symptoms occur in patients without a previous history of HF — from acutely decompensated HF (ADHF) — in which symptoms increase in patients with previously diagnosed chronic HF. This classification provides little additional information in regard to the pathophysiology of AHF but has mainly clinical implications (de novo HF requires a more extensive diagnostic process to investigate the underlying cardiac pathology than ADHF). As HF is a chronic and progressive disease, the majority of hospitalizations are related to ADHF rather than de novo AHF4,5. The clinical presentation of AHF is characterized mostly by symptoms and signs related to systemic congestion (that is, extracellular fluid accumulation, initiated by increased biventricular cardiac filling pressures)6,7. Accordingly, the initial treatment in most patients with AHF consists of non-invasive ventilation and intravenous diuretics, which are administered alone or, especially in Europe and Asia, in combination with short-acting vasodilators8. Only a minority of patients with AHF present with cardiogenic shock, a critical condition characterized by the presence of clinical signs of peripheral tissue hypoperfusion; cardiogenic shock has a tenfold higher in-hospital mortality than AHF without shock and requires specific treatments9,10.
In contrast to the substantial improvements in the treatment of chronic HFrEF, AHF is still associated with poor outcomes, with 90-day readmission rates and 1-year mortality reaching 10–30%11,12. Although AHF is not a specific disease but the shared clinical presentation of different, heterogeneous cardiac abnormalities, most patients still receive decongestive drugs only, at best tailored according to the initial haemodynamic status with little regard to the underlying pathophysiological particularities. This approach might have contributed to the multitude of neutral or negative clinical trials assessing the effect of decongestive treatments on survival and to the persistence of poor outcomes in AHF. Thus, there is an unmet need for increased individualization and continuation of treatment after hospital discharge to improve long-term outcomes. This Primer reviews current concepts of epidemiology, pathophysiology, diagnosis and management of AHF to stimulate advances in research and clinical practice to improve patient outcomes. As cardiogenic shock is a separate entity with specific features, it is not discussed in this Primer.
Epidemiology
Prevalence
There are several reasons why global data on AHF are very limited. Differential coding of the syndrome, coupled with nuanced differences in case definitions, defies simple regional comparison. The International Classification of Disease (ICD) system classifies AHF and chronic HF as intermediate conditions and not underlying causes of death. The ICD also does not distinguish between de novo HF and ADHF as reasons for hospital admission. No global data on the proportion of HFrEF and HFpEF as underlying causes of AHF are available. The Global Burden of Disease (GBD) collaborators reported on global, regional and national age-specific and sex-specific mortality of 282 causes of death in 195 countries for the period 1980–2017, including cardiovascular diseases such as rheumatic heart disease, ischaemic heart disease and cardiomyopathy, but they did not list AHF13. The latest estimate by the GBD team in 2010 was 37.7 million cases of prevalent HF worldwide, leading to an average of 4.2 years lived with this disability for each patient, but data on the global incidence of AHF were not reported14. Data on annual hospitalizations for HF are only available for the USA and Europe and exceed 1 million in both regions4,5. Among these hospitalizations, >90% were due to symptoms and signs of fluid accumulation (indicating AHF). In addition, up to one in four patients (24%) are readmitted within 30 days, readmission rates in the first 3 months after hospitalization for AHF may reach 30% in the USA and in other countries4 and one in two patients (50%) are readmitted within 6 months4,5. Recurrent fluid accumulation in patients with HF has uniformly been associated with worse outcomes independent of age and renal function15. In multiple studies of the 30-day to 90-day post-discharge period, ~25–30% of patients with AHF are readmitted during this time frame16,17,18,19,20. However, a substantial proportion of these patients are readmitted for a non-HF-related cause21,22. Medical comorbidities precipitate rehospitalization and, when poorly managed, contribute to worsening HF over time22. Psychosocial factors such as anxiety, depression, cognitive impairment and social isolation also confer increased risk of unplanned recurrent readmission or death of patients following hospitalization for AHF23.
There are no national data on the prevalence of AHF or chronic HF in low-income and middle-income countries. All registries of HF for these regions are based on hospital registries that included only patients admitted for AHF, without separating de novo HF from ADHF. Data from some of the key registries have recently been summarized24 but focus on aetiology, risk factors, sociodemographic profile and mortality. The INTER-CHF study, one of the largest registries, reported on 5,823 patients with HF from 108 centres in six geographical regions25. The overall 1-year mortality was 16.5%, with the highest mortality in Africa (34%) and India (23%), about average mortality in southeast Asia (15%) and the lowest mortality in China (7%), South America (9%) and the Middle East (9%)25.
Risk factors
A systematic review of worldwide risk factors for HF found that ischaemic heart disease was the major underlying contributor to AHF admissions in >50% of patients in high-income regions, as well as eastern and central European regions26. In Asia Pacific high-income regions and Latin America, ischaemic heart disease contributed to 30–40% of admissions26, whereas in sub-Saharan Africa it contributed to <10%27. Hypertension was a consistent contributor to HF globally (17%)26. Of the other commonly reported risk factors, rheumatic heart disease was particularly prevalent in East Asia (34%) and sub-Saharan Africa (14%)26. The heterogeneous group of cardiomyopathies (which can include familial, peripartum, infective (for example, due to HIV infection), autoimmune, post-myocarditis and idiopathic cardiomyopathy, amongst others) were particularly prevalent in Africa (25.7%), with Chagas disease-associated cardiomyopathy being a specific cause in Latin America26. Chagas disease-associated acute myocarditis is commonly (>50% cases) associated with a substantial pericardial effusion, but it usually leads to AHF in only 1–5 of every 10,000 infected people28. However, Chagas disease remains common in Latin America and is the cause of HF in 10% of patients in the RAMADHF study and 28% in the GESICA study29,30.
In high-income regions with associated high scores in the human development index (a statistical tool that takes into account life expectancy, education and income), patients with AHF typically have a median age of >75 years at presentation, whereas in other areas, such as Latin America and sub-Saharan Africa, the median age of patients with AHF is up to two decades lower25. This difference could be due to poorly treated hypertension, ischaemic heart disease and late diagnosed rheumatic heart disease leading to HF presentation in younger age groups. In addition, there are differences between regions in the sex distribution; for example, rheumatic heart disease commonly affects women more than men31,32, and peripartum cardiomyopathy is particularly common in Africa33. As the obesity epidemic also affects women disproportionately, hypertensive heart disease leading to HF is commonly more prevalent in women than men25.
Morbidity and mortality
Globally, in-hospital AHF mortality hovers at ~4%, rises to ~10% within 60 to 90 days after discharge and increases further to 25–30% at 1 year16,17,18,34,35. The INTER-CHF prospective cohort study showed striking global variations in HF-associated mortality, with the highest 1-year overall and HF-related mortality in the countries with the youngest populations, such as India and African countries25. However, there was no analysis of HFpEF versus HFrEF as the underlying condition in the HF group.
Data from the THESUS-HF registry (a prospective study of AHF in nine sub-Saharan countries) were analysed to determine the predictors of readmission and outcome (including death) after an AHF event35. Similar to results in high-income countries, the predictors of 180-day mortality included malignancy, severe lung disease, smoking history, systolic blood pressure and heart rate either below or above their physiological ranges and symptoms and signs of congestion (orthopnoea (dyspnoea when lying flat), peripheral oedema and rales) at admission, kidney dysfunction, anaemia and HIV positivity. The risks predicted by calibration plots, comparing observed event rates with those predicted by the models, were generally low for all risk factors considered, suggesting that the main factors contributing to adverse outcomes in patients with AHF are still largely unknown35.
Mechanisms/pathophysiology
Pathophysiological mechanisms of AHF
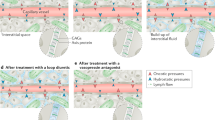
An underlying structural or functional cardiac condition is a prerequisite for AHF and includes a multitude of different acute (for example, myocardial infarction) or chronic (for example, dilated cardiomyopathy and ischaemic heart disease) cardiac pathologies. The underlying cardiac disease leads to the activation of several pathophysiological pathways (at first adaptive responses, which with time become maladaptive) that counter the negative effects of HF on oxygen delivery to the peripheral tissues, but such pathways can also eventually cause systemic congestion, ventricular remodelling and organ dysfunction36. Furthermore, some acute diseases can act as precipitating factors and trigger AHF either by directly impairing cardiac diastolic and/or systolic function or by further promoting systemic congestion36. Systemic congestion has a major effect on the clinical presentation in the majority of patients with AHF and is a relevant determinant of multi-organ dysfunction occurring in AHF (Fig. 1). The pathophysiology of AHF is heterogeneous, as it is greatly affected by the nature of the underlying cardiac disease. It is perhaps not surprising, therefore, that the responses to treatment may vary and that different patients may respond best to distinct treatment strategies that depend on the underlying pathophysiology.
Acute heart failure (HF) results from the combination of an underlying but newly diagnosed cardiac dysfunction and precipitating factors or the onset of a new cardiac dysfunction (de novo HF) or the combination of an underlying chronic cardiac dysfunction and one or more precipitating factors (acutely decompensated HF (ADHF), that is, decompensation of chronic HF). Precipitating factors may directly affect left ventricular (LV) or right ventricular (RV) function (for example, myocardial ischaemia and arrhythmias) or may contribute to the development of congestion (for example, infection, hypertension and non-compliance with treatment recommendations). LV dysfunction (diastolic dysfunction in HF with preserved ejection fraction (HFpEF) or diastolic and systolic dysfunction in HF with reduced ejection fraction (HFrEF)) leads to pulmonary congestion, which in turn contributes to RV dysfunction and systemic congestion. Systemic congestion, neurohumoral activation and inflammation negatively affect ventricular function and further contribute to self-perpetuating congestion.
LV systolic and diastolic dysfunction
An acute change in cardiac function, mostly a worsening of left ventricular (LV) diastolic function, which in turn leads to an increase in LV filling pressures and pulmonary congestion, can result in AHF37; an example of such sudden changes is acute myocardial ischaemia. Several pathophysiological mechanisms underlie the link between ischaemia, LV systolic and diastolic dysfunction and pulmonary congestion. LV contraction is highly dependent on oxidative energy generation and, therefore, ischaemia triggers systolic impairment, which leads to an increased residual LV end-diastolic volume and filling pressure. LV filling normally occurs in two phases, an early rapid phase that is highly dependent upon fast myocardial relaxation and a later phase that is dependent on left atrial contraction and the atrial-to-ventricular pressure gradient, which in turn is affected by the physical properties of the LV (for example, stiffness). Myocardial relaxation is also an active energy-requiring process that involves removing cytoplasmic calcium, mostly via re-uptake into the sarcoplasmic reticulum by the sarcoplasmic reticulum Ca2+ ATPase (SERCA) pump and in part via extrusion across the cardiomyocyte plasma membrane. The end-diastolic properties of the LV are affected by the residual LV end-diastolic volume, structural changes (for example, fibrosis) and also by extremely delayed relaxation. The reduction in oxidative ATP generation in cardiomyocytes with the onset of severe acute ischaemia rapidly impairs myocardial relaxation, thereby affecting early LV filling and further increasing filling pressures. The presence of any coexisting conditions in which relaxation is already impaired or end-diastolic LV stiffness is increased will increase the likelihood of AHF. Conditions in which end-diastolic LV stiffness may be increased (and, therefore, also conditions with an increased risk of AHF precipitated by ischaemia) include chronic LV systolic dysfunction with raised LV end-diastolic volume and structural fibrosis and/or hypertrophy, both of which could result from diabetes mellitus, chronic hypertension, chronic kidney disease, chronic aortic stenosis and ageing38. LV filling may also be impaired by the sudden development of atrial fibrillation with the accompanying loss of atrial contraction, which may substantially increase filling pressures when there is already pre-existing diastolic dysfunction. For example, severe mitral stenosis (which is a common manifestation of rheumatic heart disease) is a type of diastolic dysfunction due to the valve abnormality rather than LV structural disease, and it can also induce atrial fibrillation, thereby increasing the risk of triggering AHF.
Fluid retention
In HF, an increase in the volume of extracellular fluid (referred to as fluid retention or fluid accumulation) and/or a change in the compliance of venous beds (which results in fluid redistribution without an increase in the overall volume) can lead to an increase in filling pressures. In fact, in the majority of patients, AHF occurs without acute changes in cardiac function but is induced by fluid accumulation and/or redistribution, which results in systemic congestion, especially in the presence of an underlying diastolic dysfunction39. The interactions between intravascular and interstitial fluid volumes are complex, and there is no linear correlation between central haemodynamics and volume changes40. Animal studies have shown that marked intravascular volume expansion does not lead to increased cardiac filling pressures if sympathetic activity is low41,42, and in patients with HF intravascular volume is only marginally reduced after diuretic therapy despite large reductions in body weight40. By contrast, only half of the patients exhibit a weight gain of >0.9 kg over the month preceding hospital presentation for ADHF, indicating that changes in the compliance state of the venous beds are also important drivers of congestion43. The majority of the retained sodium is stored in the extracellular compartment, which consists of both the intravascular compartment and the interstitium44. In healthy individuals, increased total body sodium is usually not accompanied by oedema formation, as a large quantity of sodium may be buffered by the interstitial glycosaminoglycan networks without compensatory water retention45. Moreover, the interstitial glycosaminoglycan networks display low compliance (limited elastic properties), which prevents fluid accumulation in the interstitium46. In patients with HF, when sodium accumulation persists, the glycosaminoglycan networks may become dysfunctional, resulting in reduced buffering capacity, increased interstitial compliance and oedema formation even in the presence of mildly elevated hydrostatic pressures44.
Fluid retention is frequently related to increased neurohumoral activation (that is, activation of the renin–angiotensin–aldosterone system and the vasopressin system) leading to renal salt and water retention, although it can also be iatrogenic (for example, caused by the administration of inappropriately large amounts of intravenous fluids). The neurohumoral pathway is already activated above the physiological baseline level early during disease progression in patients with chronic HF (even before the development of symptoms) or kidney disease, and, therefore, these patients are particularly prone to fluid accumulation. Mechanisms and consequences of neurohumoral activation have been extensively reviewed elsewhere47. Importantly, the resulting organ dysfunction contributes to self-perpetuation of congestion.
In HF, alterations in both proximal and distal nephron segments increase kidney sodium avidity48, which is already increased even before clinical symptoms of HF occur49,50. Furthermore, in several studies increased central venous pressure has been associated with worsening of renal function (WRF), often resulting in a further drop in natriuresis51,52,53. However, changes in renal function during AHF need to be interpreted within the specific clinical context, as this approach helps to correctly assess risk and determine further treatment strategies. In fact, it is possible that changes in renal function parameters occurring during AHF that would typically indicate WRF do not correspond to ‘true’ WRF, when accompanied by simultaneous favourable ongoing diuresis and improvement in HF status. Currently, misinterpretation of WRF in the AHF setting is a leading cause of decongestion not being achieved in AHF. To distinguish between ‘true’ WRF and ‘pseudo’ WRF during AHF, renal evaluation should include the assessment not only of changes in glomerular function (indicating the development of WRF), but also of the tubular response to diuretic therapy (diuretic response and/or efficiency), that is, the ability to eliminate residual congestion and the administered therapy.
Fluid redistribution
Sympathetic stimulation can induce a transient vasoconstriction leading to a sudden displacement of volume from the splanchnic and peripheral venous system to the pulmonary circulation, without exogenous fluid retention — that is, fluid redistribution54. Large veins physiologically contain one-quarter of the total blood volume and stabilize cardiac preload, buffering fluid retention55. Preload indicates the degree of stretch of cardiomyocytes at the end of diastole and correlates with the end-diastolic volume and pressure. By contrast, afterload indicates the pressure that the heart has to overcome to eject blood during ventricular contraction and correlates with systolic arterial pressure. A mismatch in the ventricular–vascular coupling relationship with increased afterload and decreased venous capacitance (leading to increased preload and end-diastolic volume) may excessively increase cardiac workload and exacerbate pulmonary and systemic congestion56. Finally, acute mechanical factors may also increase ventricular preload and cause AHF; for example, the sudden occurrence of mitral valve regurgitation due to ruptured papillary muscle chords or the sudden development of a ventricular septal defect.
Fluid accumulation and fluid redistribution both produce systemic congestion in AHF, but their relative contributions probably vary according to different clinical scenarios, and the decongestive therapy should be tailored accordingly (see Management)36.
Precipitating factors of AHF
The onset and increase in systemic congestion that precede AHF may develop over hours up to days, and can be triggered by several factors, either directly through stimulation of pathophysiological mechanisms leading to fluid accumulation or redistribution or indirectly through a worsening of cardiac diastolic or systolic function. The understanding of the pathophysiology involved in the development of AHF is important for providing the appropriate treatment. Although in many patients a progressive increase in body weight and pulmonary pressures may be observed as early as several days before hospital admission, in a relevant proportion of patients AHF is associated with only a minimal increase in body weight39,43. Several registries, including the North American OPTIMIZE-HF registry and the Euro-Asian registry of the GREAT network, have investigated the presence of precipitants in patients with AHF57,58. Acute coronary syndromes, arrhythmias (in particular atrial fibrillation), infections (in particular airway infections), uncontrolled hypertension and non-compliance with dietary recommendations and drug prescriptions are the most common identified precipitants57,58. Of note, in a relevant proportion of patients (~40–50%), no precipitants could be identified, whereas a combination of multiple factors were present in ~5–20% of patients57,58.
The identification of precipitants provides prognostic information, as highlighted by several studies showing an association between precipitating factors and both mortality and readmission rates57,58,59,60. AHF precipitated by acute coronary syndromes or infection is associated with higher short-term mortality than AHF precipitated by atrial fibrillation or uncontrolled hypertension57,58. Notably, although patients with AHF precipitated by acute coronary syndromes and those with AHF precipitated by infection have similar unfavourable prognoses, the risk of death changes with time differently in the two patient groups: it is the highest during the first days after admission in the first group and peaks ~3 weeks after admission in the second58,61. The explanation for this phenomenon is speculative; we might suggest a complex interaction between infection and a combination of endothelial dysfunction, atherosclerotic plaque instability, activated coagulation, fluid retention, inflammatory and ischaemic myocardial injury, arrhythmias and the risk of other precipitating non-cardiac illnesses that may lead to death58. Finally, and most importantly, the identification of precipitating factors enables the delivery of specific treatments directed towards the underlying causes of AHF, in addition to decongestive therapy.
Congestion and organ dysfunction
In the heart, elevated ventricular filling pressures lead to increased ventricular wall tension, myocardial stretch and remodelling, contributing to a progressive worsening in cardiac contractility, valvular regurgitation and systemic congestion62. In response to the increased wall tension, circulating natriuretic peptides (which stimulate diuresis and vasodilation) are physiologically released by atrial and ventricular cardiomyocytes as a compensatory mechanism, and often high-sensitivity cardiac troponins are detectable in a large proportion of patients with AHF, revealing nonischaemic myocyte injury or necrosis63. Increases in left atrial pressure and mitral valve regurgitation will increase the hydrostatic pressure in the pulmonary capillaries, thereby increasing fluid filtration rate from the capillaries to the pulmonary interstitium, causing lung stiffness and dyspnoea64. Notably, the relationship between hydrostatic pressure and interstitial fluid content is rather complex, as other mechanisms are involved in fluid homeostasis. For example, the lymphangiogenic factor VEGF-D has been found to regulate and mitigate pulmonary and systemic congestion in patients with HF or renal failure65,66,67. Indeed, in the early stage of lung congestion, the lymphatic system can cope with the large volume of interstitial fluid, but eventually, the drainage capacity is exceeded. Hence, fluid moves to pleural and intra-alveolar spaces causing pleural effusion and pulmonary oedema68.
Systemic congestion is a central feature in most patients with AHF6. In addition to poor cardiac function, numerous organs play a part in the development and propagation of congestion69. Congestion is the essential pathophysiological mechanism of impaired organ function in AHF, and hypoperfusion — if present — might cause further deterioration in organ function and is associated with increased mortality risk6. Improvement in organ function with decongestive therapies has been associated with a reduced risk of death, and, therefore, prevention and treatment of organ dysfunction is a key therapeutic target in patients with AHF.
AHF is associated with WRF. Elevated central venous pressure leads to renal venous hypertension, which in turn increases renal interstitial pressure. Ultimately, the hydrostatic pressure in the renal interstitium exceeds the intratubular hydrostatic pressure, resulting in the collapse of tubules and, therefore, reduced glomerular filtration rate70. In addition, renal venous hypertension induces a reduction in renal blood flow, renal hypoxia and ultimately interstitial fibrosis51,52,71. Other contributors to AHF-induced renal dysfunction include inflammatory processes, iatrogenic factors (for example, contrast media and nephrotoxic medications), impaired cardiac output and elevated intra-abdominal pressure7,72. Of note, an increase in plasma creatinine is often interpreted by clinicians as a sign of hypovolaemia, prompting a reduction in decongestive therapy, on the basis that excessive decongestion might result in renal tubular damage; however, this is not always the case, as discussed above (see Fluid retention)73,74. In patients with an increase in creatinine during decongestive therapy, it is recommended that decongestive therapy is pursued until euvolaemia is achieved75, as clinical outcomes are extremely poor if patients are discharged with ongoing congestion in the presence of WRF76. By contrast, relying exclusively on serial measurements of levels of biomarkers (such as circulating natriuretic peptides) to assess changes in volume might lead to inappropriate dose escalation of loop diuretics in patients without substantial residual congestion. This dose escalation may lead to adverse effects such as hypotension and/or further WRF. A multiparameter-based evaluation of congestion before discharge would be of benefit in patients with HF. In addition to biomarkers, clinical assessment at rest and during dynamic manoeuvres, supplemented with technical assessments (such as echocardiography or measurement of pulmonary pressures), is probably the best strategy, although it needs prospective evaluation75.
In patients with liver congestion, elevations in alkaline phosphatase, bilirubin and/or γ-glutamyl transferase (also known as glutathione hydrolase 1 proenzyme) are often observed77,78,79. Centrilobular necrosis and markedly elevated transaminases (alanine aminotransferase and aspartate aminotransferase) owing to hypoperfusion in the setting of hypoxic hepatitis are observed in severe hypoperfusion states such as cardiogenic shock78.
Splanchnic congestion results in increased intra-abdominal pressure and ischaemia of villi, which modify intestinal morphology, and alters intestinal permeability, nutrient absorption and the bacterial biolayer, possibly contributing to chronic inflammation and malnutrition80,81,82. Additionally, venous congestion and/or hypoperfusion impairs the splanchnic microcirculation and increases the risk of bowel ischaemia, enabling lipopolysaccharide or endotoxin produced by Gram-negative gut bacteria to enter the circulatory system and increase the pro-inflammatory environment of AHF56. Finally, congestion per se also results in endothelial activation, which further promotes a pro-inflammatory environment83,84.
Diagnosis, screening and prevention
The management of patients with HF is strikingly heterogeneous across the world according to sociocultural disparities and differences in health-care systems. Many cardiology societies have endeavoured to increase awareness of HF among the population in different countries and to educate health-care professionals to improve the management of patients with HF. The following sections about diagnosis and treatment of AHF reflect current recommendations in high-income countries and may be substantially different from management standards in low-income or developing countries depending on local availability of resources. The modern management of patients with AHF also includes an optimal interplay between accurate diagnosis, rapid implementation of disease-modifying drugs and devices, specific treatment of the underlying cardiac disease and frequent outpatient follow-up visits. Whereas loop diuretics to relieve congestion are inexpensive and widely available, disease-modifying drugs (particularly sacubitril (a neprilysin inhibitor)–valsartan (an angiotensin receptor blocker)85, which promotes vasodilation and natriuresis, and sodium-glucose cotransporter 2 inhibitors, which reduce blood glucose levels in patients with diabetes mellitus and have also been shown to have beneficial effects in patients with HF)86 and cardiac devices are usually available only in high-income areas. Furthermore, accurate diagnosis of the underlying cardiac diseases and specific treatments often require multimodal imaging techniques, as well as interventional and surgical procedures, which are mostly available in high-volume centres in developed countries. Finally, frequent follow-up visits to reduce the need for hospital readmissions are only feasible in countries with an established network of health-care providers with sufficient expertise in the treatment of patients with HF.
Initial diagnosis
Clinical presentation
Symptoms and signs related to systemic congestion characterize the clinical picture of patients presenting with AHF, to a similar extent regardless of LVEF87. The most common symptoms include dyspnoea during exercise or at rest, orthopnoea, fatigue and reduced exercise tolerance; symptoms are often accompanied by clinical signs such as peripheral oedema, jugular vein distension, the presence of a third heart sound (known as “S3 gallop”, an early diastolic low-frequency sound that may be present under different haemodynamic conditions and might represent termination of the rapid filling of the left ventricle), and pulmonary rales88. In patients presenting with chest discomfort, the differentiation between AHF and acute coronary syndrome may be challenging. Symptoms and signs related to peripheral hypoperfusion, such as cold and clammy skin, altered mental status and oliguria, characterize cardiogenic shock. Cardiogenic shock, as well as respiratory failure, myocardial infarction and arrhythmia, should be rapidly excluded during the initial triage of patients admitted for suspected AHF because these conditions require an appropriate level of monitoring and specific treatments9,89. Commonly accepted criteria for hospitalization in an intensive care unit or a cardiac care unit include haemodynamic instability (heart rate <40 beats per minute or >130 beats per minute, systolic blood pressure <90 mmHg or evidence of hypoperfusion) and respiratory distress (respiratory rate >25 breaths per minute, peripheral oxygen saturation <90% despite supplemental oxygen, use of accessory muscles for breathing or need for mechanical ventilatory support)90.
Several algorithms and scores, most of which include clinical variables and biomarkers, have been developed to predict in-hospital death, but most of these tools have not been adequately prospectively tested for triage or resources allocation purposes. The ADHERE risk tree is used to classify patients on the basis of whether three parameters collected at admission (that is, blood urea nitrogen, systolic blood pressure and serum creatinine) are above or below specific cut-off values; this tool enables patient stratification into five groups with substantially different in-hospital mortality ranging from 2% to 22%91. The GWTG-HF score is computed by adding the points derived from seven variables (age, systolic blood pressure, heart rate, blood urea nitrogen, plasma sodium, history of chronic obstructive pulmonary disease and black ethnicity) and enables stratification into nine categories with in-hospital risk of death ranging from <1% to >50%92. The MEESSI-AHF score includes 13 independent risk factors and may be used to estimate the 30-day mortality in patients with AHF93.
Diagnostic work-up
The clinical picture of AHF is neither sensitive nor specific enough for confirming or ruling out the diagnosis; thus, additional tests are required94. Cardiovascular biomarkers play a crucial part in the diagnostic process of AHF. Patients presenting with suspected AHF should undergo measurement of plasma natriuretic peptides (for example, brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP) or mid-regional pro-atrial natriuretic peptide (MR-proANP)). Although no diagnostic test can on its own reliably differentiate AHF from chronic HF, as all cardiovascular biomarkers are impaired in both patient groups, natriuretic peptides display high sensitivity for detecting underlying cardiac disease in patients presenting with acute dyspnoea. In patients with AHF, levels of circulating natriuretic peptides are elevated compared with levels in patients with shortness of breath of non-cardiac origin95,96,97; thus the measurement of natriuretic peptides provides higher diagnostic accuracy than clinical evaluation alone98. By contrast, dyspnoea in patients with normal (or unchanged) circulating natriuretic peptides is very likely to be of non-cardiac origin. The measurement of natriuretic peptides is recommended in patients with suspected AHF upon admission1,89. In patients with chronically elevated natriuretic peptides owing to chronic HF, a relevant increase in circulating natriuretic peptides may indicate AHF. Additional tests, such as echocardiography or other imaging procedures, are required to confirm the diagnosis of AHF in patients with elevated natriuretic peptides. Several new biomarkers reflecting different pathophysiological aspects of AHF (for example, myocardial injury, systemic congestion, inflammation and fibrosis) may be useful for diagnostic or prognostic purposes, but their role in routine clinical practice is still not well established.
The initial diagnostic process should include a comprehensive evaluation not only of the clinical phenotype but also of the underlying cardiac disorders, precipitating factors and comorbidities. Our (M.A.) group has proposed a ‘7-P’ protocol for guiding evaluation and personalization of treatment. The seven elements are phenotype, pathophysiology, precipitants, pathology, polymorbidity, potential iatrogenic harms and patient preferences99 (Box 1). The diagnosis of AHF is frequently made clinically based on history and clinical signs assisted by measuring circulating natriuretic peptides. The role of imaging for the initial assessment of AHF is limited to patients in whom the underlying cardiac condition is unknown (for example, patients with de novo HF, who require a more extensive diagnostic process than patients with ADHF) or the detection of congestion is uncertain. In these patients, echocardiography and lung ultrasonography may add valuable information. Transthoracic echocardiography should be performed in all patients with de novo HF or in patients with ADHF when a relevant change in cardiac pathology is suspected, to estimate LV and RV function and exclude severe valve disease or pericardial tamponade. Lung ultrasonography has emerged as a valuable modality to detect and monitor pulmonary congestion in patients with AHF. This bedside technique enables the detection of interstitial fluid in the pulmonary parenchyma in a rapid, inexpensive and reliable manner100,101. An ischaemic trigger of AHF, such as acute coronary syndromes, should be ruled out by electrocardiography and (serial) measurement of cardiac troponins; arrhythmias can be evaluated by electrocardiography, continuous electrocardiographic monitoring or interrogation of implantable cardioverter–defibrillator interrogation in selected patients; and infections by measurement of inflammatory markers (for example, C-reactive protein and procalcitonin) and additional investigations according to the clinical presentation (for example, analysis of microbiological specimens and imaging). Additional imaging modalities (for example, MRI) are rarely needed during the initial work-up but may be helpful during further investigations. The initial laboratory evaluation should also include a basic assessment of the function of other organ systems (for example, kidney, liver and blood).
Current recommendations on the management of AHF are mainly based on expert opinion rather than robust evidence, as randomized controlled trials are either lacking or their results are neutral or negative1,3,9. Recent data have shown that timely initiation of therapy may be a crucial factor in the treatment of AHF, with a positive association between short time from admission to diuretic administration and improved in-hospital survival. For this reason, the initial treatment should be delivered as soon as possible, ideally as early as during the diagnostic work-up102. However, because short-term intravenous therapy with diuretics or vasodilators is unlikely to change the mid-term and long-term clinical course in patients with AHF, the choice of initial treatment should take into account not only the clinical phenotype but also the underlying cardiac disorders, precipitating factors and comorbidities.
Screening and prevention
As mentioned above, AHF can arise de novo or in patients with previously diagnosed HF (ADHF). The prospective STOP-HF study investigated the efficacy of a natriuretic peptide-based screening programme and collaborative care in reducing newly diagnosed HF in an at-risk population103. However, although this study showed a significant reduction in the rate of emergency hospitalization for major cardiovascular events in the screening group, the reduction in the incidence of HF did not reach statistical significance. Thus, the role of screening in preventing HF — and more specifically AHF — has yet to be determined, and screening is not recommended by current guidelines1.
By contrast, prevention of decompensation in patients with previously diagnosed HF is of major importance. Hospital readmissions are frequent — in particular during the first months after hospital discharge for AHF — and are associated with adverse outcomes and relevant health-care expenditure12. The optimal strategy for reducing hospital readmission has not been prospectively validated in clinical trials. Residual congestion and lack of disease-modifying treatment implementation before hospital discharge have been associated with worse post-discharge outcomes104,105. Patient education and empowerment may play a crucial part: patients should understand the importance of compliance with medical treatment, be able to recognize symptoms or signs of worsening HF, have a plan about when and how to start or increase diuretic treatment, and know when to contact their cardiologist or the medical emergency system to avoid unnecessary delay. Furthermore, particular attention should be given to avoid self-medication or initiation of contraindicated drugs (for example, NSAIDs) by other physicians who are unaware of the HF diagnosis. Finally, a continuation of the chronic treatment of HF (diuretics and disease-modifying drugs) without interruption should be ensured, although this goal may be challenging, in particular in low-income countries and in the absence of insurance coverage for medical treatments.
Management
Pre-hospital early management
There is a growing body of evidence that delayed treatment delivery is associated with poor outcomes in AHF102. For this reason, current guidelines advocate a ‘time-to-treatment’ concept, similar to those for acute myocardial infarctions or cerebrovascular accidents, and recommend early initiation of treatment in patients with AHF, optimally before hospital admission1,9,89. In the pre-hospital setting, patients with AHF should benefit from adequate non-invasive monitoring (that is, continuous electrocardiography and measurement of blood pressure and peripheral oxygen saturation (SpO2)), oxygen supplementation in case of hypoxia (SpO2 <90%) or non-invasive ventilation in case of respiratory distress. Preclinical non-invasive ventilation treatment can reduce intubation rates and improve short-term outcome in patients with cardiogenic pulmonary oedema106. When the clinical diagnosis of AHF is straightforward, intravenous treatment (mostly vasodilators and/or diuretics) based on the clinical phenotype and involved pathophysiology should be delivered without waiting for additional testing. Diuretics are mainly used in the presence of fluid retention, whereas vasodilators are administered to reduce filling pressures and modulate ventricular–vascular coupling in the presence of fluid redistribution and preserved systolic blood pressure (>110 mmHg; caution should be used if the systolic blood pressure is 90–110 mmHg)1,3. The use of vasodilators is recommended by current guidelines1,3. However, in light of the new results of randomized clinical trials (such as RELAX-AHF-2, TRUE-AHF and GALACTIC) showing no prognostic benefit of vasodilatory agents in AHF, these recommendations may change. The use of inotropes should be restricted to patients in cardiogenic shock due to impaired myocardial contractility, as their inappropriate use is associated with arrhythmias, increased morbidity and mortality107. Notably, pre-hospital treatment should not delay rapid transfer to hospital, preferably to a site with a cardiology and cardiac care unit and/or an intensive care unit. Upon arrival at the hospital, patients should be triaged to exclude cardiopulmonary instability (that is, cardiogenic shock and respiratory failure) and undergo a detailed clinical evaluation.
In-hospital management
Individuals with AHF are at risk of death not only from cardiovascular failure but also from the consequences of organ dysfunction due to congestion and hypoperfusion; thus, it is imperative that the treatment strategy addresses both these issues. Despite the fact that there is little evidence from randomized controlled trials that tackling congestion improves survival, the effect of diuretics on symptoms and organ congestion are evident. Once oxygen saturation has been restored (with oxygen supplementation, non-invasive ventilation or mechanical ventilation), the initial treatment goals in patients presenting with AHF consist of achieving decongestion without residual fluid retention, optimizing perfusion pressures to preserve organ perfusion and maintaining or initiating disease-modifying oral therapies directed towards neurohumoral activation, as these medications also increase diuretic response and improve long-term survival108,109 (Fig. 2)
Congestion is assessed on the basis of the presence of compatible clinical signs (for example, pulmonary rales, distended jugular veins and peripheral oedema), evidence of organ congestion on chest X-ray radiography or lung ultrasonography and elevated filling pressures on invasive monitoring. Abnormal peripheral perfusion is assessed on the basis of the presence of compatible clinical signs (for example, cold and clammy skin, oliguria and altered mental status) and other evidence of altered oxygen transport (for example, increased blood lactate and low central venous or mixed venous oxygen saturation). The response to fluid challenge (that is, change in cardiac output after administration of 250–500 ml of fluids), positive inotropic agents (that is, intravenous drugs that increase cardiac contractility) and vasopressors (that is, intravenous drugs that increase arterial blood pressure by causing peripheral vasoconstriction) should be closely assessed by measuring changes in stroke volume, either by echocardiography or by other invasive monitoring systems. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MCS, mechanical circulatory support; SBP, systolic blood pressure.
Decongestive therapy
As patients with AHF present with a similar congestion profile irrespective of their LVEF87, the decongestive therapy is similar in patients with HFrEF or HFpEF1. The decongestive treatment should be tailored according to the haemodynamic phenotype and the underlying pathophysiology and administered (intravenously, to overcome reduced enteral absorption owing to gastrointestinal congestion) as soon as possible after presentation to increase its success. The practical approach to diuretic treatment has been extensively described in a consensus statement of the Heart Failure Association of the European Society of Cardiology75. Because loop diuretics are >90% protein-bound by albumin in the blood and need to be secreted into the proximal convoluted tubule through several organic anion transporters, when renal blood flow is reduced (such as in AHF), diuretic dosing needs to be adjusted to achieve a plasma concentration sufficient to obtain the desired effect. Furthermore, the peak effect of intravenous loop diuretics occurs within the first hours, with sodium excretion returning to baseline by 6–8 hours; however, to maintain the decongestive effect, the administration of diuretics should continue until euvolaemia is achieved, with three or four daily doses or continuous infusion.
The diuretic response may be evaluated by measuring the urinary volume output and spot urinary sodium content within the first hours after loop diuretic administration75. The measurement of spot urinary sodium content is particularly useful in patients with low to medium urine output. Whereas in patients producing high urinary volumes natriuresis is almost universally high, more-recent data indicate that in patients with a low to medium urine output, spot urinary sodium content offers independent prognostic information in addition to urinary volume output110. In patients with congestion, an hourly urine output of <100–150 ml during the first 6 hours and/or a spot urinary sodium content of <50–70 mmol 2 hours after loop diuretic administration generally indicates an inadequate response to diuretics75. Early evaluation of the diuretic response is recommended to identify patients with diuretic resistance, enabling rapid intensification (such as doubling) of the loop diuretic dose to attain the ceiling (maximum) dose quickly. As increasing the loop diuretic dose any further than the ceiling dose does not induce incremental diuresis and/or natriuresis, the addition of another diuretic agent with a different mode of action should be considered (sequential nephron blockade). In refractory forms, renal replacement therapy may be considered, although these technologies — despite being very effective in volume removal — have not been shown to improve outcomes111,112,113. Mechanisms and treatment approaches to diuretic resistance have been extensively reviewed elsewhere114.
Decongestive treatments should be continued until euvolaemia has been achieved and the medications are switched to an oral form. Loop diuretic therapy should then be reduced to the lowest dose that can maintain euvolaemia1,115. The quantification of fluid excess and the determination of euvolaemia may be challenging in clinical practice and may require a multimodal approach including symptoms, clinical signs, imaging (such as echocardiography, chest X-ray radiography and lung ultrasonography) and biomarkers75. Other techniques, such as data from implanted cardiac devices, pulmonary artery pressure sensors, bioelectrical impedance analysis and indicator dilution techniques, may provide additional valuable information, but their widespread use is limited by technical reasons and cost.
Comprehensive therapy
Specific treatments for the underlying cardiac disease and the precipitating factors should be implemented during hospitalization. For example, myocardial revascularization and optimal antimicrobial treatment should not be delayed when AHF is precipitated by myocardial ischaemia or infection, respectively. On the basis of the comorbidities identified during the initial evaluation and treatment, clinicians should be able to anticipate the need for particular drugs for some specific forms of HF (for example, HF associated with amyloidosis), surgical procedures (for example, for valvular heart disease), mechanical circulatory support (such as LV assist device) or cardiac transplantation. Finally, enrolment of patients in a comprehensive multidisciplinary HF care management programme, promoting medication adherence, up-titration of disease-modifying therapy, cardiac rehabilitation, treatment of underlying comorbidities and timely follow-up with the health-care team, is essential1.
Long-term management
Management goals and pre-discharge management
Individuals who survive the first episode of AHF are at increased risk of experiencing another episode12. Thus, the management goals include improving survival and reducing the risk of hospital readmission due to subsequent episodes of AHF. Ensuring that the individual’s condition is sufficiently stabilized for a safe hospital discharge is the central element of pre-discharge management. Patients with AHF are considered ready for discharge after achieving adequate decongestion and stable renal function on guideline-directed oral therapy1. Congestion is the most common cause of AHF readmission, and persistent congestion and renal dysfunction are known markers of a poor post-discharge prognosis69. A variety of clinical markers (such as weight and fluid loss) and biochemical markers (such as natriuretic peptides) are used as proxies of congestion, but because HF decompensation can occur owing to both fluid accumulation and redistribution, these biomarkers cannot be applied uniformly across patients with AHF. Several studies have demonstrated the usefulness of natriuretic peptides and cardiac troponins in predicting the risk of death and readmission for HF116,117,118. Patients with AHF who have markedly elevated pre-discharge natriuretic peptide levels have worse clinical outcomes, including all-cause and cardiovascular mortality and morbidity, than patients with lower levels. However, the benefits of achieving specific natriuretic peptide target values prior to discharge have not been demonstrated. Abnormally elevated cardiac troponins are often detected in patients with AHF in the absence of overt myocardial ischaemia and are similarly associated with poor outcomes116,117. Another biomarker of myocardial fibrosis, soluble ST2 receptor (also known as IL-1 receptor-like 1, a protein involved in the process of myocardial fibrosis and hypertrophy) has been correlated with disease severity and a poor prognosis in patients with AHF119. ST2, along with other biomarkers of oxidative stress, inflammation and remodelling, requires further study and remains in preclinical exploration120. Overall, defining and achieving satisfactory decongestion remains the major hurdle in AHF management.
In addition to achieving adequate decongestion, implementation of the medical treatment of precipitating factors is recommended to improve post-discharge outcome. In patients with HFrEF, disease-modifying oral HF therapy according to HF guidelines (consisting of β-adrenergic receptor blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor–neprilysin inhibitors, and mineralocorticoid receptor antagonists) should be continued or started during hospitalization and gradually titrated thereafter1, as it is associated with improved outcomes105. In patients with HFpEF, optimal control of comorbidities and precipitating factors is recommended1. Additional treatments, including appropriate drugs for some specific forms of HF or surgical procedures, should be evaluated during hospitalization.
Finally, pillars of pre-discharge management include ensuring a deliberate transition to outpatient care and creating a plan to assess and improve post-discharge prognosis. Care coordination for patients with HF is highly complex as clinicians, patients, care-givers and ancillary services must collaborate to titrate pharmacological therapy, monitor fluid volume status and electrolytes, treat comorbidities, initiate lifestyle changes and establish plans for adherence to treatment and emergency care1,120. Conversations regarding illness severity, barriers to self-care and advance care planning should be introduced before discharge.
Post-discharge management
In addition to continued supervised medical therapy, post-discharge management should incorporate efforts to improve symptoms and quality of life (QOL), delay disease progression and attempt to triage and prognosticate using a risk assessment framework to prevent hospital readmission and death. Generally, post-discharge prognostic tools are prediction models that take several patient clinical variables (for example, age, vital signs during hospitalization, laboratory data and comorbidities) into account and relate them to 30-day and 1-year mortality. Regardless of the time period considered, patients with AHF remain at persistently high risk of rehospitalization and death121. Thus, the American College of Cardiology Foundation–American Heart Association guideline for the management of HF recommends the first post-discharge telephone contact within 3 days and a follow-up visit 7–14 days after discharge, and the European Society of Cardiology guidelines recommend the first follow-up outpatient visit within 7 days of discharge1,120. Despite the complexity of factors associated with rehospitalization for HF, the readmission rate is a ubiquitous metric used to elucidate patient factors (as mentioned above) and health-care system factors that contribute to HF-related morbidity and mortality. Such health-care system factors include, for example, the quality of care provided, patient education, transitional support and medication reconciliation (that is, ensuring that the list of all medications a patient is taking is always as accurate and up-to-date as possible, to facilitate adjustments to the therapy whenever the patient is admitted to, or transferred or discharged from, a hospital). The public health and financial burdens of HF readmissions continue to grow, and evidence is surfacing that some national health policies, for example the Hospital Readmissions Reduction Program in the USA, which were intended to reduce these readmissions, may have had the unintended consequence of increasing post-discharge mortality122.
Clinicians should attempt to identify patients with AHF at high risk of readmission by incorporating clinical, laboratory, imaging and haemodynamic data into a comprehensive assessment. Concerning clinical characteristics in the post-discharge phase include multiple comorbidities (for example, chronic obstructive pulmonary disease, anaemia and chronic renal disease), low systolic blood pressure, high heart rate, progressive orthopnoea and jugular vein distension; laboratory parameters that should raise concerns include low serum sodium, elevated blood urea nitrogen and serum creatinine, low serum albumin and elevated natriuretic peptides123,124,125. In addition to traditional echocardiographic parameters used to evaluate biventricular filling pressures, other imaging techniques, such as lung ultrasonography and point-of-care ultrasonographic assessment of right internal jugular vein compliance, have shown promise in prediction of AHF rehospitalization in patients admitted with AHF126,127. Clinicians should prioritize a comprehensive clinical assessment of patients with AHF with close surveillance for these hallmarks of decompensation and perform targeted interventions focused on decongestion and patient education in the vulnerable early post-discharge phase128.
Implantable pulmonary artery pressure sensors to monitor the haemodynamic status and guide therapy can reduce the risk HF-related hospitalization in patients with HFrEF and HFpEF, but questions regarding true device efficacy remain, owing to concerns about potential bias and misconduct during trial execution129,130,131,132,133. Remote care using intrathoracic impedance monitoring has been associated with an increased risk of HF-related hospitalization134. Thus, the 2016 European Society of Cardiology guidelines provide a weak recommendation for the use of wireless implantable haemodynamic monitoring systems in patients with HF to reduce the risk of recurrent HF hospitalization1. Ultimately, prevention of readmission after an AHF hospitalization remains a challenge. Reliable identification of high-risk patients and of effective interventions to reduce the risk of rehospitalization has been elusive, as high-quality studies in representative patient cohorts are still needed.
Innovative care delivery models are being increasingly investigated as tools to improve post-discharge outcomes in patients with HF; however, results thus far have been disappointing. Telemonitoring alone did not reduce HF readmission in large multicentre and multinational trials135,136,137,138. Patient-centred transitional care approaches that include structured education, communication, clinical care and close surveillance did not improve outcomes compared with usual care models139. Questions remain regarding whether the use of these techniques alone can benefit certain subpopulations of patients and whether proving their efficacy will require a combination of patient-centred strategies.
Quality of life
Patients with AHF and chronic HF cope with numerous physical and psychological symptoms that adversely affect their QOL. Dyspnoea, fatigue, dry mouth, orthopnoea, sleep disturbance and difficulty concentrating are highly prevalent, distressing and burdensome and are predictive of reduced QOL in this population140 (Fig. 3). Depression is more common among patients with HF than in the general population, with at least 20% of patients with HF meeting criteria for major depression141. Prevalence estimates of depression in the HF population vary widely, ranging from 9% to 60%, and such variation is thought to be largely due to differences in outcome ascertainment methods (that is, interviews versus self-reported questionnaires) and in HF severity at the time of assessment141,142. Patients with HF with more severe depression have increased health-care utilization, rehospitalization rates and mortality141,143,144,145. For clinicians, differentiating between symptoms due to HF and those due to depression can be challenging, highlighting a crucial need for a pragmatic and standardized approach to QOL assessment in routine clinical care.
In addition to the physiological alterations in patients with AHF, the stressors of the acute care environment can exacerbate physical and psychological impairments and lead to further declines in QOL146. Elderly hospitalized patients with AHF have a markedly higher symptom burden and worse QOL than age-matched cohorts with stable HFpEF and stable HFrEF146,147. For example, in a prospective, comprehensive, multicentre and multidimensional assessment of 27 patients of ≥60 years of age hospitalized with ADHF compared with three age-matched ambulatory cohorts with stable HF, 78% of the ADHF cohort had cognitive impairment and 30% had depressed mood, but only 11% had a previous diagnosis of depression, suggesting substantial under-recognition of depression in this population. In a sex-stratified analysis of several large international studies on chronic HF, disproportionately worse disease-specific and general QOL was observed in women than in men148. This sex-related difference was unexplained — possible hypotheses included differences in the perception of the effect of the disease between women and men and sex-related confounders that were not measured in this study (for example, access to health care, socioeconomic and educational factors, level of care-giver support, living alone or with other people and proactive help-seeking behaviour). In a global study of patients with LVEF <40% hospitalized with AHF, 13% of patients reported persistently unfavourable QOL, defined by Kansas City Cardiomyopathy Questionnaire (KCCQ) scores of <45, at 1 and 24 weeks after hospital discharge149. QOL issues also affect patient adherence to pharmacological and non-pharmacological treatment and place extraordinary stress on care-givers. Although many studies examining the QOL of patients with chronic stable HF have been published, there is a notable dearth of evidence regarding QOL in patients with AHF.
Similarly, interventions aimed at improving QOL in patients with AHF are not well studied. Guideline-directed medical therapy decreases symptoms and improves QOL in patients with HFrEF. Non-pharmacological and non-device-based or surgical strategies, such as multidisciplinary team management, exercise training, self-care education and lifestyle modifications, have been examined more rigorously in ambulatory patients with chronic HF, but have not been effective in improving QOL independently1. In a small single-centre study of hospitalized patients with HF, inpatient palliative care consultation was associated with improved symptom burden, depressive symptoms and QOL for up to 3 months after hospitalization150. Patient-centred outcomes such as QOL are increasingly incorporated into HF trials and recognized as predictors of clinical events. Further research into tools to assess and strategies to improve QOL in the AHF population should be prioritized as the global population of patients with HF continues to grow.
Outlook
The development of new, effective interventions for the treatment of AHF has been unsuccessful since the 1990s. In contrast to substantial progress achieved in other fields of cardiology and oncology, for example, no new medication or device has been approved for AHF treatment. Many therapies have been tested in the setting of AHF, including inotropic agents (for example, levosimendan and omecamtiv mecarbil), vasodilators (for example, nesiritide, ularitide and serelaxin) and diuretics (for example, tolvaptan)151,152,153,154, but the results of these studies were neutral, and it is still unclear whether this neutrality was due to the inactivity of the tested drugs or inadequacy of the study designs. For instance, determining the best time to administer a tested drug is still a challenge. Few studies have assessed early end points and seem to indicate the use of effective agents as early as possible. On the one hand, if drugs that improve cardiac function are given as early as possible (for example, within 6 hours of presentation to the emergency department), they might prevent worsening of organ dysfunction and death. On the other hand, mortality in the first hours and days is related to severe and irreversible alteration in organ function, that is, excess congestion, hypoxia and/or hypoperfusion, and drugs that aim to improve heart function might not prevent death. Hence, studies have suggested that tested HF drugs should be administered within 48 hours of presentation. Furthermore, choosing the most appropriate primary end point also remains a challenge. For years, regulatory agencies sought ‘improvement in survival rate’ as the primary efficacy end point in both patients with AHF and patients with chronic HF, although intravenous drugs tested in patients with AHF were usually administered for 48 hours only, whereas oral therapy was given every day for years in patients with chronic HF. Because no drug has been shown to improve the survival rate in patients with AHF, experts and patient associations are asking to designate improvements in morbidity as the primary efficacy end point and mortality as a safety end point rather than a primary one.
Several new medications are being tested in AHF. These drugs act by modulating endothelial cell function via the adrenomedullin pathway (adrenomedullin is involved in the maintenance of the endothelial barrier function and in the regulation of the renin–angiotensin–aldosterone system and may have protective properties against fluid retention in AHF)155 or improving cardiovascular function via the modulation of intracellular enzymes, such as dipeptidyl-peptidase 3 (a cytosolic enzyme involved in angiotensin II and enkephalin cleavage that has myocardial depressant properties and whose inhibition may improve haemodynamics)156,157, that are released into the circulation during cell necrosis. While these studies are ongoing, two challenges remain in the management of AHF. First, the implementation of disease-modifying oral HF therapy in patients with HFrEF is still a major challenge worldwide. Only a minority of patients receive the right classes and the right doses of angiotensin-converting enzyme inhibitors, β-adrenergic receptor blockers and mineralocorticoid receptor antagonists. Achieving this goal will certainly minimize episodes of AHF. The second challenge is the post-discharge medication for patients with AHF with HFpEF. Except for treating cardiovascular and metabolic comorbidities that are very frequent in these patients, no drug is recommended after discharge to prevent readmission for a new episode of acute dyspnoea.
Circulating biomarkers, such as natriuretic peptides, are increasingly used in the treatment of patients with AHF. However, during the acute episode, they indicate myocardial stretch but neither venous nor whole-body congestion. Furthermore, although observational studies have shown that a rapid decrease in natriuretic peptides levels is associated with improved outcomes, a recent trial showed no benefit from intensifying therapy to achieve low levels of natriuretic peptides158. Thus, a multimarker strategy based on serially evaluated biomarkers, such as natriuretic peptides, high-sensitivity cardiac troponins, soluble ST2, growth differentiation factor 15, cystatin-C, galectin-3 and high-sensitivity C-reactive protein, may provide increased prognostic accuracy and risk prediction but requires further investigation in different cohorts of patients with HF159. This multimarker strategy might identify high-risk patients who may benefit from novel therapies.
QOL is the main issue for individuals who survive an episode of AHF. Readmissions for dyspnoea are frequent in the months and years following an AHF episode, in particular if the patient does not have optimal doses of disease-modifying HF therapies and does not receive the appropriate devices when needed. Thus, patients seem to favour a rapid improvement in QOL, measured as the number of days out of hospital after discharge, rather than an improvement in survival rate with a bad QOL.
Basic and translational research is also needed to decipher mechanisms of decompensation in chronic HFrEF and HFpEF. AHF is associated with stimulation of the neuroendocrine system and worsening in congestion that harms many organs, including the lungs, kidney and liver. Studies need to elucidate the mechanisms that lead to organ dysfunctions in AHF to prevent worsening in organ function during AHF episodes.
In summary, AHF is a very frequent event that affects the QOL and survival in patients with chronic HFrEF or HFpEF. Signs and symptoms are often related to congestion and in a few patients to hypoperfusion. Mechanisms of decompensation are still unknown. The administration of symptomatic and causal treatments is recommended. Optimizing disease-modifying HF therapies as early as possible is probably the most effective way to prevent AHF episodes. Further research to decipher mechanisms of cardiac and neuroendocrine decompensation and to identify new treatments is needed.
References
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 37, 2129–2200 (2016). The guidelines of the European Society of Cardiology provide evidence-based recommendations on diagnosis and treatment of chronic HF and AHF.
Braunwald, E. Heart failure. JACC Heart Fail. 1, 1–20 (2013).
Mebazaa, A. et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur. Heart J. 36, 1958–1966 (2015). This consensus document contains contemporary recommendations endorsed by different professional societies on the management of AHF.
Ambrosy, A. P. et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 63, 1123–1133 (2014).
Crespo-Leiro, M. G. et al. European Society of Cardiology Heart Failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 18, 613–625 (2016).
Mentz, R. J. & O’Connor, C. M. Pathophysiology and clinical evaluation of acute heart failure. Nat. Rev. Cardiol. 13, 28–35 (2015).
Ishihara, S. et al. Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin. Res. Cardiol. 105, 971–980 (2016).
Mebazaa, A. et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 37, 290–301 (2010).
Mebazaa, A. et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 42, 147–163 (2015).
Mebazaa, A. et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 44, 760–773 (2018).
Follath, F. et al. Clinical presentation, management and outcomes in the acute heart failure global survey of standard treatment (ALARM-HF). Intensive Care Med. 37, 619–626 (2011).
Greene, S. J. et al. The vulnerable phase after hospitalization for heart failure. Nat. Rev. Cardiol. 12, 220–229 (2015). This paper reviews the topic of the vulnerable phase after an AHF episode, which is characterized by high mortality and readmission rates.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 (2018).
Vos, T. et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196 (2012).
Setoguchi, S., Stevenson, L. W. & Schneeweiss, S. Repeated hospitalizations predict mortality in the community population with heart failure. Am. Heart J. 154, 260–266 (2007).
Gheorghiade, M. et al. Acute heart failure syndromes: current state and framework for future research. Circulation 112, 3958–3968 (2005).
Hamo, C. E. et al. A critical appraisal of short-term endpoints in acute heart failure clinical trials. J. Card. Fail. 24, 783–792 (2018).
Cleland, J. et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 1: patient characteristics and diagnosis. Eur. Heart J. 24, 442–463 (2003).
Cuffe, M. S. et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 287, 1541–1547 (2002).
Dharmarajan, K. et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 309, 355–363 (2013).
Fudim, M. et al. Aetiology, timing and clinical predictors of early vs. late readmission following index hospitalization for acute heart failure: insights from ASCEND–HF. Eur. J. Heart Fail. 20, 304–314 (2018).
Giamouzis, G. et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J. Card. Fail. 17, 54–75 (2011).
Sokoreli, I. et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA–HF study. Eur. J. Heart Fail. 20, 689–696 (2018).
Ferreira, J. P. et al. World Heart Federation Roadmap for heart failure. Glob. Heart 14, 197–214 (2019).
Dokainish, H. et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob. Health 5, e665–e672 (2017). This study provides epidemiological data on mortality associated with HF across different continents.
Khatibzadeh, S., Farzadfar, F., Oliver, J., Ezzati, M. & Moran, A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int. J. Cardiol. 168, 1186–1194 (2013).
Damasceno, A. et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch. Intern. Med. 172, 1386–1394 (2012).
Parada, H., Carrasco, H. A., Añez, N., Fuenmayor, C. & Inglessis, I. Cardiac involvement is a constant finding in acute Chagas’ disease: a clinical, parasitological and histopathological study. Int. J. Cardiol. 60, 49–54 (1997).
Bocchi, E. A. et al. Long-term prospective, randomized, controlled study using repetitive education at six-month intervals and monitoring for adherence in heart failure outpatients: the REMADHE trial. Circ. Heart Fail. 1, 115–124 (2008).
Doval, H. C. et al. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet 344, 493–498 (1994).
Zühlke, L. et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY Study). Circulation 134, 1456–1466 (2016).
Sliwa, K. et al. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the Heart of Soweto study. Eur. Heart J. 31, 719–727 (2010).
Bauersachs, J. et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 21, 827–843 (2019).
Abraham, W. T. et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J. Am. Coll. Cardiol. 52, 347–356 (2008).
Sliwa, K. et al. Readmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry. Eur. Heart J. 34, 3151–3159 (2013).
Arrigo, M., Parissis, J. T., Akiyama, E. & Mebazaa, A. Understanding acute heart failure: pathophysiology and diagnosis. Eur. Heart J. Suppl. 18, G11–G18 (2016). This review summarizes the pathophysiology and the diagnostic process in AHF with special attention to treatment-relevant aspects.
Shah, A. M. Ventricular remodeling in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 10, 341–349 (2013).
Wang, M. & Shah, A. M. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J. Mol. Cell. Cardiol. 83, 101–111 (2015).
Zile, M. R. et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 118, 1433–1441 (2008). This study provides unique insights into the pathophysiology of AHF using data obtained from continuous monitoring of intracardiac pressures.
Miller, W. L. & Mullan, B. P. Understanding the heterogeneity in volume overload and fluid distribution in decompensated heart failure is key to optimal volume management: role for blood volume quantitation. JACC Heart Fail. 2, 298–305 (2014).
Kaye, D. M. et al. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J. Am. Coll. Cardiol. 23, 570–578 (1994).
Fallick, C., Sobotka, P. A. & Dunlap, M. E. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ. Heart Fail. 4, 669–675 (2011).
Chaudhry, S. I., Wang, Y., Concato, J., Gill, T. M. & Krumholz, H. M. Patterns of weight change preceding hospitalization for heart failure. Circulation 116, 1549–1554 (2007).
Nijst, P. et al. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 65, 378–388 (2015).
Titze, J. et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am. J. Physiol. Heart Circ. Physiol. 287, H203–H208 (2004).
Guyton, A. C. Interstitial fluid presure. II. Pressure-volume curves of interstitial space. Circ. Res. 16, 452–460 (1965).
Hartupee, J. & Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14, 30–38 (2017).
Mullens, W., Verbrugge, F. H., Nijst, P. & Tang, W. H. W. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur. Heart J. 38, 1872–1882 (2017).
McKie, P. M. et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J. Am. Coll. Cardiol. 58, 2095–2103 (2011).
Mullens, W. & Tang, W. H. W. The early intertwining of the heart and the kidney through an impaired natriuretic response to acute volume expansion. J. Am. Coll. Cardiol. 58, 2104–2105 (2011).
Mullens, W. et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 53, 589–596 (2009). This study highlights the impact of congestion (instead of reduced cardiac output) on renal function in patients with AHF.
Damman, K. et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 53, 582–588 (2009).
Nijst, P., Martens, P., Dupont, M., Tang, W. H. W. & Mullens, W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 5, 672–681 (2017).
Cotter, G., Metra, M., Milo-Cotter, O., Dittrich, H. C. & Gheorghiade, M. Fluid overload in acute heart failure – re-distribution and other mechanisms beyond fluid accumulation. Eur. J. Heart Fail. 10, 165–169 (2014).
Greenway, C. V. & Lister, G. E. Capacitance effects and blood reservoir function in the splanchnic vascular bed during non-hypotensive haemorrhage and blood volume expansion in anaesthetized cats. J. Physiol. 237, 279–294 (1974).
Verbrugge, F. H. et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 62, 485–495 (2013).
Fonarow, G. C. et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch. Intern. Med. 168, 847–854 (2008).
Arrigo, M. et al. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur. J. Heart Fail. 19, 201–208 (2017). This study shows the impact of precipitating factors on prognosis of patients with AHF in a large intercontinental registry.
Arrigo, M. et al. Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC. Heart Fail. 3, 115–121 (2016).
Platz, E. et al. Prevalence and prognostic importance of precipitating factors leading to heart failure hospitalization: recurrent hospitalizations and mortality. Eur. J. Heart Fail. 20, 295–303 (2018).
Miró, Ò. et al. Time-pattern of adverse outcomes after an infection-triggered acute heart failure decompensation and the influence of early antibiotic administration and hospitalisation: results of the PAPRICA-3 study. Clin. Res. Cardiol. 109, 34–45 (2020).
Parrinello, G. et al. Water and sodium in heart failure: a spotlight on congestion. Heart Fail. Rev. 20, 13–24 (2015).
Volpe, M., Carnovali, M. & Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin. Sci. 130, 57–77 (2016).
MacIver, D. H., Adeniran, I., MacIver, I. R., Revell, A. & Zhang, H. Physiological mechanisms of pulmonary hypertension. Am. Heart J. 180, 1–11 (2016).
Borné, Y. et al. Vascular endothelial growth factor D, pulmonary congestion, and incidence of heart failure. J. Am. Coll. Cardiol. 71, 580–582 (2018).
Houston, B. A. et al. Relation of lymphangiogenic factor vascular endothelial growth factor-D to elevated pulmonary artery wedge pressure. Am. J. Cardiol. 124, 756–762 (2019).
von Moos, S. et al. Vascular endothelial growth factor D is a biomarker of fluid overload in haemodialysis patients. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfz281 (2020).
Ware, L. B. & Matthay, M. A. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 353, 2788–2796 (2005).
Harjola, V.-P. et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 19, 821–836 (2017). This review summarizes pathophysiology, diagnosis and management of organ dysfunction occurring in patients with AHF.
Braam, B., Cupples, W. A., Joles, J. A. & Gaillard, C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail. Rev. 17, 161–175 (2012).
Legrand, M., Mebazaa, A., Ronco, C. & Januzzi, J. L. When cardiac failure, kidney dysfunction, and kidney injury intersect in acute conditions: the case of cardiorenal syndrome. Crit. Care Med. 42, 2109–2117 (2014).
Mullens, W. et al. Elevated intra-abdominal pressure in acute decompensated heart failure. J. Am. Coll. Cardiol. 51, 300–306 (2008).
Ahmad, T. et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 137, 2016–2028 (2018).
Maisel, A. S. et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKINESIS study. J. Am. Coll. Cardiol. 68, 1420–1431 (2016).
Mullens, W. et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 21, 137–155 (2019). This position statement from the Heart Failure Association of the European Society of Cardiology provides practical guidance on the use of diuretics to relieve congestion in patients with AHF.
Metra, M. et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ. Heart Fail. 5, 54–62 (2012).
Auer, J. What does the liver tell us about the failing heart? Eur. Heart J. 34, 711–714 (2013).
Møller, S. & Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 34, 2804–2811 (2013).
Samsky, M. D. et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J. Am. Coll. Cardiol. 61, 2397–2405 (2013).
Rogler, G. & Rosano, G. The heart and the gut. Eur. Heart J. 35, 426–430 (2014).
Sandek, A. et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 50, 1561–1569 (2007).
Valentova, M. et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur. Heart J. 37, 1684–1691 (2016).
Colombo, P. C. et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur. Heart J. 35, 448–454 (2014).
Colombo, P. C. et al. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr. Heart Fail. Rep. 12, 215–222 (2015).
McMurray, J. J. V. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
Van Aelst, L. N. L. et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur. J. Heart Fail. 20, 738–747 (2018).
Van de Werf, F. et al. Diastolic properties of the left ventricle in normal adults and in patients with third heart sounds. Circulation 69, 1070–1078 (1984).
Mebazaa, A. et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur. J. Heart Fail. 17, 544–558 (2015).
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. J. Heart Fail. 18, 891–975 (2016).
Fonarow, G. C. et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293, 572–580 (2005). This study provides one easy-to-use risk score (among many others available) to stratify patients admitted with AHF.
Peterson, P. N. et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association Get With the Guidelines program. Circ. Cardiovasc. Qual. Outcomes 3, 25–32 (2010).
Miró, Ò. et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann. Intern. Med. 167, 698–705 (2017).
Gheorghiade, M. et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 12, 423–433 (2010). This position statement from the Heart Failure Association of the European Society of Cardiology provides a statement on the assessment and quantification of congestion in AHF.
Maisel, A. S. et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 347, 161–167 (2002).
Januzzi, J. L. Jr et al. The N-terminal Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Am. J. Cardiol. 95, 948–954 (2005).
Maisel, A. et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea. J. Am. Coll. Cardiol. 55, 2062–2076 (2010).
McCullough, P. A. et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) multinational study. Circulation 106, 416–422 (2002).
Arrigo, M., Nijst, P. & Rudiger, A. Optimising heart failure therapies in the acute setting. Card. Fail. Rev. 4, 38–42 (2018).
Platz, E. et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur. Heart J. 37, 1244–1251 (2016).
Aras, M. A. & Teerlink, J. R. Lung ultrasound: a ‘B-line’ to the prediction of decompensated heart failure. Eur. Heart J. 37, 1252–1254 (2016).
Matsue, Y. et al. Time-to-furosemide treatment and mortality in patients hospitalized with acute heart failure. J. Am. Coll. Cardiol. 69, 3042–3051 (2017). This study shows a beneficial association between early administration of decongestive treatment (loop diuretics) and mortality in AHF.
Ledwidge, M. et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 310, 66–74 (2013).
Rubio-Gracia, J. et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int. J. Cardiol. 258, 185–191 (2018).
Gayat, E. et al. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity-score matched study. Eur. J. Heart Fail. 20, 345–354 (2018). This study shows in a propensity-matched cohort a positive association between pre-discharge implementation of neuro-humoral blockers (β-adrenergic receptor blockers and renin–angiotensin inhibitors) and survival in patients with AHF.
Plaisance, P., Pirracchio, R., Berton, C., Vicaut, E. & Payen, D. A randomized study of out-of-hospital continuous positive airway pressure for acute cardiogenic pulmonary oedema: physiological and clinical effects. Eur. Heart J. 28, 2895–2901 (2007).
Arrigo, M. & Mebazaa, A. Understanding the differences among inotropes. Intensive Care Med. 41, 912–915 (2015).
Butler, J., Gheorghiade, M. & Metra, M. Moving away from symptoms-based heart failure treatment: misperceptions and real risks for patients with heart failure. Eur. J. Heart Fail. 18, 350–352 (2016).
Kula, A. J. et al. Influence of titration of neurohormonal antagonists and blood pressure reduction on renal function and decongestion in decompensated heart failure. Circ. Heart Fail. 9, e002333 (2016).
Brinkley, D. M. et al. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J. Card. Fail. 24, 349–354 (2018).
Costanzo, M. R. et al. Extracorporeal ultrafiltration for fluid overload in heart failure. J. Am. Coll. Cardiol. 69, 2428–2445 (2017).
Costanzo, M. R. et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol. 49, 675–683 (2007).
Bart, B. A. et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N. Engl. J. Med. 367, 2296–2304 (2012).
ter Maaten, J. M. et al. Diuretic response in acute heart failure–pathophysiology, evaluation and therapy. Nat. Rev. Cardiol. 12, 184–192 (2015). This article reviews the mechanisms and the treatment of diuretic resistance occurring in AHF.
Martens, P., Nijst, P. & Mullens, W. Current approach to decongestive therapy in acute heart failure. Curr. Heart Fail. Rep. 12, 367–378 (2015).
Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136, e137–e161 (2017).
Peacock, W. F. et al. Cardiac troponin and outcome in acute heart failure. N. Engl. J. Med. 358, 2117–2126 (2008).
Logeart, D. et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J. Am. Coll. Cardiol. 43, 635–641 (2004).
Rehman, S. U., Mueller, T. & Januzzi, J. L. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J. Am. Coll. Cardiol. 52, 1458–1465 (2008).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 62, 1495–1539 (2013).
Passantino, A., Monitillo, F., Iacoviello, M. & Scrutinio, D. Predicting mortality in patients with acute heart failure: role of risk scores. World J. Cardiol. 7, 902–911 (2015).
Wadhera, R. K. et al. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA 320, 2542–2552 (2018).
Gheorghiade, M. et al. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail. Rev. 17, 485–509 (2012).
Grodin, J. L. et al. Prognostic implications of changes in amino-terminal Pro-B-type natriuretic peptide in acute decompensated heart failure: insights from ASCEND-HF. J. Card. Fail. 25, 703–711 (2019).
Mueller, C. et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 21, 715–731 (2019).
Simon, M. A., Schnatz, R. G., Romeo, J. D. & Pacella, J. J. Bedside ultrasound assessment of jugular venous compliance as a potential point-of-care method to predict acute decompensated heart failure 30-day readmission. J. Am. Heart Assoc. 7, e008184 (2018).
Platz, E. et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail. 7, 849–858 (2019).
Palazzuoli, A. et al. Early readmission for heart failure: an avoidable or ineluctable debacle? Int. J. Cardiol. 277, 186–195 (2019).
Abraham, W. T. et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377, 658–666 (2011).
Adamson, P. B. et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ. Heart Fail. 7, 935–944 (2014).
Givertz, M. M. et al. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 70, 1875–1886 (2017).
Krahnke, J. S. et al. Heart failure and respiratory hospitalizations are reduced in patients with heart failure and chronic obstructive pulmonary disease with the use of an implantable pulmonary artery pressure monitoring device. J. Card. Fail. 21, 240–249 (2015).
Loh, J. P., Barbash, I. M. & Waksman, R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J. Am. Coll. Cardiol. 61, 1571–1576 (2013).
Van Veldhuisen, D. J. et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation 124, 1719–1726 (2011).
Chaudhry, S. I. et al. Telemonitoring in patients with heart failure. N. Engl. J. Med. 363, 2301–2309 (2010).
Jayaram, N. M. et al. Impact of telemonitoring on health status. Circ. Cardiovasc. Qual. Outcomes 10, e004148 (2017).
Koehler, F. et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur. J. Heart Fail. 12, 1354–1362 (2010).
Cleland, J. G. F. et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 45, 1654–1664 (2005).
Van Spall, H. G. C. et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 321, 753–761 (2019).
Zambroski, C. H., Moser, D. K., Bhat, G. & Ziegler, C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur. J. Cardiovasc. Nurs. 4, 198–206 (2005).
Rutledge, T., Reis, V. A., Linke, S. E., Greenberg, B. H. & Mills, P. J. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J. Am. Coll. Cardiol. 48, 1527–1537 (2006).
Freedland, K. E. et al. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom. Med. 65, 119–128 (2003).
Adelborg, K. et al. Mortality risk among heart failure patients with depression: a nationwide population-based cohort study. J. Am. Heart Assoc. 5, e004137 (2016).
Sullivan, M., Simon, G., Spertus, J. & Russo, J. Depression-related costs in heart failure care. Arch. Intern. Med. 162, 1860–1866 (2002).
O’Connor, C. M. & Joynt, K. E. Depression: are we ignoring an important comorbidity in heart failure? J. Am. Coll. Cardiol. 43, 1550–1552 (2004).
Reeves, G. R. et al. Comparison of frequency of frailty and severely impaired physical function in patients ≥60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am. J. Cardiol. 117, 1953–1958 (2016).
Warraich, H. J. et al. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults ≥60 years with acute decompensated heart failure with preserved versus reduced ejection fraction: insights from the REHAB-HF trial. Circ. Heart Fail. 11, e005254 (2018).
Dewan, P. et al. Differential impact of heart failure with reduced ejection fraction on men and women. J. Am. Coll. Cardiol. 73, 29–40 (2019).
Allen, L. A. et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ. Cardiovasc. Qual. Outcomes 4, 389–398 (2011).
Sidebottom, A. C., Jorgenson, A., Richards, H., Kirven, J. & Sillah, A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J. Palliat. Med. 18, 134–142 (2015).
Mebazaa, A. et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 297, 1883–1891 (2007).
Teerlink, J. R. et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure. J. Am. Coll. Cardiol. 67, 1444–1455 (2016).
Packer, M. et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N. Engl. J. Med. 376, 1956–1964 (2017).
Konstam, M. A. et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 297, 1319–1331 (2007).
Voors, A. A. et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur. J. Heart Fail. 21, 163–171 (2019).
Deniau, B. et al. Circulating dipeptidyl peptidase 3 is a myocardial depressant factor: dipeptidyl peptidase 3 inhibition rapidly and sustainably improves haemodynamics. Eur. J. Heart Fail. 14, e0220866 (2019).
Takagi, K. et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial. Eur. J. Heart Fail. https://doi.org/10.1002/ejhf.1600 (2019).
Troughton, R., Michael Felker, G. & Januzzi, J. L. Natriuretic peptide-guided heart failure management. Eur. Heart J. 35, 16–24 (2014).
Demissei, B. G. et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur. J. Heart Fail. 19, 1001–1010 (2017).
Acknowledgements
N.R. is supported by the National Institutes of Health, National Human Genome Research Institute, Ruth L. Kirschstein Institutional National Research Service T32 Award in Genomic Medicine (T32 HG009495).
Author information
Authors and Affiliations
Contributions
Introduction (A.M. and M.A.); Epidemiology (K.S.); Mechanisms/pathophysiology (M.A., W.M. and A.M.S.); Diagnosis, screening and prevention (A.M. and M.A.); Management (M.J., W.M. and N.R.); Quality of life (M.J. and N.R.); Outlook (A.M.); Overview of Primer (A.M.).
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks A. Palazzuoli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arrigo, M., Jessup, M., Mullens, W. et al. Acute heart failure. Nat Rev Dis Primers 6, 16 (2020). https://doi.org/10.1038/s41572-020-0151-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0151-7
This article is cited by
-
Risk factors and prognosis of perioperative acute heart failure in elderly patients with hip fracture: case-control studies and cohort study
BMC Musculoskeletal Disorders (2024)
-
Diagnostic and prognostic value of plasma miR-106a-5p levels in patients with acute heart failure
Journal of Cardiothoracic Surgery (2024)
-
Associations between static and dynamic changes of platelet counts and in-hospital mortality in critical patients with acute heart failure
Scientific Reports (2024)
-
The management of heart failure cardiogenic shock: an international RAND appropriateness panel
Critical Care (2024)
-
Timing of previous heart failure hospitalization as a prognostic factor for emergency department heart failure patients
Internal and Emergency Medicine (2024)