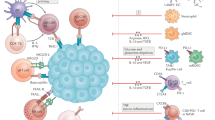
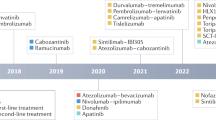
Abstract
Liver cancer, specifically hepatocellular carcinoma (HCC), is the sixth most common cancer and the third leading cause of cancer mortality worldwide. The development of effective systemic therapies, particularly those involving immune-checkpoint inhibitors (ICIs), has substantially improved the outcomes of patients with advanced-stage HCC. Approximately 30% of patients are diagnosed with early stage disease and currently receive potentially curative therapies, such as resection, liver transplantation or local ablation, which result in median overall survival durations beyond 60 months. Nonetheless, up to 70% of these patients will have disease recurrence within 5 years of resection or local ablation. To date, the results of randomized clinical trials testing adjuvant therapy in patients with HCC have been negative. This major unmet need has been addressed with the IMbrave 050 trial, demonstrating a recurrence-free survival benefit in patients with a high risk of relapse after resection or local ablation who received adjuvant atezolizumab plus bevacizumab. In parallel, studies testing neoadjuvant ICIs alone or in combination in patients with early stage disease have also reported efficacy. In this Review, we provide a comprehensive overview of the current approaches to manage patients with early stage HCC. We also describe the tumour immune microenvironment and the mechanisms of action of ICIs and cancer vaccines in this setting. Finally, we summarize the available evidence from phase II/III trials of neoadjuvant and adjuvant approaches and discuss emerging clinical trials, identification of biomarkers and clinical trial design considerations for future studies.
Key points
-
Approximately 30% of patients with hepatocellular carcinoma (HCC) undergo resection or local ablation as primary treatment. However, the probability of recurrence at 3 years is 30–50% and is associated with the size of the main tumour, microvascular invasion and poor differentiation degree.
-
In the phase III IMbrave 050 trial, patients with HCC at high risk of recurrence after resection or local ablation who received adjuvant atezolizumab plus bevacizumab had significantly improved recurrence-free survival compared with those who had active surveillance.
-
Neoadjuvant exposure to immunotherapies enables more-efficient interactions among T cells, antigen-presenting cells and cancer cells owing to a larger tumour burden compared with the adjuvant approach.
-
Neoadjuvant and adjuvant administration of immunotherapies results in significantly improved outcomes compared with adjuvant administration alone in patients with melanoma or non-small-cell lung cancer.
-
Phase II trials of cancer vaccines in combination with immune-checkpoint inhibitors in patients with melanoma or pancreatic adenocarcinoma have shown signals of efficacy; these approaches are currently being explored in HCC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 7 (2021).
Llovet, J. M. et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 3, 386–401 (2022).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022).
Cappuyns, S., Virginia, C., Yarchoan, M., Finn, R. S. & Llovet, J. M. Critical appraisal of guideline recommendations on systemic therapies for advanced hepatocellular carcinoma. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2023.2677 (2023).
Singal, A. G. et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 78, 1922–1965 (2023).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Patel, S. P. et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N. Engl. J. Med. 388, 813–823 (2023).
Spaander, M. C. W. et al. Young-onset colorectal cancer. Nat. Rev. Dis. Prim. 9, 22 (2023).
Haber, P. K. et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology 161, 879–898 (2021).
Qin, S. et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave 050): a randomised, open-label, multicentre, phase 3 trial. Lancet 402, 1835–1847 (2023).
Marron, T. U. et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 219–229 (2022).
Ho, W. J. et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat. Cancer 2, 891–903 (2021).
Kaseb, A. O. et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 208–218 (2022).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous V.2.2023 (2023).
Cercek, A. et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Yang, X. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 379, e14 (2018).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Akinboro, O. et al. US Food and Drug Administration approval summary: nivolumab plus platinum-doublet chemotherapy for the neoadjuvant treatment of patients with resectable non-small-cell lung cancer. J. Clin. Oncol. 41, 3249–3259 (2023).
Galle, P. R. et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Kudo, M. et al. Management of hepatocellular carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 10, 181–223 (2021).
Xie, D., Shi, J., Zhou, J., Fan, J. & Gao, Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Chinese perspective. Clin. Mol. Hepatol. 29, 206–216 (2023).
Goh, M. J. et al. Clinical practice guideline and real-life practice in hepatocellular carcinoma: a Korean perspective. Clin. Mol. Hepatol. 29, 197–205 (2023).
Roayaie, S. et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 62, 440–451 (2015).
Llovet, J. M., Schwartz, M. & Mazzaferro, V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 25, 181–200 (2005).
Reveron-Thornton, R. F. et al. Global and regional long-term survival following resection for HCC in the recent decade: a meta-analysis of 110 studies. Hepatol. Commun. 6, 1813–1826 (2022).
Rumgay, H. et al. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 161, 108–118 (2022).
Wolf, E., Rich, N. E., Marrero, J. A., Parikh, N. D. & Singal, A. G. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology 73, 713–725 (2021).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
Tsoulfas, G. Surgical Challenges in the Management of Liver Disease. https://doi.org/10.5772/intechopen.76553 (IntechOpen, 2019).
Franssen, B. et al. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: a retrospective analysis of a single North American center. Ann. Surg. 260, 650–658 (2014).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2, 16018 (2016).
Llovet, J. M. et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 20, 487–503 (2023).
Kudo, M. Surveillance, diagnosis, and treatment outcome of hepatocellular carcinoma in Japan: 2023 update. Liver Cancer 12, 95–102 (2023).
Shan, T. et al. Disparities in stage at diagnosis for liver cancer in China. J. Natl Cancer Cent. 3, 7–13 (2023).
Ishizawa, T. et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 134, 1908–1916 (2008).
Di Benedetto, F. et al. Safety and efficacy of robotic vs open liver resection for hepatocellular carcinoma. JAMA Surg. 158, 46–54 (2023).
Llovet, J. M. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313 (2021).
Lin, S.-M., Lin, C.-J., Lin, C.-C., Hsu, C.-W. & Chen, Y.-C. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 54, 1151–1156 (2005).
Doyle, A. et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J. Hepatol. 70, 866–873 (2019).
Shiina, S. et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 107, 569–577 (2012).
Charalel, R. A. et al. Long-term survival after surgery versus ablation for early liver cancer in a large, nationally representative cohort. J. Am. Coll. Radiol. 19, 1213–1223 (2022).
Pompili, M. et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤ 3 cm. Results of a multicenter Italian survey. J. Hepatol. 59, 89–97 (2013).
Kudo, M. et al. Report of the 22nd nationwide follow-up Survey of Primary Liver Cancer in Japan (2012–2013). Hepatol. Res. 52, 5–66 (2022).
Yoon, J. S. et al. Hepatocellular carcinoma in Korea between 2008 and 2011: an analysis of Korean Nationwide Cancer Registry. J. Liver Cancer 20, 41–52 (2020).
Meloni, M. F. et al. Use of contrast‐enhanced ultrasound in ablation therapy of HCC. J. Ultrasound Med. 40, 879–894 (2021).
Jie, T., Guoying, F., Gang, T., Zhengrong, S. & Maoping, L. Efficacy and safety of fusion imaging in radiofrequency ablation of hepatocellular carcinoma compared to ultrasound: a meta-analysis. Front. Surg. 8, 728098 (2021).
Feng, Q., Chi, Y., Liu, Y., Zhang, L. & Liu, Q. Efficacy and safety of percutaneous radiofrequency ablation versus surgical resection for small hepatocellular carcinoma: a meta-analysis of 23 studies. J. Cancer Res. Clin. Oncol. 141, 1–9 (2015).
Takayama, T. et al. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: a randomized controlled trial (SURF Trial). Liver Cancer 11, 209–218 (2022).
Sheta, E. et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur. J. Gastroenterol. Hepatol. 28, 1198–1203 (2016).
Peng, Z.-W. et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J. Clin. Oncol. 31, 426–432 (2013).
Tabrizian, P., Jibara, G., Shrager, B., Schwartz, M. & Roayaie, S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann. Surg. 261, 947–955 (2015).
Vibert, E., Schwartz, M. & Olthoff, K. M. Advances in resection and transplantation for hepatocellular carcinoma. J. Hepatol. 72, 262–276 (2020).
Chen, R. et al. Recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Front. Oncol. 13, 1–11 (2023).
Zhu, Y. et al. Factors influencing early recurrence of hepatocellular carcinoma after curative resection. J. Int. Med. Res. 48, 0300060520945552 (2020).
Xu, X.-F. et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 154, 209–217 (2019).
Ringelhan, M., Pfister, D., O’Connor, T., Pikarsky, E. & Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 19, 222–232 (2018).
Zheng, C. et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169, 1342–1356.e16 (2017).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845.e20 (2019).
Geh, D. et al. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 257–273 (2022).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135, 234–243 (2008).
Han, Y. et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 59, 567–579 (2014).
Finkin, S. et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 16, 1235–1244 (2015).
Ramadori, P., Kam, S. & Heikenwalder, M. T cells: friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology 75, 1038–1049 (2022).
Jardim, D. L., Goodman, A., de Melo Gagliato, D. & Kurzrock, R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 39, 154–173 (2021).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Bassaganyas, L. et al. Copy-number alteration burden differentially impacts immune profiles and molecular features of hepatocellular carcinoma. Clin. Cancer Res. 26, 6350–6361 (2020).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Haber, P. K. et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology 164, 72–88.e18 (2023).
Ruiz de Galarreta, M. et al. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 (2019).
Moeini, A. et al. An immune gene expression signature associated with development of human hepatocellular carcinoma identifies mice that respond to chemopreventive agents. Gastroenterology 157, 1383–1397.e11 (2019).
Xu, Y. et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 25, 301–311 (2019).
Li, J. et al. Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J. Clin. Invest. 130, 2712–2726 (2020).
Shen, J. et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 24, 556–562 (2018).
Montironi, C. et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut 72, 129–140 (2023).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469 (2020).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 1–11 (2017).
Magen, A. et al. Intratumoral dendritic cell–CD4+ T helper cell niches enable CD8+ T cell differentiation following PD-1 blockade in hepatocellular carcinoma. Nat. Med. 29, 1389–1399 (2023).
Pinyol, R., Sia, D. & Llovet, J. M. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin. Cancer Res. 25, 2021–2023 (2019).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37, 429–442 (2003).
Okita, K. et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: a randomized double-blind placebo-controlled study. J. Gastroenterol. 50, 191–202 (2015).
Yoshida, H. et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 54, 532–540 (2011).
Mazzaferro, V. et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 44, 1543–1554 (2006).
Chen, L.-T. et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann. Surg. 255, 8–17 (2012).
Raoul, J. et al. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology 26, 1156–1161 (1997).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Geissler, E. K. et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation 100, 116–125 (2016).
Li, S.-H. et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III randomized study. J. Clin. Oncol. 41, 1898–1908 (2023).
Wang, Z. et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin. Cancer Res. 24, 2074–2081 (2018).
Lee, J. H. et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391.e6 (2015).
Wu, C.-Y. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 308, 1906 (2012).
Reig, M. et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 65, 719–726 (2016).
Singal, A. G. et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology 157, 1253–1263.e2 (2019).
Yin, J. et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J. Clin. Oncol. 31, 3647–3655 (2013).
Jørgensen, J. T. The current landscape of the FDA approved companion diagnostics. Transl. Oncol. 14, 101063 (2021).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e14 (2021).
Pfister, D. et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592, 450–456 (2021).
Llovet, J. M. Exploring a new pathway for biomarker-based approval of immunotherapies. Nat. Rev. Clin. Oncol. 20, 279–280 (2023).
Topalian, S. L. et al. Neoadjuvant immune checkpoint blockade: a window of opportunity to advance cancer immunotherapy. Cancer Cell 41, 1551–1566 (2023).
Garg, M. et al. Tumour gene expression signature in primary melanoma predicts long-term outcomes. Nat. Commun. 12, 1137 (2021).
Lucas, M. W., Versluis, J. M., Rozeman, E. A. & Blank, C. U. Personalizing neoadjuvant immune-checkpoint inhibition in patients with melanoma. Nat. Rev. Clin. Oncol. 20, 408–422 (2023).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Oba, T., Kajihara, R., Yokoi, T., Repasky, E. A. & Ito, F. Neoadjuvant in situ immunomodulation enhances systemic antitumor immunity against highly metastatic tumors. Cancer Res. 81, 6183–6195 (2021).
Hughes, E. et al. Primary breast tumours but not lung metastases induce protective anti-tumour immune responses after Treg-depletion. Cancer Immunol. Immunother. 69, 2063–2073 (2020).
Pai, C.-C. S. et al. Clonal deletion of tumor-specific T cells by interferon-γ confers therapeutic resistance to combination immune checkpoint blockade. Immunity 50, 477–492.e8 (2019).
Friedman, J. et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin. Cancer Res. 26, 679–689 (2020).
Cottrell, T. R. et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 29, 1853–1860 (2018).
Rozeman, E. A. et al. LBA75 — 18-months relapse-free survival (RFS) and biomarker analyses of OpACIN-neo: a study to identify the optimal dosing schedule of neoadjuvant (neoadj) ipilimumab (IPI) + nivolumab (NIVO) in stage III melanoma. Ann. Oncol. 30, v910 (2019).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Tetzlaff, M. T. et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 29, 1861–1868 (2018).
Rozeman, E. A. et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 20, 948–960 (2019).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
Springfeld, C. et al. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 20, 318–337 (2023).
Allard, M.-A. et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J. Hepatol. 63, 83–92 (2015).
Llovet, J. M. et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 73, 158–191 (2021).
Wakelee, H. et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 389, 491–503 (2023).
Topalian, S. L. et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 5, 1411–1420 (2019).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Robert, C. et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 36, 1668–1674 (2018).
Spicer, J. et al. Surgical outcomes from the phase 3 CheckMate 816 trial: nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 39, 8503 (2021).
Lissoni, P. et al. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory T lymphocytes in cancer patients. Anticancer Res. 29, 1847–1852 (2009).
Tang, F., Tie, Y., Tu, C. & Wei, X. Surgical trauma-induced immunosuppression in cancer: recent advances and the potential therapies. Clin. Transl. Med. 10, 199–223 (2020).
Bakos, O., Lawson, C., Rouleau, S. & Tai, L.-H. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 6, 86 (2018).
Marron, T. U. et al. Neoadjuvant clinical trials provide a window of opportunity for cancer drug discovery. Nat. Med. 28, 626–629 (2022).
Marron, T. U. et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J. Immunother. Cancer 9, e001901 (2021).
Schiller, J. T. & Lowy, D. R. Vaccines to prevent infections by oncoviruses. Annu. Rev. Microbiol. 64, 23–41 (2010).
Yarchoan, M., Johnson, B. A., Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 17, 209–222 (2017).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Dolgin, E. The tangled history of mRNA vaccines. Nature 597, 318–324 (2021).
Szebeni, J. et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 17, 337–346 (2022).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
Precision medicine meets cancer vaccines. Nat. Med. 29, 1287 (2023).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
mRNA vaccine slows melanoma recurrence. Cancer Discov. 13, 1278 (2023).
Weber et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet 403, 632–644 (2024).
Yarchoan, M. et al. Personalized DNA neoantigen vaccine in combination with plasmid IL-12 and pembrolizumab for the treatment of patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 39, TPS2680 (2021).
Liu, C. et al. mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023).
Llovet, J. M., Brú, C. & Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19, 329–338 (1999).
de Haas, R. J. et al. Curative salvage liver transplantation in patients with cirrhosis and hepatocellular carcinoma: an intention-to-treat analysis. Hepatology 67, 204–215 (2018).
Mehta, N. et al. Downstaging outcomes for hepatocellular carcinoma: results from the multicenter evaluation of reduction in tumor size before liver transplantation (MERITS-LT) consortium. Gastroenterology 161, 1502–1512 (2021).
Mazzaferro, V. et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 21, 947–956 (2020).
Kudo, M. et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child–Pugh a liver function: a proof-of-concept study. Cancers 11, 1084 (2019).
Abou-Alfa, G. K. et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 40, 379 (2022).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Wong, J. S. L. et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J. Immunother. Cancer 9, e001945 (2021).
von Felden, J., Garcia-Lezana, T., Schulze, K., Losic, B. & Villanueva, A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 69, 2025–2034 (2020).
Roayaie, S. et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology 57, 1426–1435 (2013).
Zhu, Q. et al. Hepatocellular carcinoma in a large medical center of China over a 10-year period: evolving therapeutic option and improving survival. Oncotarget 6, 4440–4450 (2015).
Aroldi, F. & Lord, S. R. Window of opportunity clinical trial designs to study cancer metabolism. Br. J. Cancer 122, 45–51 (2020).
Hu, C. & Dignam, J. J. Biomarker-driven oncology clinical trials: key design elements, types, features, and practical considerations. JCO Precis. Oncol. https://doi.org/10.1200/PO.19.00086 (2019).
Acknowledgements
The authors thank M. Zeitlhoefler (Icahn School of Medicine at Mount Sinai and IDIBAPS) for his help in preparing the tables for this manuscript. J.M.L. is supported by grants from Asociación Española Contra el Cáncer (Proyectos Generales: PRYGN223117LLOV), the European Commission (Horizon Europe-Mission Cancer, THRIVE, Ref. 101136622), by an Accelarator Award from Cancer Research UK, Fondazione per la Ricerca sul Cancro (AIRC) and Fundación Científica de la Asociación Española Contra el Cáncer (FAECC) (HUNTER, Ref. C9380/A26813), Generalitat de Catalunya (AGAUR, 2021-SGR 01347), Acadèmia de Ciències Mèdiques i de la Salut de Catalunya i Balears; NIH (R01-CA273932-01, RO1DK56621 and RO1DK128289), the Samuel Waxman Cancer Research Foundation and the Spanish National Health Institute (MICINN, PID2022-139365OB-I00).
Author information
Authors and Affiliations
Contributions
All the authors contributed to all aspects of preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
J.M.L. receives research support from Bayer HealthCare Pharmaceuticals, Eisai Inc. and Sagimet; has received consulting fees from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol–Myers Squibb, Eisai Inc., Exelixis, Genentech, Glycotest, Merck, Moderna and Roche. M.Y. has received institutional research support from Bristol–Myers Squibb, Genentech and Incyte; honoraria from Astrazeneca, Eisai, Exelixis, Genentech, Hepion and Replimune; and is a co-founder of and holds equity in Adventris Pharmaceuticals. A.G.S. has served as a consultant or on advisory boards for AstraZeneca, Bayer, Boston Scientific, Eisai, Exact Sciences, Exelixis, Freenome, FujiFilm Medical Sciences, GRAIL, Genentech, Glycotest, Roche and Universal Dx. T.U.M. has served on advisory and/or data safety monitoring boards for AbbVie, Arcus, Astellas, AstraZeneca, Atara, Boehringer Ingelheim, Bristol–Meyers Squibb, Celldex, Chimeric, DBV Technologies, DrenBio, G1 Therapeutics, Genentech, Glenmark, Merck, NGMbio, Regeneron, Rockefeller University, Simcere and Surface; and received research grants from Boehringer Ingelheim, Bristol–Myers Squibb, Merck and Regeneron. M.K. has received research support from Bayer Pharmaceutical, Chugai, Eisai, Ono Pharmaceutical and Takeda; consultancy or lecture fees from AbbVie, AstraZeneca, Bayer, Chugai, EA Pharma, Eisai, Eli Lilly, GE Healthcare, Gilead Sciences, Merck, Otsuka, Roche, Sumitomo Dainippon Pharma and Takeda. R.P., M.S., E.P. and R.S.F. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S.-Q. Cheng; A. Kaseb, who co-reviewed with M. LaPelusa; T. Pawlik; and S. Qin for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Llovet, J.M., Pinyol, R., Yarchoan, M. et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat Rev Clin Oncol 21, 294–311 (2024). https://doi.org/10.1038/s41571-024-00868-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-024-00868-0