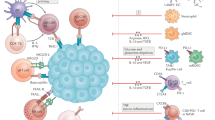
Abstract
Immune-checkpoint inhibitors (ICIs) are now widely used for the treatment of patients with advanced-stage hepatocellular carcinoma (HCC). Two different ICI-containing regimens, atezolizumab plus bevacizumab and tremelimumab plus durvalumab, are now approved standard-of-care first-line therapies in this setting. However, and despite substantial improvements in survival outcomes relative to sorafenib, most patients with advanced-stage HCC do not derive durable benefit from these regimens. Advances in genome sequencing including the use of single-cell RNA sequencing (both of tumour material and blood samples), as well as immune cell identification strategies and other techniques such as radiomics and analysis of the microbiota, have created considerable potential for the identification of novel predictive biomarkers enabling the accurate selection of patients who are most likely to derive benefit from ICIs. In this Review, we summarize data on the immunology of HCC and the outcomes in patients receiving ICIs for the treatment of this disease. We then provide an overview of current biomarker use and developments in the past 5 years, including gene signatures, circulating tumour cells, high-dimensional flow cytometry, single-cell RNA sequencing as well as approaches involving the microbiome, radiomics and clinical markers. Novel concepts for further biomarker development in HCC are then discussed including biomarker-driven trials, spatial transcriptomics and integrated ‘big data’ analysis approaches. These concepts all have the potential to better identify patients who are most likely to benefit from ICIs and to promote the development of new treatment approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
International Agency for Research on Cancer. Estimated number of new cases in 2020, World, both sexes, all ages (excl. NMSC). Cancer Today https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (2020).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6 (2021).
Cheng, A. L. et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76, 862–873 (2022).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Greten, T. F. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J. Immunother. Cancer 9, e002794 (2021).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Abou-Alfa, G. K. et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 1 (8), https://doi.org/10.1056/EVIDoa2100070 (2022).
Yau, T. et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23, 77–90 (2022).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 38, 193–202 (2020).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Monge, C., Xie, C., Steinberg, S. M. & Greten, T. F. Clinical indicators for long-term survival with immune checkpoint therapy in advanced hepatocellular carcinoma. J. Hepatocell. Carcinoma 8, 507–512 (2021).
Pinato, D. J. et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur. J. Cancer 157, 140–152 (2021).
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18, 223–238 (2021).
Marrero, J. A. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68, 723–750 (2018).
Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major. Probl. Clin. Surg. 1, 1–85 (1964).
Reig, M. et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 76, 681–693 (2022).
Bruix, J., Chan, S. L., Galle, P. R., Rimassa, L. & Sangro, B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J. Hepatol. 75, 960–974 (2021).
Kudo, M. et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer 10, 181–223 (2021).
Zhou, J. et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 9, 682–720 (2020).
Vogel, A. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv238–iv255 (2018).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Ren, Z. et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 22, 977–990 (2021).
Qin, S. et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study [abstract]. J. Clin. Oncol. 40 (4 Suppl.), 383 (2022).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Sangro, B. et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 72, 320–341 (2020).
Chow, P. et al. IMbrave050: phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation [abstract]. Cancer Res. 83 (8 Suppl.), CT003 (2023).
Kaseb, A. O. et al. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 208–218 (2022).
Ho, W. J. et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat. Cancer 2, 891–903 (2021).
Shu, D. H. et al. 12-chemokine gene signature identifies major pathologic response in patients with hepatocellular carcinoma treated with neoadjuvant nivolumab and cabozantinib [abstract]. Cancer Res. 82 (12 Suppl.), 1323 (2022).
Marron, T. U. et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 219–229 (2022).
Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
McKean, W. B., Moser, J. C., Rimm, D. & Hu-Lieskovan, S. Biomarkers in precision cancer immunotherapy: promise and challenges. Am. Soc. Clin. Oncol. Educ. Book. 40, e275–e291 (2020).
Han, Y., Liu, D. & Li, L. PD-1/PD-L1 pathway: current researches in cancer. Am. J. Cancer Res. 10, 727–742 (2020).
Paver, E. C. et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology 53, 141–156 (2021).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Ng, H. H. M. et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J. Immunother. Cancer 8, e000987 (2020).
Ang, C. et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 10, 4018–4025 (2019).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Cancer Genome Atlas Research Network Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e23 (2017).
Chaisaingmongkol, J. et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 32, 57–70.e3 (2017).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Haber, P. K. et al. Molecular markers of response to anti-PD1 therapy in advanced hepatocellular carcinoma. Gastroenterology 164, 72–88.e18 (2023).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469 (2020).
Hong, J. Y. et al. Hepatocellular carcinoma patients with high circulating cytotoxic T cells and intra-tumoral immune signature benefit from pembrolizumab: results from a single-arm phase 2 trial. Genome Med. 14, 1 (2022).
Huang, M. et al. The influence of immune heterogeneity on the effectiveness of immune checkpoint inhibitors in multifocal hepatocellular carcinomas. Clin. Cancer Res. 26, 4947–4957 (2020).
Budhu, A. et al. Tumor biology and immune infiltration define primary liver cancer subsets linked to overall survival after immunotherapy. Cell Rep. Med. 4, 101052 (2023).
Vanhersecke, L. et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Yu, S. et al. Tumor-infiltrating immune cells in hepatocellular carcinoma: Tregs is correlated with poor overall survival. PLoS ONE 15, e0231003 (2020).
Montironi, C. et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut 72, 129–140 (2022).
Ge, P. L. et al. Prognostic values of immune scores and immune microenvironment-related genes for hepatocellular carcinoma. Aging 12, 5479–5499 (2020).
Martin-Serrano, M. A. et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 72, 736–748 (2023).
Ma, L. et al. Single-cell atlas of tumor cell evolution in response to therapy in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Hepatol. 75, 1397–1408 (2021).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845.e20 (2019).
Zheng, L. et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science 374, abe6474 (2021).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018).
Ma, L. et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36, 418–430.e6 (2019).
Bian, J. et al. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am. J. Cancer Res. 10, 4585 (2020).
Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19, 151–172 (2022).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 19, 133–150 (2019).
Ho, D. W. et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat. Commun. 12, 3684 (2021).
Liu, Y. et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 78, 770–782 (2023).
Nguyen, P. H. D. et al. Trajectory of immune evasion and cancer progression in hepatocellular carcinoma. Nat. Commun. 13, 1441 (2022).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology 135, 234–243 (2008).
Liu, M. et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 69, 365–379 (2020).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147 (2022).
Geh, D. et al. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 257–273 (2022).
Ma, L. et al. Multiregional single-cell dissection of tumor and immune cells reveals stable lock-and-key features in liver cancer. Nat. Commun. 13, 7533 (2022).
Murai, H. et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 1, 77–91 (2022).
Provine, N. M. & Klenerman, P. MAIT cells in health and disease. Annu. Rev. Immunol. 38, 203–228 (2020).
Ruf, B. et al. Tumor-associated macrophages trigger MAIT cell dysfunction at the HCC invasive margin. Cell 186, 3686–3705.e32 (2023).
Nguyen, P. H. D. et al. Intratumoural immune heterogeneity as a hallmark of tumour evolution and progression in hepatocellular carcinoma. Nat. Commun. 12, 227 (2021).
Zhang, S. et al. Spatial transcriptomics analysis of neoadjuvant cabozantinib and nivolumab in advanced hepatocellular carcinoma identifies independent mechanisms of resistance and recurrence. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2023.01.10.523481v1 (2023).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Hu, X., Chen, R., Wei, Q. & Xu, X. The landscape of alpha fetoprotein in hepatocellular carcinoma: where are we? Int. J. Biol. Sci. 18, 536–551 (2022).
Galle, P. R. et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 39, 2214–2229 (2019).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 282–296 (2019).
Zhu, A. X. et al. Alpha-fetoprotein as a potential surrogate biomarker for atezolizumab + bevacizumab treatment of hepatocellular carcinoma. Clin. Cancer Res. 28, 3537–3545 (2022).
Shao, Y. Y. et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 39, 2184–2189 (2019).
Lee, P. C. et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 12, 182 (2020).
Spahn, S. et al. Clinical and genetic tumor characteristics of responding and non-responding patients to PD-1 inhibition in hepatocellular carcinoma. Cancers 12, 3830 (2020).
Scheiner, B. et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy – development and validation of the CRAFITY score. J. Hepatol. 76, 353–363 (2022).
Hatanaka, T. et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol. Int. 16, 1150–1160 (2022).
Teng, W. et al. Combination of CRAFITY score with alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am. J. Cancer Res. 12, 1899–1911 (2022).
Sun, X. et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer 21, 775 (2021).
Myojin, Y. et al. Interleukin-6 is a circulating prognostic biomarker for hepatocellular carcinoma patients treated with combined immunotherapy. Cancers 14, 883 (2022).
Yang, H. et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 5, 100672 (2023).
Feun, L. G. et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 125, 3603–3614 (2019).
Feun, L. G. et al. Circulating biomarkers to predict antitumor response to immunotherapy in advanced unresectable hepatoma [abstract]. Cancer Res. 82 (12 Suppl.), 2771 (2022).
Li, X. S., Li, J. W., Li, H. & Jiang, T. Prognostic value of programmed cell death ligand 1 (PD-L1) for hepatocellular carcinoma: a meta-analysis. Biosci. Rep. 40, BSR20200459 (2020).
Wang, T., Denman, D., Bacot, S. M. & Feldman, G. M. Challenges and the evolving landscape of assessing blood-based PD-L1 expression as a biomarker for anti-PD-(L)1 immunotherapy. Biomedicines 10, 1181 (2022).
Lin, Z. F., Qin, L. X. & Chen, J. H. Biomarkers for response to immunotherapy in hepatobiliary malignancies. Hepatobiliary Pancreat. Dis. Int. 21, 413–419 (2022).
Pallozzi, M. et al. Non-invasive biomarkers for immunotherapy in patients with hepatocellular carcinoma: current knowledge and future perspectives. Cancers 14, 4631 (2022).
Dharmapuri, S. et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 9, 4962–4970 (2020).
Hung, H. C. et al. Response prediction in immune checkpoint inhibitor immunotherapy for advanced hepatocellular carcinoma. Cancers 13, 1607 (2021).
Muhammed, A. et al. The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers 14, 186 (2021).
Mei, J. et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J. Inflamm. Res. 14, 3879–3890 (2021).
Wu, Y. L. et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancers 14, 5834 (2022).
Kim, C. et al. Association of high levels of antidrug antibodies against atezolizumab with clinical outcomes and T-cell responses in patients with hepatocellular carcinoma. JAMA Oncol. 8, 1825–1829 (2022).
Chew, V. et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc. Natl Acad. Sci. USA 114, E5900–E5909 (2017).
Zheng, C. et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell 169, 1342–1356.e16 (2017).
Sun, Y. et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 184, 404–421 e416 (2021).
Heinrich, B. et al. The tumour microenvironment shapes innate lymphoid cells in patients with hepatocellular carcinoma. Gut 71, 1161–1175 (2022).
Spitzer, M. H. & Nolan, G. P. Mass cytometry: single cells, many features. Cell 165, 780–791 (2016).
Monge, C. et al. Phase I/II study of PexaVec in combination with immune checkpoint inhibition in refractory metastatic colorectal cancer. J. Immunother. Cancer 11, e005640 (2023).
Gohil, S. H., Iorgulescu, J. B., Braun, D. A., Keskin, D. B. & Livak, K. J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 244–256 (2021).
Krieg, C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 24, 144–153 (2018).
Agdashian, D. et al. The effect of anti-CTLA4 treatment on peripheral and intra-tumoral T cells in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 68, 599–608 (2019).
Hung, Y. P. et al. Potential of circulating immune cells as biomarkers of nivolumab treatment efficacy for advanced hepatocellular carcinoma. J. Chin. Med. Assoc. 84, 144–150 (2021).
Heinrich, B. et al. Checkpoint inhibitors modulate plasticity of innate lymphoid cells in peripheral blood of patients with hepatocellular carcinoma. Front. Immunol. 13, 849958 (2022).
Ruf, B., Heinrich, B. & Greten, T. F. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol. Immunol. 18, 112–127 (2021).
Barsch, M. et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 77, 397–409 (2022).
Chuah, S. et al. Uncoupling immune trajectories of response and adverse events from anti-PD-1 immunotherapy in hepatocellular carcinoma. J. Hepatol. 77, 683–694 (2022).
Sidiropoulos, D. N. et al. Integrated T cell cytometry metrics for immune-monitoring applications in immunotherapy clinical trials. JCI Insight 7, e160398 (2022).
Alix-Panabieres, C. & Pantel, K. Liquid biopsy: from discovery to clinical application. Cancer Discov. 11, 858–873 (2021).
Schroers-Martin, J. G. et al. Molecular monitoring of lymphomas. Annu. Rev. Pathol. 18, 149–180 (2023).
von Felden, J., Garcia-Lezana, T., Schulze, K., Losic, B. & Villanueva, A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 69, 2025–2034 (2020).
Klein, E. A. et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 32, 1167–1177 (2021).
Tran, N. H., Kisiel, J. & Roberts, L. R. Using cell-free DNA for HCC surveillance and prognosis. JHEP Rep. 3, 100304 (2021).
Kaseb, A. O. et al. Molecular profiling of hepatocellular carcinoma using circulating cell-free DNA. Clin. Cancer Res. 25, 6107–6118 (2019).
von Felden, J. et al. Mutations in circulating tumor DNA predict primary resistance to systemic therapies in advanced hepatocellular carcinoma. Oncogene 40, 140–151 (2021).
Matsumae, T. et al. Circulating cell-free DNA profiling predicts the therapeutic outcome in advanced hepatocellular carcinoma patients treated with combination immunotherapy. Cancers 14, 3367 (2022).
Harding, J. J. et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin. Cancer Res. 25, 2116–2126 (2019).
An, H. J., Chon, H. J. & Kim, C. Peripheral blood-based biomarkers for immune checkpoint inhibitors. Int. J. Mol. Sci. 22, 9414 (2021).
Tamminga, M. et al. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 7, 173 (2019).
Winograd, P. et al. Hepatocellular carcinoma-circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors. Hepatol. Commun. 4, 1527–1540 (2020).
Budhu, A. et al. Integrated metabolite and gene expression profiles identify lipid biomarkers associated with progression of hepatocellular carcinoma and patient outcomes. Gastroenterology 144, 1066–1075.e1 (2013).
Pomyen, Y. et al. Tumor metabolism and associated serum metabolites define prognostic subtypes of Asian hepatocellular carcinoma. Sci. Rep. 11, 12097 (2021).
Breeur, M. et al. Pan-cancer analysis of pre-diagnostic blood metabolite concentrations in the European Prospective Investigation into Cancer and Nutrition. BMC Med. 20, 351 (2022).
Fujiwara, N. et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med 2, 836–850.e10 (2021).
Hung, M. H. et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat. Commun. 12, 1455 (2021).
Wu, H. et al. Dynamic microbiome and metabolome analyses reveal the interaction between gut microbiota and anti-PD-1 based immunotherapy in hepatocellular carcinoma. Int. J. Cancer 151, 1321–1334 (2022).
Gong, X. Q. et al. Progress of MRI radiomics in hepatocellular carcinoma. Front. Oncol. 11, 698373 (2021).
Dercle, L. et al. Artificial intelligence and radiomics: fundamentals, applications, and challenges in immunotherapy. J. Immunother. Cancer 10, e005292 (2022).
Dercle, L. et al. Emerging and evolving concepts in cancer immunotherapy imaging. Radiology 306, 32–46 (2023).
Martinino, A. et al. Artificial intelligence in the diagnosis of hepatocellular carcinoma: a systematic review. J. Clin. Med. 11, 6368 (2022).
Tao, Y. Y. et al. Radiomic analysis based on magnetic resonance imaging for predicting PD-L2 expression in hepatocellular carcinoma. Cancers (Basel) 15, 365 (2023).
Chen, S. et al. Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur. Radiol. 29, 4177–4187 (2019).
Hectors, S. J. et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur. Radiol. 30, 3759–3769 (2020).
Yuan, G. et al. Development and validation of a contrast-enhanced CT-based radiomics nomogram for prediction of therapeutic efficacy of anti-PD-1 antibodies in advanced HCC patients. Front. Immunol. 11, 613946 (2020).
Castilla-Lievre, M. A. et al. Diagnostic value of combining 11C-choline and 18F-FDG PET/CT in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 43, 852–859 (2016).
European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018).
Wei, W. et al. ImmunoPET: concept, design, and applications. Chem. Rev. 120, 3787–3851 (2020).
Bell, M., Turkbey, E. B. & Escorcia, F. E. Radiomics, radiogenomics, and next-generation molecular imaging to augment diagnosis of hepatocellular carcinoma. Cancer J. 26, 108–115 (2020).
Mena, E. et al. Functional imaging of liver cancer (FLIC): study protocol of a phase 2 trial of 18F-DCFPyL PET/CT imaging for patients with hepatocellular carcinoma. PLoS ONE 17, e0277407 (2022).
Rizzo, A. et al. PSMA radioligand uptake as a biomarker of neoangiogenesis in solid tumours: diagnostic or theragnostic factor? Cancers 14, 4309 (2022).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371, eabc4552 (2021).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20, e77–e91 (2019).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Schwabe, R. F. & Greten, T. F. Gut microbiome in HCC – mechanisms, diagnosis and therapy. J. Hepatol. 72, 230–238 (2020).
Silveira, M. A. D., Bilodeau, S., Greten, T. F., Wang, X. W. & Trinchieri, G. The gut–liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 8, 583–597 (2022).
Myojin, Y. & Greten, T. F. The microbiome and liver cancer. Cancer J. 29, 57–60 (2023).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Zhang, L. et al. The association between antibiotic use and outcomes of HCC patients treated with immune checkpoint inhibitors. Front. Immunol. 13, 956533 (2022).
Fulgenzi, C. A. M. et al. Effect of early antibiotic exposure on survival of patients receiving atezolizumab plus bevacizumab but not sorafenib for unresectable HCC: a sub-analysis of the phase III IMbrave150 study. J. Clin. Oncol. 41, 597–597 (2023).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Lee, W. S., Yang, H., Chon, H. J. & Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular–immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 52, 1475–1485 (2020).
Zhang, Y. et al. VEGFR2 activity on myeloid cells mediates immune suppression in the tumor microenvironment. JCI Insight 6, e150375 (2021).
Kudo, M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers 12, 1089 (2020).
Neely, J. et al. Genomic and transcriptomic analyses related to the clinical efficacy of first-line nivolumab in advanced hepatocellular carcinoma from the phase 3 CheckMate 459 trial [abstract]. Cancer Res. 82 (12 Suppl.), 2145 (2022).
Ruiz de Galarreta, M. et al. β-Catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 (2019).
Kaseb, A. O. et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol. Res. 7, 1390–1395 (2019).
Jiang, P. et al. Big data in basic and translational cancer research. Nat. Rev. Cancer 22, 625–639 (2022).
Boehm, K. M., Khosravi, P., Vanguri, R., Gao, J. & Shah, S. P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer 22, 114–126 (2022).
Cohen, Y. C. et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 27, 491–503 (2021).
Echle, A. et al. Deep learning in cancer pathology: a new generation of clinical biomarkers. Br. J. Cancer 124, 686–696 (2021).
Kato, S. et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 11, 4965 (2020).
Tamborero, D. et al. The molecular tumor board portal supports clinical decisions and automated reporting for precision oncology. Nat. Cancer 3, 251–261 (2022).
Vanguri, R. S. et al. Multimodal integration of radiology, pathology and genomics for prediction of response to PD-(L)1 blockade in patients with non-small cell lung cancer. Nat. Cancer 3, 1151–1164 (2022).
Hoang, D.-T. et al. Synthetic lethality-based prediction of cancer treatment response from histopathology images. Cell 3, 2487–2502.e13 (2023).
Shi, A. et al. Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome. Sci. Adv. 6, eabb3461 (2020).
Cao, Y. et al. Predicting tumor immune microenvironment and checkpoint therapy response of head & neck cancer patients from blood immune single-cell transcriptomics. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2023.01.17.524455v1 (2023).
Singal, A. G. et al. International liver cancer association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology 160, 2572–2584 (2021).
Liu, J. et al. A viral exposure signature defines early onset of hepatocellular carcinoma. Cell 182, 317–328.e10 (2020).
Lo, Y. M. D., Han, D. S. C., Jiang, P. & Chiu, R. W. K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 372, eaaw3616 (2021).
Foda, Z. H. et al. Detecting liver cancer using cell-free DNA fragmentomes. Cancer Discov. 13, 616–631 (2022).
Dudani, J. S., Ibrahim, M., Kirkpatrick, J., Warren, A. D. & Bhatia, S. N. Classification of prostate cancer using a protease activity nanosensor library. Proc. Natl Acad. Sci. USA 115, 8954–8959 (2018).
Canady, T. D. et al. Digital-resolution detection of microRNA with single-base selectivity by photonic resonator absorption microscopy. Proc. Natl Acad. Sci. USA 116, 19362–19367 (2019).
Zhao, B. et al. Digital-resolution and highly sensitive detection of multiple exosomal small RNAs by DNA toehold probe-based photonic resonator absorption microscopy. Talanta 241, 123256 (2022).
Qin, S. et al. Final analysis of RATIONALE-301: randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma [abstract LBA36]. Ann. Oncol. 33 (Suppl. 7), S1402–S1403 (2022).
Qin, S. et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial [abstract LBA35]. Ann. Oncol. 33 (Suppl. 7), S1401–S1402 (2022).
Kelley, R. K. et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 23, 995–1008 (2022).
Finn, R. S. et al. Primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC) [abstract LBA34]. Ann. Oncol. 33 (Suppl. 7), S1401 (2022).
Qin, S. et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 39, 3002–3011 (2021).
Qin, S. et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 6, 559–568 (2021).
Qin, S. et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J. Clin. Oncol. 41, 1434–1443 (2023).
Verset, G. et al. Pembrolizumab monotherapy for previously untreated advanced hepatocellular carcinoma: data from the open-label, phase II KEYNOTE-224 trial. Clin. Cancer Res. 28, 2547–2554 (2022).
Yau, T. et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: results from cohort 6 of the CheckMate 040 trial. J. Clin. Oncol. 41, 1747–1757 (2023).
Xu, J. et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin. Cancer Res. 27, 1003–1011 (2021).
Finn, R. S. et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 38, 2960–2970 (2020).
Kelley, R. K. et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 39, 2991–3001 (2021).
Author information
Authors and Affiliations
Contributions
A.V., F.K., M.Y., L.M., E.R. and X.W.W researched data for the manuscript and made a substantial contribution to discussions of content. All authors wrote the manuscript, and A.V., F.K., B.R. M.Y., L.M. and E.R. edited and/or reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
A.V. has acted as a consultant and/or adviser for Astra Zeneca, BMS, Eisai, FirstWorld, Genentech, Natera, NGM Pharmaceuticals, Pioneering Medicine and Roche; has received research support from Eisai; has stock options from Espervita; and is listed as an inventor on a patent related to early detection of HCC (PCT/US20/61441). M.Y. has acted as a consultant and/or adviser for AstraZeneca, Eisai, Exelixis, Genentech, Hepion and Replimune; has received research funding from Bristol-Myers Squibb, Genentech and Incyte; and holds equity in Adventris Pharmaceuticals. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks L. Rimassa, A. Rizzo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greten, T.F., Villanueva, A., Korangy, F. et al. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat Rev Clin Oncol 20, 780–798 (2023). https://doi.org/10.1038/s41571-023-00816-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00816-4