Abstract
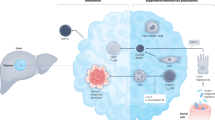
In the past 5 years, important advances have been made in the scientific understanding and clinical management of cholangiocarcinoma (CCA). The cellular immune landscape of CCA has been characterized and tumour subsets with distinct immune microenvironments have been defined using molecular approaches. Among these subsets, the identification of ‘immune-desert’ tumours that are relatively devoid of immune cells emphasizes the need to consider the tumour immune microenvironment in the development of immunotherapy approaches. Progress has also made in identifying the complex heterogeneity and diverse functions of cancer-associated fibroblasts in this desmoplastic cancer. Assays measuring circulating cell-free DNA and cell-free tumour DNA are emerging as clinical tools for detection and monitoring of the disease. Molecularly targeted therapy for CCA has now become a reality, with three drugs targeting oncogenic fibroblast growth factor receptor 2 (FGFR2) fusions and one targeting neomorphic, gain-of-function variants of isocitrate dehydrogenase 1 (IDH1) obtaining regulatory approval. By contrast, immunotherapy using immune-checkpoint inhibitors has produced disappointing results in patients with CCA, underscoring the requirement for novel immune-based treatment strategies. Finally, liver transplantation for early stage intrahepatic CCA under research protocols is emerging as a viable therapeutic option in selected patients. This Review highlights and provides in-depth information on these advances.
Key points
-
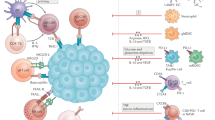
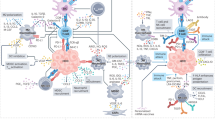
Cholangiocarcinomas (CCAs) are highly desmoplastic cancers characterized by a tumour microenvironment that is poorly immunogenic, with an abundance of immunosuppressive cell types such as myeloid-derived suppressor cells and tumour-associated macrophages.
-
The desmoplasia of CCAs is associated with an abundance of heterogeneous cancer-associated fibroblasts (CAFs), with several transcriptomically diverse CAF subtypes identified.
-
Assessments of circulating tumour cells and cell-free tumour DNA are becoming important in the diagnosis and monitoring of CCA.
-
Potent oncogenic drivers of intrahepatic CCAs (iCCAs) include fibroblast growth factor receptor 2 (FGFR2) gene fusions and neomorphic, gain-of-function variants of isocitrate dehydrogenase 1 (IDH1). FGFR2 fusions and IDH1 mutations are targetable, and approved molecularly targeted therapies are now available for the treatment of CCAs harbouring these genetic aberrations.
-
The results of immune-checkpoint inhibitor monotherapy in patients with CCA have been disappointing, although the combination of gemcitabine, cisplatin and durvalumab has been approved in the first-line setting for the treatment of advanced-stage CCA.
-
Liver transplantation is an emerging option for the subset of patients with early stage iCCA occurring in the setting of cirrhosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Banales, J. M. et al. Cholangiocarcinoma: state-of-the-art knowledge and challenges. Liver Int. 39, 5–6 (2019).
Brindley, P. J. et al. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 7, 65 (2021).
Banales, J. M. et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 17, 557–588 (2020).
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K. & Gores, G. J. Cholangiocarcinoma– evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15, 95–111 (2018).
Affo, S. et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 39, 883 (2021).
Yang, J. D. et al. DNA methylation markers for detection of cholangiocarcinoma: discovery, validation, and clinical testing in biliary brushings and plasma. Hepatol. Commun. 5, 1448–1459 (2021).
Zill, O. A. et al. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov. 5, 1040–1048 (2015).
Wu, M. J., Shi, L., Merritt, J., Zhu, A. X. & Bardeesy, N. Biology of IDH mutant cholangiocarcinoma. Hepatology 75, 1322–1337 (2022).
Kelley, R. K., Bridgewater, J., Gores, G. J. & Zhu, A. X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 72, 353–363 (2020).
Abou-Alfa, G. K. et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684 (2020).
Javle, M. et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 6, 803–815 (2021).
Goyal, L. et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N. Engl. J. Med. 388, 228–239 (2023).
US Food and Drug Administration. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (2020).
US Food and Drug Administration. FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (2021).
US Food and Drug Administration. FDA grants accelerated approval to futibatinib for cholangiocarcinoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma (2022).
Abou-Alfa, G. K. et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807 (2020).
US Food and Drug Administration. FDA approves ivosidenib for advanced or metastatic cholangiocarcinoma. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma (2022).
Gravely, A. K., Vibert, E. & Sapisochin, G. Surgical treatment of intrahepatic cholangiocarcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2022.01.004 (2022).
Wang, J., Loeuillard, E., Gores, G. J. & Ilyas, S. I. Cholangiocarcinoma: what are the most valuable therapeutic targets – cancer-associated fibroblasts, immune cells, or beyond T cells? Expert. Opin. Ther. Targets 25, 835–845 (2021).
Loeuillard, E. et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Invest. 130, 5380–5396 (2020).
Ruffolo, L. I. et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 71, 1386–1398 (2022).
Zhang, Q. et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 11, 1248–1267 (2021).
Gani, F. et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 23, 2610–2617 (2016).
Zhou, G. et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J. Hepatol. 71, 753–762 (2019).
Ghidini, M. et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur. J. Cancer 86, 158–165 (2017).
Ma, L. et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36, 418–430.e6 (2019).
Fabris, L., Sato, K., Alpini, G. & Strazzabosco, M. The tumor microenvironment in cholangiocarcinoma progression. Hepatology 73, 75–85 (2021).
Piha-Paul, S. A. et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 147, 2190–2198 (2020).
Buettner, S. et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 164, 411–418 (2018).
Peng, D. et al. Lymphocyte to monocyte ratio predicts resectability and early recurrence of Bismuth-Corlette type IV hilar cholangiocarcinoma. J. Gastrointest. Surg. 24, 330–340 (2020).
Tsilimigras, D. I. et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB 22, 1667–1674 (2020).
Sun, D. et al. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ 8, e8458 (2020).
Yuan, D. et al. Kupffer cell-derived TNF triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 31, 771–789.e6 (2017).
Boulter, L. et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Invest. 125, 1269–1285 (2015).
Dwyer, B. J. et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J. Hepatol. 74, 860–872 (2021).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018).
Xu, X. D. et al. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 15, 99–105 (2016).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257 (2014).
Song, G. et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat. Commun. 13, 1642 (2022).
Bao, X. et al. Molecular subgroups of intrahepatic cholangiocarcinoma discovered by single-cell RNA sequencing-assisted multiomics analysis. Cancer Immunol. Res. 10, 811–828 (2022).
Keenan, B. P. et al. Circulating monocytes associated with anti-PD-1 resistance in human biliary cancer induce T cell paralysis. Cell Rep. 40, 111384 (2022).
Tavazoie, M. F. et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e18 (2018).
Vonderheide, R. H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020).
Diggs, L. P. et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol. 74, 1145–1154 (2021).
Wabitsch, S. et al. Anti-PD-1 in combination with trametinib suppresses tumor growth and improves survival of intrahepatic cholangiocarcinoma in mice. Cell Mol. Gastroenterol. Hepatol. 12, 1166–1178 (2021).
Affo, S., Yu, L. X. & Schwabe, R. F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. 12, 153–186 (2017).
Brivio, S., Cadamuro, M., Strazzabosco, M. & Fabris, L. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J. Hepatol. 9, 455–468 (2017).
Sirica, A. E., Campbell, D. J. & Dumur, C. I. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 27, 276–284 (2011).
Sirica, A. E. & Gores, G. J. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 59, 2397–2402 (2014).
Fabris, L. et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 39, 63–78 (2019).
Mertens, J. C. et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 73, 897–907 (2013).
Chen, Y., McAndrews, K. M. & Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 18, 792–804 (2021).
Campbell, D. J., Dumur, C. I., Lamour, N. F., Dewitt, J. L. & Sirica, A. E. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol. Res. 42, 1119–1130 (2012).
Biffi, G. & Tuveson, D. A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 101, 147–176 (2021).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Bhattacharjee, S. et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Invest. https://doi.org/10.1172/JCI146987 (2021).
Martin-Serrano, M. A. et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut https://doi.org/10.1136/gutjnl-2021-326514 (2022).
Zhang, M. et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 73, 1118–1130 (2020).
Ravichandra, A., Bhattacharjee, S. & Affo, S. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma progression and therapeutic resistance. Adv. Cancer Res. 156, 201–226 (2022).
Chen, Y. et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39, 548–565.e6 (2021).
Liu, M. C. et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 31, 745–759 (2020).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Wasenang, W., Chaiyarit, P., Proungvitaya, S. & Limpaiboon, T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenetics 11, 39 (2019).
Ettrich, T. J. et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci. Rep. 9, 13261 (2019).
Mody, K. et al. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precis. Oncol. 3, 1–9 (2019).
Moreno Luna, L. E. et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 131, 1064–1072 (2006).
Barr Fritcher, E. G. et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology 149, 1813–1824.e1 (2015).
Gonda, T. A. et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin. Gastroenterol. Hepatol. 15, 913–919.e1 (2017).
Singhi, A. D. et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 69, 52–61 (2020).
Arechederra, M. et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 71, 1141–1151 (2022).
Andresen, K. et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology 61, 1651–1659 (2015).
Loi, E. et al. HOXD8 hypermethylation as a fully sensitive and specific biomarker for biliary tract cancer detectable in tissue and bile samples. Br. J. Cancer 126, 1783–1794 (2022).
Shigehara, K. et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE 6, e23584 (2011).
Han, H. S. et al. Bile-derived circulating extracellular miR-30d-5p and miR-92a-3p as potential biomarkers for cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 19, 41–50 (2020).
Berchuck, J. E. et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann. Oncol. 33, 1269–1283 (2022).
Andersen, J. B. et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 142, 1021–1031.e15 (2012).
Montal, R. et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 73, 315–327 (2020).
Sia, D. et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 144, 829–840 (2013).
Job, S. et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 72, 965–981 (2020).
Lowery, M. A. et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin. Cancer Res. 24, 4154–4161 (2018).
Weinberg, B. A. et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol. 10, 652–662 (2019).
Wardell, C. P. et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 68, 959–969 (2018).
Jusakul, A. et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 7, 1116–1135 (2017).
Nakamura, H. et al. Genomic spectra of biliary tract cancer. Nat. Genet. 47, 1003–1010 (2015).
Boscoe, A. N., Rolland, C. & Kelley, R. K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J. Gastrointest. Oncol. 10, 751–765 (2019).
Chaisaingmongkol, J. et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 32, 57–70.e3 (2017).
Chan-On, W. et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet. 45, 1474–1478 (2013).
Bekaii-Saab, T. S., Bridgewater, J. & Normanno, N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol. 32, 1111–1126 (2021).
Lamarca, A. et al. Molecular profiling in daily clinical practice: practicalities in advanced cholangiocarcinoma and other biliary tract cancers. J. Clin. Med. https://doi.org/10.3390/jcm9092854 (2020).
National Comprehensive Cancer Network. NCCN guidelines: hepatobiliary cancer. NCCN https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438 (2022).
Haugsten, E. M., Wiedlocha, A., Olsnes, S. & Wesche, J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 8, 1439–1452 (2010).
Babina, I. S. & Turner, N. C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 17, 318–332 (2017).
Wu, Y. M. et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013).
Graham, R. P. et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 45, 1630–1638 (2014).
Arai, Y. et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 59, 1427–1434 (2014).
Becker, Z. BridgeBio says it’s undeterred after Truseltiq partner withdraws application, discontinues drug. FIERCE Pharma https://www.fiercepharma.com/pharma/bridgebios-cancer-drug-truseltiqs-future-unclear-partner-helsinn-withdrawing-nda (2022).
Droz Dit Busset, M. et al. Derazantinib for patients with intrahepatic cholangiocarcinoma harboring FGFR2 fusions/rearrangements: primary results from the phase II study FIDES-01 [abstract 47P]. Ann. Oncol. 32 (Suppl. 5), S376 (2021).
Krook, M. A. et al. Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Mol. Cancer Ther. 19, 847–857 (2020).
Krook, M. A. et al. Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb. Mol. Case Stud. https://doi.org/10.1101/mcs.a004002 (2019).
Goyal, L. et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 7, 252–263 (2017).
Goyal, L. et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 9, 1064–1079 (2019).
Sootome, H. et al. Futibatinib is a novel irreversible FGFR 1-4 inhibitor that shows selective antitumor activity against FGFR-deregulated tumors. Cancer Res. 80, 4986–4997 (2020).
Meric-Bernstam, F. et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 12, 402–415 (2022).
Casaletto, J. et al. RLY-4008, a novel precision therapy for FGFR2-driven cancers designed to potently and selectively inhibit FGFR2 and FGFR2 resistance mutations [abstract]. Cancer Res. 81 (Suppl. 13), 1455 (2021).
Franovic, A. et al. Activity of KIN-3248, a next-generation pan-FGFR inhibitor, against acquired FGFR-gatekeeper and molecular-brake drug resistance mutations [abstract]. J. Clin. Oncol. 40 (Suppl. 4), 461 (2022).
Goyal, L. et al. First results of RLY-4008, a potent and highly selective FGFR2 inhibitor in a first-in-human study in patients with FGFR2-altered cholangiocarcinoma and multiple solid tumors [abstract]. Mol. Cancer Ther. 20 (Suppl. 12), P02-02 (2021).
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012).
Inoue, S. et al. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell 30, 337–348 (2016).
Sulkowski, P. L. et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aal2463 (2017).
Valle, J. W., Lamarca, A., Goyal, L., Barriuso, J. & Zhu, A. X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 7, 943–962 (2017).
Subbiah, V. et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 21, 1234–1243 (2020).
US Food and Drug Administration. FDA grants accelerated approval of dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (2022).
Subbiah, V. et al. Pan-cancer efficacy of vemurafenib in BRAFV600-mutant non-melanoma cancers. Cancer Discov. 10, 657–663 (2020).
Galdy, S. et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 36, 141–157 (2017).
Javle, M. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 22, 1290–1300 (2021).
Ohba, A. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial) [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4006 (2022).
Lee, C. K. et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol. Hepatol. https://doi.org/10.1016/S2468-1253(22)00335-1 (2022).
Harding, J. J. et al. Targeting HER2 mutation-positive advanced biliary tract cancers with neratinib: final results from the phase 2 SUMMIT basket trial. J. Clin. Oncol. 40, 4079–4079 (2022).
Meric-Bernstam, F. et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): results from a phase I study [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 299 (2021).
Westphalen, C. B. et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol. 5, 69 (2021).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
US Food and Drug Administration. FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 (2018).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 21, 271–282 (2020).
US Food and Drug Administration. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (2019).
Parimi, V. et al. Genomic landscape of 891 RET fusions detected across diverse solid tumor types. NPJ Precis. Oncol. 7, 10 (2023).
Subbiah, V. et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 28, 1640–1645 (2022).
Subbiah, V. et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 23, 1261–1273 (2022).
Valle, J. W., Kelley, R. K., Nervi, B., Oh, D. Y. & Zhu, A. X. Biliary tract cancer. Lancet 397, 428–444 (2021).
Lamarca, A., Edeline, J. & Goyal, L. How I treat biliary tract cancer. ESMO Open 7, 100378 (2022).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Maio, M. et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase 2 KEYNOTE-158 study. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.05.519 (2022).
US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (2017).
US Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (2020).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Ma, K. et al. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol. Lett. 14, 250–256 (2017).
Zhu, Y. et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. Cancer Manag. Res. 10, 4113–4123 (2018).
Fluxa, P. et al. High CD8+ and absence of Foxp3+ T lymphocytes infiltration in gallbladder tumors correlate with prolonged patients survival. BMC Cancer 18, 243 (2018).
Goeppert, B. et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer 113, 1343–1349 (2015).
Sun, D. et al. Anti-PD-1 therapy combined with chemotherapy in patients with advanced biliary tract cancer. Cancer Immunol. Immunother. 68, 1527–1535 (2019).
Sahai, V. et al. A multicenter randomized phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4582 (2020).
Liu, T. et al. Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: a preliminary analysis of safety and efficacy of an open-label phase II clinical study [abstract 53P]. Ann. Oncol. 31 (Suppl. 4), S261 (2020).
Oh, D. Y. et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 7, 522–532 (2022).
Oh, D.-Y. et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. https://doi.org/10.1056/EVIDoa2200015 (2022).
Burris, H. A. et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4070 (2022).
He, A. et al. Outcomes by primary tumour location in patients with advanced biliary tract cancer treated with durvalumab or placebo plus gemcitabine and cisplatin in the phase 3 TOPAZ-1 study [abstract O-1]. Ann. Oncol. 33 (Suppl. 4), S378 (2022).
Okusaka, T. et al. Outcomes by disease status in patients with advanced biliary tract cancer treated wtih durvalumab or placebo plus gemcitabine and cisplatin in the phase 3 TOPAZ-1 study [abstract 93P]. Ann. Oncol. 33 (Suppl. 9), S1471 (2022).
Vogel, A. et al. Regional subgroup analysis of the phase 3 TOPAZ-1 study of durvalumab (D) plus gemcitabine and cisplatin (GC) in advanced biliary tract cancer (BTC) [abstract]. J. Clin. Oncol. 40 (Suppl. 16), 4075 (2022).
US Food and Drug Administration. FDA D.I.S.C.O. burst edition: FDA approval of Imfinzi (durvalumab) for adult patients with locally advanced or metastatic biliary tract cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-imfinzi-durvalumab-adult-patients-locally-advanced-or (2022).
Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet https://doi.org/10.1016/S0140-6736(23)00727-4 (2023).
Villaneuva, L. et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 321 (2021).
Sapisochin, G. et al. Transplant oncology in primary and metastatic liver tumors: principles, evidence, and opportunities. Ann. Surg. 273, 483–493 (2021).
McMillan, R. R. et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transpl. 22, 823–832 (2022).
Ivanics, T., Toso, C., Ilyas, S. I. & Sapisochin, G. Transplant oncology in locally advanced intrahepatic cholangiocarcinoma: one more step on a long road. Am. J. Transpl. 22, 685–686 (2022).
Jain, A. et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis. Oncol. 2, 1–12 (2018).
Franssen, S. et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg. https://doi.org/10.1001/jamasurg.2022.1298 (2022).
Sapisochin, G. et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am. J. Transpl. 14, 660–667 (2014).
Sapisochin, G. et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 64, 1178–1188 (2016).
Jung, D. H. et al. Clinicopathological features and prognosis of intrahepatic cholangiocarcinoma after liver transplantation and resection. Ann. Transpl. 22, 42–52 (2017).
De Martin, E. et al. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl. 26, 785–798 (2020).
Lunsford, K. E. et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol. Hepatol. 3, 337–348 (2018).
Ito, T. et al. A 3-decade, single-center experience of liver transplantation for cholangiocarcinoma: impact of era, tumor size, location, and neoadjuvant therapy. Liver Transpl. 28, 386–396 (2022).
Acknowledgements
The work of the authors is supported by the US National Institutes of Health (NIH)/National Cancer Institute (NCI) grants SPORE P50 CA210964 (to S.I.I. and G.J.G.) and 1K08CA236874 (to S.I.I.). The work of S.A. is supported by a fellowship from “la Caixa” Foundation (ID 100010434) and by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 847648. The work of L.G. is supported by American Cancer Society Clinical Scientist Development Grant 134013‐CSDG‐19‐163‐01‐TBG and NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003. A.L. has received funding from The Christie Charity and the European Union’s Horizon 2020 research and innovation programme (grant no. 82551, ESCALON). J.D.Y. has received an American College of Gastroenterology Junior Faculty Development Award and a U.S. Department of Defense Peer Reviewed Cancer Research Program Career Development Award (CA 191051).
Author information
Authors and Affiliations
Contributions
S.I.I., J.D.Y. and G.J.G. contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission. All authors contributed to researching data for the article and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.I.I. serves as an adviser and consultant to AstraZeneca. L.G. has received research funding (via her institution) from Adaptimmune, Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, Genentech, Incyte, Leap Therapeutics, Loxo Oncology, Macrogenics, Merck, Novartis, Nucana, QED Therapeutics, Relay Therapeutics, Servier and Taiho Oncology, and serves as an adviser or consultant to Alentis Therapeutics, AstraZeneca, Black Diamond, Exelixis, Genentech, H3Biomedicine, Incyte, QED Therapeutics, Servier, Sirtex Medical, Taiho Oncology and Transthera Bio. A.L. declares travel and educational support from Advanced Accelerator Applications, Bayer, Delcath, Ipsen, Mylan, Novartis, Pfizer and SirtEx; speaker’s honoraria from Advanced Accelerator Applications, AstraZeneca, Eisai, Incyte, Ipsen, Merck, Pfizer, QED Therapeutics and Servier; and advisory or consultancy roles with Albireo Pharma, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Eisai, GENFIT, Ipsen, Nutricia, QED Therapeutics, Roche, Servier and TransThera Biosciences; and is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. G.S. has received grant support from Roche, and declares consultancy roles with AstraZeneca, Chiesi, Evidera, Integra, Novartis and Roche. S.A., J.D.Y. and G.J.G. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C. Berasain, H. Francis, T. Greten, and J. Harding for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilyas, S.I., Affo, S., Goyal, L. et al. Cholangiocarcinoma — novel biological insights and therapeutic strategies. Nat Rev Clin Oncol 20, 470–486 (2023). https://doi.org/10.1038/s41571-023-00770-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00770-1