Abstract
In the past few years, international treatment guidelines for chronic myeloid leukaemia have incorporated recommendations for attempting discontinuation of treatment with tyrosine-kinase inhibitors (TKIs) outside of the setting of a clinical trial with the aim of a treatment-free remission (TFR). Physicians involved in the treatment of chronic myeloid leukaemia need to be sufficiently well informed to guide patients through decision-making about the discontinuation of treatment with TKIs targeting BCR–ABL1 by providing a balanced assessment of the potential risks and benefits of stopping or continuing therapy. These guidelines also seek to ensure that the risks associated with being off treatment are kept to a minimum. In this Review, we summarize the clinical studies of TFR and how their results can guide routine clinical practice with a focus on specific aspects such as molecular monitoring and the pregnancy-specific risks associated with a TFR attempt in female patients. We also address the development of predictors of outcome after TKI discontinuation and present strategies that warrant further consideration to enable more patients to enter TFR.
Key points
Effective treatment with tyrosine-kinase inhibitors (TKIs) leads the majority of patients with chronic myeloid leukaemia to achieve a deep molecular response after ≥5 years of treatment.
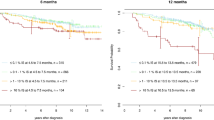
Approximately 50% of patients with a sustained deep molecular response can discontinue the TKI and remain in treatment-free remission.
The availability of sensitive, standardized quantitative reverse transcriptase PCR to detect BCR–ABL1 mRNA in peripheral blood, with rapid follow-up of results, is an essential requirement before TKI discontinuation can be offered to patients.
Loss of a major molecular response (BCR–ABL1 mRNA > 0.1%) should trigger the resumption of TKI treatment and leads to restoration of a deep molecular response in >90% of patients.
The biological and clinical factors that influence the outcome after TKI discontinuation are under investigation, with possible predictors including duration of treatment and/or response, depth of molecular response and immunological factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Deininger, M. W., Goldman, J. M. & Melo, J. V. The molecular biology of chronic myeloid leukemia. Blood 96, 3343–3356 (2000).
Branford, S. et al. Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia 33, 1835–1850 (2019).
Swerdlow, S. H. et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn (IARC, 2017).
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Saussele, S. et al. Impact of comorbidities on overall survival in patients with chronic myeloid leukemia: results of the randomized CML study IV. Blood 126, 42–49 (2015).
Hochhaus, A. et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 30, 1044–1054 (2016).
Cortes, J. E. et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J. Clin. Oncol. 34, 2333–2340 (2016).
Lipton, J. H. et al. Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 17, 612–621 (2016).
Hochhaus, A. et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N. Eng. J. Med. 376, 917–927 (2017).
Goldman, J. & Gordon, M. Why do chronic myelogenous leukemia stem cells survive allogeneic stem cell transplantation or imatinib: does it really matter? Leuk. Lymphoma 47, 1–7 (2006).
Guilhot, F. et al. French Chronic Myeloid Leukemia Study Group. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. N. Engl. J. Med. 337, 223–229 (1997).
O’Brien, S. G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Baccarani, M. et al. The proportion of different BCR-ABL1 transcript types in chronic myeloid leukemia. An international overview. Leukemia 33, 1173–1183 (2019).
Hughes, T. et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 108, 28–37 (2006).
Hughes, T. P. et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 349, 1423–1432 (2003).
Branford, S. et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin. Cancer Res. 13, 7080–7085 (2007).
Branford, S. et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia 20, 1925–1930 (2006).
Cross, N. C. et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 29, 999–1003 (2015).
Hehlmann, R. et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J. Clin. Oncol. 32, 415–423 (2014).
Rousselot, P. et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 109, 58–60 (2007).
National Comprehensive Cancer Network. Chronic Myeloid Leukemia www.nccn.org (2017).
Hochhaus, A. et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28 (Suppl. 4), iv41–iv51 (2017).
Hughes, T. P. & Ross, D. M. Moving treatment-free remission into mainstream clinical practice in CML. Blood 128, 17–23 (2016).
Mahon, F. X. et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 11, 1029–1035 (2010).
Etienne, G. et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J. Clin. Oncol. 35, 298–305 (2017).
Ross, D. M. et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 122, 515–522 (2013).
Ross, D. M. et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia 32, 2572–2579 (2018).
Nicolini, F. E. et al. Evaluation of residual disease and tki duration are critical predictive factors for molecular recurrence after stopping imatinib first-line in chronic phase CML patients. Clin. Cancer Res. 25, 6606–6613 (2019).
Fujisawa, S. et al. Feasibility of the imatinib stop study in the Japanese clinical setting: delightedly overcome CML expert stop TKI trial (DOMEST Trial). Int. J. Clin. Oncol. 24, 445–453 (2019).
Rousselot, P. et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 32, 424–430 (2014).
Lee, S. E. et al. Predictive factors for successful imatinib cessation in chronic myeloid leukemia patients treated with imatinib. Am. J. Hematol. 88, 449–454 (2013).
Mori, S. et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am. J. Hematol. 90, 910–914 (2015).
Takahashi, N. et al. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int. J. Hematol. 107, 185–193 (2018).
Saussele, S. et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 19, 747–757 (2018).
Clark, R. E. et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 4, e310–e316 (2017).
Clark, R. E. et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 6, e375–e383 (2019).
Branford, S. et al. BCR-ABL1 doubling times more reliably assess the dynamics of CML relapse compared with the BCR-ABL1 fold rise: implications for monitoring and management. Blood 119, 4264–4271 (2012).
Lee, S. E. et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica 101, 717–723 (2016).
Kimura, S. et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): a single-arm, multicentre, phase 2 trial. Lancet Haematol. 7, e218–e225 (2020).
Imagawa, J. et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2, e528–e535 (2015).
Okada, M. et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin. Lymphoma Myeloma Leuk. 18, 353–360 (2018).
Kumagai, T. et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 109, 182–192 (2018).
Shah, N. P. et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk. Lymphoma 61, 650–659 (2020).
Rea, D. et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood 129, 846–854 (2017).
Takahashi, N. et al. Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan. Haematologica 103, 1835–1842 (2018).
Mahon, F. X. et al. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann. Intern. Med. 168, 461–470 (2018).
Hochhaus, A. et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia 31, 1525–1531 (2017).
Ross, D. M. et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J. Cancer Res. Clin. Oncol. 144, 945–954 (2018).
Baccarani, M. et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 108, 1809–1820 (2006).
Baccarani, M. et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 27, 6041–6051 (2009).
Baccarani, M. et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 122, 872–884 (2013).
Radich, J. P. et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 16, 1108–1135 (2018).
Shanmuganathan, N. et al. Modeling the safe minimum frequency of molecular monitoring for CML patients attempting treatment-free remission. Blood 134, 85–89 (2019).
Pagani, I. S. et al. BCR-ABL1 genomic DNA PCR response kinetics during first-line imatinib treatment of chronic myeloid leukemia. Haematologica 103, 2026–2032 (2018).
Falchi, L. et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am. J. Hematol. 88, 1024–1029 (2013).
Goldman, J. M. et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J. Clin. Oncol. 28, 1888–1895 (2010).
Sekhri, A. et al. Very late relapse of chronic myelogenous leukemia after allogeneic bone marrow transplantation. Leuk. Res. 33, 1291–1293 (2009).
Papalexandri, A. et al. Blast crisis of CML after TKI discontinuation in a patient with previous stable deep molecular response: is it safe to stop? HemaSphere 2, e157 (2018).
Rea, D. et al. Prognostication of molecular relapses after dasatinib or nilotinib discontinuation in chronic myeloid leukemia (CML): a FI-LMC STOP 2G-TKI study update. Blood 134, 30 (2019).
Benjamini, O. et al. Patient-driven discontinuation of tyrosine kinase inhibitors: single institution experience. Leuk. Lymphoma 55, 2879–2886 (2014).
Richter, J. et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J. Clin. Oncol. 32, 2821–2823 (2014).
Shah, N. P. et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk. Lymphoma 61, 650–659 (2020).
Berger, M. G. et al. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. Br. J. Haematol. 187, 337–346 (2019).
Katagiri, S. et al. Musculoskeletal pain after stopping tyrosine kinase inhibitor in patients with chronic myeloid leukemia: a questionnaire survey. Rinsho Ketsueki 57, 873–876 (2016).
Villemagne Sanchez, L. A. et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk. Lymphoma 59, 406–415 (2018).
Breccia, M. et al. Adherence and future discontinuation of tyrosine kinase inhibitors in chronic phase chronic myeloid leukemia. A patient-based survey on 1133 patients. Leuk. Res. 39, 1055–1059 (2015).
Jiang, Q., Liu, Z. C., Zhang, S. X. & Gale, R. P. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J. Cancer Res. Clin. Oncol. 142, 1539–1547 (2016).
Barnes, D. J. et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 65, 8912–8919 (2005).
Legros, L. et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer 123, 4403–4410 (2017).
Pye, S. M. et al. The effects of imatinib on pregnancy outcome. Blood 111, 5505–5508 (2008).
Cortes, J. E. et al. The impact of dasatinib on pregnancy outcomes. Am. J. Hematol. 90, 1111–1115 (2015).
Berman, E., Druker, B. J. & Burwick, R. Chronic myelogenous leukemia: pregnancy in the era of stopping tyrosine kinase inhibitor therapy. J. Clin. Oncol. 36, 1250–1256 (2018).
Ross, D. M., Burbury, K. L., Grigg, A. P., Hughes, T. P. & Seymour, J. F. Management of pregnancy in women with chronic myeloid leukemia. J. Clin. Oncol. 36, 2657–2658 (2018).
Claudiani, S. et al. E14a2 BCR-ABL1 transcript is associated with a higher rate of treatment-free remission in individuals with chronic myeloid leukemia after stopping tyrosine kinase inhibitor therapy. Haematologica 102, e297–e299 (2017).
Sokal, J. E. et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 63, 789–799 (1984).
Pfirrmann, M. et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 30, 48–56 (2016).
D’Adda, M. et al. The e13a2 BCR-ABL transcript negatively affects sustained deep molecular response and the achievement of treatment-free remission in patients with chronic myeloid leukemia who receive tyrosine kinase inhibitors. Cancer 125, 1674–1682 (2019).
Ross, D. M. et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia 24, 1719–1724 (2010).
Score, J. et al. Analysis of genomic breakpoints in p190 and p210 BCR-ABL indicate distinct mechanisms of formation. Leukemia 24, 1742–1750 (2010).
Ohyashiki, K. et al. Increased natural killer cells and decreased CD3+CD8+CD62L+ T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br. J. Haematol. 157, 254–256 (2012).
Ilander, M. et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 31, 1108–1116 (2017).
Ross, D. M. et al. Lenalidomide maintenance treatment after imatinib discontinuation: results of a phase 1 clinical trial in chronic myeloid leukaemia. Br. J. Haematol. 186, e56–e60 (2019).
Schutz, C. et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 32, 1054 (2018).
Pagani, I. S. et al. BCR-ABL1 genomic DNA PCR response kinetics during first-line imatinib treatment of chronic myeloid leukemia. Haematologica 103, 2026–2032 (2018).
Bocchia, M. et al. Residual peripheral blood CD26+ leukemic stem cells in chronic myeloid leukemia patients during TKI therapy and during treatment-free remission. Front. Oncol. 8, 194 (2018).
Chomel, J. C. et al. Leukemic stem cell persistence in chronic myeloid leukemia patients in deep molecular response induced by tyrosine kinase inhibitors and the impact of therapy discontinuation. Oncotarget 7, 35293–35301 (2016).
Houshmand, M. et al. Chronic myeloid leukemia stem cells. Leukemia 33, 1543–1556 (2019).
Ross, D. M., Hughes, T. P. & Melo, J. V. Do we have to kill the last CML cell? Leukemia 25, 193–200 (2011).
Cheah, C. Y. et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 123, 3574–3577 (2014).
Legrand, F. et al. The spectrum of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia: new insights based on a survey of 44 cases. Medicine 92, e1–e9 (2013).
Cerrano, M. et al. Long-term therapy-free remission in a patient with platelet-derived growth factor receptor beta-rearranged myeloproliferative neoplasm. Am. J. Hematol. 91, E353 (2016).
Helbig, G., Soja, A., Swiderska, A., Hus, M. & Kyrcz-Krzemien, S. Imatinib discontinuation for hypereosinophilic syndrome harboring the FIP1L1-PDGFRA transcript. Leuk. Lymphoma 57, 708–710 (2016).
Bidet, A. et al. Molecular monitoring of patients with ETV6-PDGFRB rearrangement: Implications for therapeutic adaptation. Br. J. Haematol. 182, 148–152 (2018).
McMahon, C. M. et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discov. 9, 1050–1063 (2019).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Jansen, Y. J. L. et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann. Oncol. 30, 1154–1161 (2019).
Tan, A. C. et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann. Oncol. 29, 2115–2120 (2018).
Seremet, T. et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J. Transl Med. 17, 303 (2019).
Dahlen, T. et al. Cardiovascular events associated with use of tyrosine kinase inhibitors in chronic myeloid leukemia: a population-based cohort study. Ann. Intern. Med. 165, 161–166 (2016).
Yilmaz, M. et al. Estimated glomerular filtration rate changes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. Cancer 121, 3894–3904 (2015).
Shih, Y. T., Cortes, J. E. & Kantarjian, H. M. Treatment value of second-generation BCR-ABL1 tyrosine kinase inhibitors compared with imatinib to achieve treatment-free remission in patients with chronic myeloid leukaemia: a modelling study. Lancet Haematol. 6, e398–e408 (2019).
Loeliger, E. A., van den Besselaar, A. M. & Lewis, S. M. Reliability and clinical impact of the normalization of the prothrombin times in oral anticoagulant control. Thromb. Haemost. 53, 148–154 (1985).
Acknowledgements
The authors receive financial support from the South Australian Cancer Council’s Beat Cancer Project on behalf of its donors and the State Government through the Department of Health. The authors are grateful to the many patients with chronic myeloid leukaemia who have participated in clinical studies and especially to the pioneers who participated in the earliest treatment-free remission studies. We also thank the many international colleagues whose research is cited here, and the scientific and clinical colleagues in Australia who have been instrumental to our own studies.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
D.M.R. receives research funding and honoraria from BMS and Novartis. T.P.H. receives research funding and honoraria from BMS and Novartis and is a member of the advisory board for BMS, Incyte and Novartis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ross, D.M., Hughes, T.P. Treatment-free remission in patients with chronic myeloid leukaemia. Nat Rev Clin Oncol 17, 493–503 (2020). https://doi.org/10.1038/s41571-020-0367-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0367-1
This article is cited by
-
Computational modeling reveals key factors driving treatment-free remission in chronic myeloid leukemia patients
npj Systems Biology and Applications (2024)
-
Cardiovascular Adverse Events of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: Clinical Relevance, Impact on Outcome, Preventive Measures and Treatment Strategies
Current Treatment Options in Oncology (2023)
-
Mitochondrial Dysfunction in Cardiotoxicity Induced by BCR-ABL1 Tyrosine Kinase Inhibitors -Underlying Mechanisms, Detection, Potential Therapies
Cardiovascular Toxicology (2023)
-
Identification of key microRNAs as predictive biomarkers of Nilotinib response in chronic myeloid leukemia: a sub-analysis of the ENESTxtnd clinical trial
Leukemia (2022)
-
Therapy Resistance and Disease Progression in CML: Mechanistic Links and Therapeutic Strategies
Current Hematologic Malignancy Reports (2022)