Abstract
The global burden of hepatocellular carcinoma (HCC) is increasing and might soon surpass an annual incidence of 1 million cases. Genomic studies have established the landscape of molecular alterations in HCC; however, the most common mutations are not actionable, and only ~25% of tumours harbour potentially targetable drivers. Despite the fact that surveillance programmes lead to early diagnosis in 40–50% of patients, at a point when potentially curative treatments are applicable, almost half of all patients with HCC ultimately receive systemic therapies. Sorafenib was the first systemic therapy approved for patients with advanced-stage HCC, after a landmark study revealed an improvement in median overall survival from 8 to 11 months. New drugs — lenvatinib in the frontline and regorafenib, cabozantinib, and ramucirumab in the second line — have also been demonstrated to improve clinical outcomes, although the median overall survival remains ~1 year; thus, therapeutic breakthroughs are still needed. Immune-checkpoint inhibitors are now being incorporated into the HCC treatment armamentarium and combinations of molecularly targeted therapies with immunotherapies are emerging as tools to boost the immune response. Research on biomarkers of a response or primary resistance to immunotherapies is also advancing. Herein, we summarize the molecular targets and therapies for the management of HCC and discuss the advancements expected in the near future, including biomarker-driven treatments and immunotherapies.
Key points
-
The global incidence of hepatocellular carcinoma (HCC) is increasing and might reach 1 million cases per year during the next decade.
-
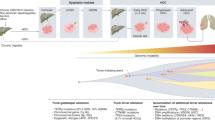
Next-generation sequencing studies have established the landscape of molecular aberrations associated with HCC; although the most common mutations (in the TERT promoter, CTNNB1, and TP53) are not clinically actionable, ~25% of HCCs harbour potentially targetable driver alterations.
-
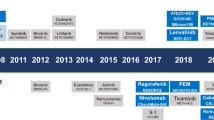
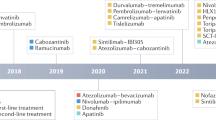
In phase III studies, survival benefits for patients with advanced-stage HCC have been demonstrated with five systemic therapies: sorafenib and lenvatinib in the first-line setting and regorafenib, cabozantinib, and ramucirumab in the second-line setting. Promising results have also been obtained with nivolumab in phase II studies in the second-line setting.
-
Prolonging the outcome of patients with advanced-stage HCC to beyond 1 year is an unmet medical need; refining the identification of patients with tumours responsive or intrinsically resistant to immunotherapy and optimizing combinations with molecularly targeted therapies are major avenues for research.
-
Proof-of-concept and biomarker-based trials of molecularly targeted agents should be implemented in both intermediate-stage and advanced-stage disease settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Figure adapted with permission from ref.4, Elsevier.



Similar content being viewed by others
References
Torre, L. A. et al. Global cancer statistics, 2012. CA. Cancer J. Clin. 65, 87–108 (2015).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2, 16018 (2016).
Zucman-Rossi, J., Villanueva, A., Nault, J. C. & Llovet, J. M. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239 (2015).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J. Hepatol. https://doi.org/10.1016/j.jhep.2018.03.019 (2018).
Bruix, J., S. M. A. A. for the S. of L. D. Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022 (2011).
Llovet, J. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Kudo, M. et al. A randomised phase 3 trial of lenvatinib versus sorafenib in firstline treatment of patients with unresectable hepatocellular carcinoma. Lancet 391, 1163–1173 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G.K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. REACH-2: A randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafe [abstract]. J. Clin. Oncol. 36, 4003 (2018).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Llovet, J. M. & Hernandez-Gea, V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res. 20, 2072–2079 (2014).
Sangiovanni, A. et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 126, 1005–1014 (2004).
Nault, J.-C. et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology 152, 880–894.e6 (2017).
Sia, D., Villanueva, A., Friedman, S. L. & Llovet, J. M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152, 745–761 (2016).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Charles Nault, J. et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 4, 2218 (2013).
Wheeler, D. A. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341 (2017).
Ahn, S.-M. et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 60, 1972–1982 (2014).
Totoki, Y. et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat. Genet. 46, 1267–1273 (2014).
Chiang, D. Y. et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 68, 6779–6788 (2008).
Martinez-Quetglas, I. et al. IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology 151, 1192–1205 (2016).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Boyault, S. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007).
Lee, J.-S. et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat. Med. 12, 410–416 (2006).
Toffanin, S. et al. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology 140, 1618–1628 (2011).
Llovet, J. M., Villanueva, A., Lachenmayer, A. & Finn, R. S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 12, 408–424 (2015).
Hoshida, Y. et al. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin. Liver Dis. 30, 35–51 (2010).
Wang, K. et al. Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology 58, 706–717 (2013).
Villanueva, A. et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology 61, 1945–1956 (2015).
Lachenmayer, A. et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin. Cancer Res. 18, 4997–5007 (2012).
Hoshida, Y. et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N. Engl. J. Med. 359, 1995–2004 (2008).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Weis, S. M. & Cheresh, D. A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 17, 1359–1370 (2011).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Yau, T. et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 146, 1691–1700.e3 (2014).
[No authors listed]. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 28, 751–755 (1998).
Sobin, L. H. & Compton, C. C. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 116, 5336–5339 (2010).
Kudo, M., Chung, H. & Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 38, 207–215 (2003).
Llovet, J. M., Brú, C. & Bruix, J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 19, 329–338 (1999).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. Oncol. 16, 1344–1354 (2015).
Llovet, J. M. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359, 1734–1739 (2002).
Lo, C.-M. et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35, 1164–1171 (2002).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37, 429–442 (2003).
Lencioni, R. et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J. Hepatol. 64, 1090–1098 (2016).
Meyer, T. et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2, 565–575 (2017).
Kudo, M. et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 60, 1697–1707 (2014).
Qin, S. et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 31, 3501–3508 (2013).
Abou-Alfa, G. K. et al. Doxorubicin plus sorafenib versus doxorubicin alone in patients with advanced hepatocellular carcinoma. JAMA 304, 2154 (2010).
Yeo, W. et al. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J. Natl Cancer Inst. 97, 1532–1538 (2005).
Chow, P. et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: a multicenter randomized controlled trial. Hepatology 36, 1221–1226 (2002).
Dalhoff, K. et al. A phase II study of the vitamin D analogue Seocalcitol in patients with inoperable hepatocellular carcinoma. Br. J. Cancer 89, 252–257 (2003).
Cheng, A.-L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34 (2009).
Reig, M. et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 61, 318–324 (2014).
Bruix, J. et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase 3 studies. J. Hepatol. 67, 999–1008 (2017).
Iavarone, M. et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 54, 2055–2063 (2011).
Ganten, T. M. et al. Sorafenib in patients with hepatocellular carcinoma—results of the Observational INSIGHT Study. Clin. Cancer Res. 23, 5720–5728 (2017).
Marrero, J. A. et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J. Hepatol. 65, 1140–1147 (2016).
Kudo, M. et al. Regional differences in sorafenib-treated patients with hepatocellular carcinoma: GIDEON observational study. Liver Int. 36, 1196–1205 (2016).
Wilhelm, S. M. et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther. 7, 3129–3140 (2008).
Llovet, J. M. et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 18, 2290–2300 (2012).
Pinyol, R. et al. Molecular predictors of recurrence prevention with sorafenib as adjuvant therapy in hepatocellular carcinoma: biomarker study of the STORM phase III trial. J. Hepatol. 66, S12–S13 (2017).
Llovet, J. M. et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl Cancer Inst. 100, 698–711 (2008).
Lencioni, R. et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 66, 1166–1172 (2017).
Montal, R., Lencioni, R. & Llovet, J. M. Reply to: mRECIST for systemic therapies: more evidence is required before recommendations could be made. J. Hepatol. 67, 196–197 (2017).
Johnson, P. J. et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 31, 3517–3524 (2013).
Cheng, A.-L. et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J. Clin. Oncol. 31, 4067–4075 (2013).
Cainap, C. et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 33, 172–179 (2015).
Zhu, aX. et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 33, 559–566 (2014).
Vilgrain, V. et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 18, 1624–1636 (2017).
Chow, P. K. H. et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J. Clin. Oncol. 36, 1913–1921 (2018).
Ricke, J. et al. The impact of combining Selective Internal Radiation Therapy (SIRT) with sorafenib on overall survival in patients with advanced hepatocellular carcinoma: the SORAMIC trial palliative cohort. J. Hepatol. 68, S102 (2018).
Matsui, J. et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin. Cancer Res. 14, 5459–5465 (2008).
Ikeda, K. et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 52, 512–519 (2017).
Zhu, A. X. et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 312, 57–67 (2014).
Zhu, A. X. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 16, 859–870 (2015).
Llovet, J. M. et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J. Clin. Oncol. 31, 3509–3516 (2013).
Rimassa, L. et al. Second-line tivantinib (ARQ 197) versus placebo in patients (Pts) with MET-high hepatocellular carcinoma (HCC): results of the METIV-HCC phase III trial. J. Clin. Oncol. 35 (Suppl. 15), 4000 (2017).
Zhu, A. X. et al. KEYNOTE-224: Phase II study of pembrolizumab in patients with previously treated advanced hepatocellular carcinoma. J. Clin. Oncol. 35 (Suppl. 4), TPS504 (2017).
Wilhelm, S. M. et al. Regorafenib (BAY 73–4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 129, 245–255 (2011).
Bruix, J. et al. Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: Multicentre, open-label, phase II safety study. Eur. J. Cancer 49, 3412–3419 (2013).
Finn, R. S. et al. Outcomes with sorafenib followed by regorafenib or placebo for HCC: additional analyses from the phase 3 RESORCE trial. J. Hepatol. https://doi.org/10.1016/j.jhep.2018.04.010 (2018).
Yakes, F. M. et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 10, 2298–2308 (2011).
Kelley, R. K. et al. Cabozantinib in hepatocellular carcinoma: Results of a phase 2 placebo-controlled randomized discontinuation study. Ann. Oncol. 28, 528–534 (2017).
Goyal, L., Muzumdar, M. D. & Zhu, A. X. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin. Cancer Res. 19, 2310–2318 (2013).
Zhu, A. X. et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin. Cancer Res. 19, 6614–6623 (2013).
Terentiev, A. A. & Moldogazieva, N. T. Alpha-fetoprotein: a renaissance. Tumor Biol. 34, 2075–2091 (2013).
Shan, Y. F. et al. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med. Oncol. 28, 1012–1016 (2011).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 451–461 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Boutros, C. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 13, 473–486 (2016).
Ribas, A. Releasing the brakes on cancer immunotherapy. N. Engl. J. Med. 373, 1490–1492 (2015).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Iñarrairaegui, M., Melero, I. & Sangro, B. Immunotherapy of hepatocellular carcinoma: facts and hopes. Clin. Cancer Res. 24, 1518–1524 (2017).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013).
El-Khoueiry, A. B. et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): subanalyses of CheckMate-040. J. Clin. Oncol. 36 (Suppl. 4), 475 (2018).
US Food & Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib (FDA, 2017).
Zhu, A. X. et al. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J. Clin. Oncol. 36 (Suppl. 4), 209 (2018).
Finn, R. S. et al. KEYNOTE-240: Randomized phase III study of pembrolizumab versus best supportive care for second-line advanced hepatocellular carcinoma. J. Clin. Oncol. 35 (Suppl. 4), TPS503 (2017).
Wainberg, Z. A. et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 35 (Suppl. 4), 4071 (2017).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–129 (2016).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Crocenzi, T. S. et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J. Clin. Oncol. 35 (Suppl. 15), 4013 (2017).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Kelley, R. K. et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J. Clin. Oncol. 35 (Suppl. 15), 4073 (2017).
Lee, C.-H. et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with renal cell carcinoma. Ann. Oncol. 28 (Suppl. 5), 295–329 (2017).
Genentech. FDA grants breakthrough therapy designation for Genentech’s TECENTRIQ in combination with avastin as first-line treatment for advanced or metastatic hepatocellular carcinoma (HCC). Genentech https://www.gene.com/media/press-releases/14736/2018-07-17/fda-grants-breakthrough-therapy-designat?utm_source=F&utm_medium=P&utm_term=15538&utm_content=TecentriqHCCBTD&utm_campaign=TecentriqHCCBTD (2018).
Collins, D. C., Sundar, R., Lim, J. S. J. & Yap, T. A. Towards precision medicine in the clinic: from biomarker discovery to novel therapeutics. Trends Pharmacol. Sci. 38, 25–40 (2017).
Santoro, A. et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 14, 55–63 (2013).
Basilico, C. et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin. Cancer Res. 19, 2381–2392 (2013).
Babina, I. S. & Turner, N. C. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 17, 318–332 (2017).
Javle, M. et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J. Clin. Oncol. 36, 276–282 (2017).
Konecny, G. E. et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 16, 686–694 (2015).
Jeong Lee, H. et al. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 21, 60–70 (2015).
Wu, X. et al. FGF19-induced hepatocyte proliferation is mediated through FGFR4 activation. J. Biol. Chem. 285, 5165–5170 (2010).
Gao, L. et al. FGF19/FGFR4 signaling contributes to the resistance of hepatocellular carcinoma to sorafenib. J. Exp. Clin. Cancer Res. 36, 1–10 (2017).
Sawey, E. T. et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell 19, 347–358 (2011).
Finn, R. S. et al. Gains in FGF19 are predictive of response to the fibroblast growth factor receptor (FGFR) small molecule tyrosine kinase inhibitor BGJ 398 in vitro [abstract 3858]. Cancer Res. 72 (Suppl. 8), 3858 (2012).
Guagnano, V. et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective Pan-FGFR inhibitor. Cancer Discov. 2, 1118–1133 (2012).
Hagel, M. et al. First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov. 5, 424–437 (2015).
Joshi, J. J. et al. H3B-6527is a potent and selective inhibitor of FGFR4 in FGF19-driven hepatocellular carcinoma. Cancer Res. 77, 6999–7013 (2017).
Harris, J. BLU-554 associated with improved response in HCC. OncLive https://www.Onclive.com/Conference-Coverage/Ilca-2017/blu554-Associated-With-Improved-Response-in-hcc (2017).
Matter, M. S., Decaens, T., Andersen, J. B. & Thorgeirsson, S. S. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J. Hepatol. 60, 855–865 (2014).
Villanueva, A. et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 135, 1972–1983 (2008).
Janku, F., Yap, T. A. & Meric-Bernstam, F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291 (2018).
Lim, H. Y. et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86–9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 20, 5976–5985 (2014).
Lim, H. Y. et al. Phase II studies with refametinib or refametinib plus sorafenib in patients with ras-mutated hepatocellular carcinoma. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-3588 (2018).
Sia, D. & Llovet, J. M. Liver cancer: Translating ‘–omics’ results into precision medicine for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 571–572 (2017).
de Gramont, A. et al. Pragmatic issues in biomarker evaluation for targeted therapies in cancer. Nat. Rev. Clin. Oncol. 12, 197–212 (2014).
Siravegna, G., Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 14, 531–548 (2017).
Xu, R. et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 16, 1155–1162 (2017).
Mullard, A. Reining in the supersized Phase I cancer trial. Nat. Rev. Drug Discov. 15, 371–373 (2016).
Hyman, D. M., Taylor, B. S. & Baselga, J. Implementing genome-driven oncology. Cell 168, 584–599 (2017).
Torrecilla, S. et al. Trunk mutational events present minimal intra- and inter-tumoral heterogeneity in hepatocellular carcinoma. J. Hepatol. 67, 1222–1231 (2017).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Weber, J. S., Yang, J. C., Atkins, M. B. & Disis, M. L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 33, 2092–2099 (2015).
Masucci, G. V. et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I — pre-analytical and analytical validation. J. Immunother. Cancer 4, 1–25 (2016).
Poh, A. First tissue-agnostic drug approval issued. Cancer Discov. 7, 656 (2017).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Acknowledgements
The work of J.M.L. is supported by grants from the European Commission Horizon 2020 programme (HEPCAR, proposal number 667273–2), the US Department of Defense (CA150272P3), the US National Cancer Institute (P30 CA196521), the Samuel Waxman Cancer Research Foundation, the Spanish National Health Institute (SAF 2016–76390), Asociación Española Contra el Cáncer (AECC), and the Generalitat de Catalunya (AGAUR, SGR-1162 and SGR-1358). The work of D.S. is supported by the Gilead Sciences Research Scholar Program in Liver Disease.
Reviewer information
Nature Reviews Clinical Oncology thanks G. Gores, J.-L. Raoul, and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to each stage of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
J.M.L. is a consultant to Bayer HealthCare, Bristol-Myers Squibb (BMS), Celsion, Eisai, Eli Lilly, Exelixis, and Ipsen and has active research funding from Bayer HealthCare, BMS, and Eisai. R.S.F. is a consultant to Bayer HealthCare, BMS, Eisai, Eli Lilly, Merck, Pfizer, and Roche. R.M. and D.S. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Llovet, J.M., Montal, R., Sia, D. et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 15, 599–616 (2018). https://doi.org/10.1038/s41571-018-0073-4
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0073-4
This article is cited by
-
Platycodin D2 enhances P21/CyclinA2-mediated senescence of HCC cells by regulating NIX-induced mitophagy
Cancer Cell International (2024)
-
Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma
Biomarker Research (2024)
-
AMPKα2 promotes tumor immune escape by inducing CD8+ T-cell exhaustion and CD4+ Treg cell formation in liver hepatocellular carcinoma
BMC Cancer (2024)
-
PDCL3 is a prognostic biomarker associated with immune infiltration in hepatocellular carcinoma
European Journal of Medical Research (2024)
-
Arvanil induces ferroptosis of hepatocellular carcinoma by binding to MICU1
Cancer Gene Therapy (2024)