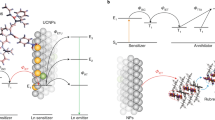
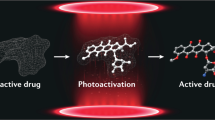
Abstract
Photon upconversion is a method for harnessing high-energy excited states from low-energy photons. Such photons, particularly in the red and near-infrared wavelength ranges, can penetrate tissue deeply and undergo less competitive absorption in coloured reaction media, enhancing the efficiency of large-scale reactions and in vivo phototherapy. Among various upconversion methodologies, the organic-based triplet–triplet annihilation upconversion (TTA-UC) stands out — demonstrating high upconversion efficiencies, requiring low excitation power densities and featuring tunable absorption and emission wavelengths. These factors contribute to improved photochemical reactions for fields such as photoredox catalysis, photoactivation, 3D printing and immunotherapy. In this Review, we explore concepts and design principles of organic TTA-UC-mediated photochemical reactions, highlighting notable advancements in the field, as well as identify challenges and propose potential solutions. This Review sheds light on the potential of organic TTA-UC to advance beyond the traditional photochemical reactions and paves the way for research in various fields and clinical applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ravetz, B. D. et al. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 565, 343–346 (2019). This letter presents the first demonstrations of near-infrared light-driven photoredox catalysis of multiple reaction types via triplet–triplet annihilation upconversion.
Tao, W. & Farokhzad, O. C. Theranostic nanomedicine in the NIR-II window: classification, fabrication, and biomedical applications. Chem. Rev. 122, 5405–5407 (2022).
Chen, Y., Wang, S. & Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Biomed. Eng. 1, 60–78 (2023).
Ravetz, B. D. et al. Development of a platform for near-infrared photoredox catalysis. ACS Cent. Sci. 6, 2053–2059 (2020).
Cabanero, D. C., Nguyen, J. A., Cazin, C. S. J., Nolan, S. P. & Rovis, T. Deep red to near-infrared light-controlled ruthenium-catalyzed olefin metathesis. ACS Catal. 13, 4384–4390 (2023).
Wang, Y. C. et al. Recent progress in near-infrared light-harvesting nanosystems for photocatalytic applications. Appl. Catal. A Gen. 644, 118836 (2022).
Glaser, F., Kerzig, C. & Wenger, O. S. Multi-photon excitation in photoredox catalysis: concepts, applications, methods. Angew. Chem. Int. Ed. 59, 10266–10284 (2020).
Richards, B. S., Hudry, D., Busko, D., Turshatov, A. & Howard, I. A. Photon upconversion for photovoltaics and photocatalysis: a critical review. Chem. Rev. 121, 9165–9195 (2021).
Ghosh, I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 346, 725–728 (2014).
Cybularczyk-Cecotka, M., Szczepanik, J. & Giedyk, M. Photocatalytic strategies for the activation of organic chlorides. Nat. Catal. 3, 872–886 (2020).
Kim, H. M. & Cho, B. R. Small-molecule two-photon probes for bioimaging applications. Chem. Rev. 115, 5014–5055 (2015).
Bando, Y., Wenzel, M. & Yuste, R. Simultaneous two-photon imaging of action potentials and subthreshold inputs in vivo. Nat. Commun. 12, 7229 (2021).
Lederhose, P. et al. Near-infrared photoinduced coupling reactions assisted by upconversion nanoparticles. Angew. Chem. Int. Ed. 55, 12195–12199 (2016).
Rocheva, V. V. et al. High-resolution 3D photopolymerization assisted by upconversion nanoparticles for rapid prototyping applications. Sci. Rep. 8, 3663 (2018).
He, L. et al. Near-infrared photoactivatable control of Ca2+ signaling and optogenetic immunomodulation. eLife 4, e10024 (2015).
Chen, S. et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 359, 679–684 (2018).
Nguyen, N. T. et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 16, 1424–1434 (2021).
Jeong, J. et al. An implantable optogenetic stimulator wirelessly powered by flexible photovoltaics with near-infrared (NIR) light. Biosens. Bioelectron. 180, 113139 (2021).
Liu, X., Yan, C. H. & Capobianco, J. A. Photon upconversion nanomaterials. Chem. Soc. Rev. 44, 1299–1301 (2015).
Dong, H. et al. Lanthanide nanoparticles: from design toward bioimaging and therapy. Chem. Rev. 115, 10725–10815 (2015).
Chen, G., Qiu, H., Prasad, P. N. & Chen, X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 114, 5161–5214 (2014).
Zeng, L., Huang, L., Han, J. & Han, G. Enhancing triplet–triplet annihilation upconversion: from molecular design to present applications. Acc. Chem. Res. 55, 2604–2615 (2022). This review reveals and summarizes the design rules of efficient triplet–triplet annihilation upconversion and its applications in emerging fields.
Bharmoria, P., Bildirir, H. & Moth-Poulsen, K. Triplet–triplet annihilation based near infrared to visible molecular photon upconversion. Chem. Soc. Rev. 49, 6529–6554 (2020). This review systematically summarizes current state of the near-infrared triplet–triplet annihilation upconversion system as well as its challenges.
Gao, C. et al. Application of triplet–triplet annihilation upconversion in organic optoelectronic devices: advances and perspectives. Adv. Mater. 33, 2100704 (2021).
Singh-Rachford, T. N. & Castellano, F. N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 254, 2560–2573 (2010).
Zhao, J., Ji, S. & Guo, H. Triplet–triplet annihilation based upconversion: from triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 1, 937–950 (2011).
Zhou, J., Liu, Q., Feng, W., Sun, Y. & Li, F. Upconversion luminescent materials: advances and applications. Chem. Rev. 115, 395–465 (2015).
Wu, Y., Li, S., Chen, Y., He, W. & Guo, Z. Recent advances in noble metal complex based photodynamic therapy. Chem. Sci. 13, 5085–5106 (2022).
Hu, W., Zhang, R., Zhang, X.-F., Liu, J. & Luo, L. Halogenated BODIPY photosensitizers: photophysical processes for generation of excited triplet state, excited singlet state and singlet oxygen. Spectrochim. Acta A Mol. Biomol. 272, 120965 (2022).
Niihori, Y., Wada, Y. & Mitsui, M. Single platinum atom doping to silver clusters enables near-infrared-to-blue photon upconversion. Angew. Chem. Int. Ed. 60, 2822–2827 (2021). This is the first example of a triplet–triplet annihilation upconversion system using noble metal clusters as photosensitizers.
Arima, D. & Mitsui, M. Structurally flexible Au–Cu alloy nanoclusters enabling efficient triplet sensitization and photon upconversion. J. Am. Chem. Soc. 145, 6994–7004 (2023).
McCusker, C. E. & Castellano, F. N. Efficient visible to near-UV photochemical upconversion sensitized by a long lifetime Cu(I) MLCT complex. Inorg. Chem. 54, 6035–6042 (2015).
Kübler, J. A., Pfund, B. & Wenger, O. S. Zinc(II) complexes with triplet charge-transfer excited states enabling energy-transfer catalysis, photoinduced electron transfer, and upconversion. JACS Au 2, 2367–2380 (2022).
Yang, M., Sheykhi, S., Zhang, Y., Milsmann, C. & Castellano, F. N. Low power threshold photochemical upconversion using a zirconium(iv) LMCT photosensitizer. Chem. Sci. 12, 9069–9077 (2021).
Wang, C. et al. Efficient triplet–triplet annihilation upconversion sensitized by a chromium(III) complex via an underexplored energy transfer mechanism. Angew. Chem. Int. Ed. 61, e202202238 (2022).
Olesund, A. et al. Approaching the spin-statistical limit in visible-to-ultraviolet photon upconversion. J. Am. Chem. Soc. 144, 3706–3716 (2022).
Cao, X. et al. Manipulating exciton dynamics toward simultaneous high-efficiency narrowband electroluminescence and photon upconversion by a selenium-incorporated multiresonance delayed fluorescence emitter. J. Am. Chem. Soc. 144, 22976–22984 (2022).
Li, J.-K., Zhang, M.-Y., Zeng, L., Huang, L. & Wang, X.-Y. NIR-absorbing B,N-heteroarene as photosensitizer for high-performance NIR-to-blue triplet–triplet annihilation upconversion. Angew. Chem. Int. Ed. 62, e202303093 (2023). This is the first example of a triplet–triplet annihilation upconversion system with a thermally activated delayed fluorescent molecule as a near-infrared light-absorbing photosensitizer.
Huang, L. et al. Highly effective near-infrared activating triplet–triplet annihilation upconversion for photoredox catalysis. J. Am. Chem. Soc. 142, 18460–18470 (2020).
Fallon, K. J. et al. Molecular engineering of chromophores to enable triplet–triplet annihilation upconversion. J. Am. Chem. Soc. 142, 19917–19925 (2020).
Pun, A. B., Campos, L. M. & Congreve, D. N. Tunable emission from triplet fusion upconversion in diketopyrrolopyrroles. J. Am. Chem. Soc. 141, 3777–3781 (2019).
Sanders, S. N. et al. Triplet fusion upconversion nanocapsules for volumetric 3D printing. Nature 604, 474–478 (2022). This article demonstrates the realization of nonlinear volumetric 3D printing with continuous light excitation using triplet–triplet annihilation upconversion nanocapsules as phototransducers.
Huang, L. et al. Long wavelength single photon like driven photolysis via triplet triplet annihilation. Nat. Commun. 12, 122 (2021). This article describes the excitation wavelength expansion of the photocleavage reaction via the triplet–triplet annihilation mechanism and the in vivo implementation of prodrug photocleavage for tumour treatment.
Huang, L. et al. Expanding anti-stokes shifting in triplet–triplet annihilation upconversion for in vivo anticancer prodrug activation. Angew. Chem. Int. Ed. 56, 14400–14404 (2017).
Zeng, L. et al. Metal-free far-red light-driven photolysis via triplet fusion to enhance checkpoint blockade immunotherapy. Angew. Chem. Int. Ed. 62, e202218341 (2023).
Awwad, N., Bui, A. T., Danilov, E. O. & Castellano, F. N. Visible-light-initiated free-radical polymerization by homomolecular triplet–triplet annihilation. Chem 6, 3071–3085 (2020). This article exhibits a single-component TTA-UC-mediated photopolymerization with zinc porphyrin and its application to surface micropattern processing.
Yoon, T. P., Ischay, M. A. & Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2, 527–532 (2010).
Theriot, J. C. et al. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352, 1082–1086 (2016).
Cambié, D., Bottecchia, C., Straathof, N. J. W., Hessel, V. & Noël, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 116, 10276–10341 (2016).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Tucker, J. W. & Stephenson, C. R. J. Shining light on photoredox catalysis: theory and synthetic applications. J. Org. Chem. 77, 1617–1622 (2012).
Singla, D., Luxami, V. & Paul, K. Eosin Y mediated photo-catalytic C–H functionalization: C–C and C–S bond formation. Org. Chem. Front. 10, 237–266 (2023).
Uji, M., Zähringer, T. J. B., Kerzig, C. & Yanai, N. Visible-to-UV photon upconversion: recent progress in new materials and applications. Angew. Chem. Int. Ed. 62, e202301506 (2023).
Mondal, P., Schwinn, K. & Huix-Rotllant, M. Impact of the redox state of flavin chromophores on the UV–Vis spectra, redox and acidity constants and electron affinities. J. Photochem. Photobiol. 387, 112164 (2020).
Losi, A. Flavin-based blue-light photosensors: a photobiophysics update. Photochem. Photobiol. 83, 1283–1300 (2007).
Hwang, S. Y. et al. Triplet–triplet annihilation-based photon-upconversion to broaden the wavelength spectrum for photobiocatalysis. Sci. Rep. 12, 9397 (2022).
Bilger, J. B., Kerzig, C., Larsen, C. B. & Wenger, O. S. A photorobust Mo(0) complex mimicking [Os(2,2′-bipyridine)3]2+ and its application in red-to-blue upconversion. J. Am. Chem. Soc. 143, 1651–1663 (2021).
Lechner, V. M. et al. Visible-light-mediated modification and manipulation of biomacromolecules. Chem. Rev. 122, 1752–1829 (2022).
O’Hagan, M. P. et al. Photocleavable ortho-nitrobenzyl-protected DNA architectures and their applications. Chem. Rev. 123, 6839–6887 (2023).
Klán, P. et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 113, 119–191 (2013).
Liu, L., Zhang, D., Johnson, M. & Devaraj, N. K. Light-activated tetrazines enable precision live-cell bioorthogonal chemistry. Nat. Chem. 14, 1078–1085 (2022).
Glickman, R. D. Ultraviolet phototoxicity to the retina. Eye Contact Lens 37, 196–205 (2011).
Vickerman, B. M., Zywot, E. M., Tarrant, T. K. & Lawrence, D. S. Taking phototherapeutics from concept to clinical launch. Nat. Rev. Chem. 5, 816–834 (2021).
Wang, W. et al. Efficient triplet–triplet annihilation-based upconversion for nanoparticle phototargeting. Nano Lett. 15, 6332–6338 (2015).
Emiliani, V. et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Prim. 2, 55 (2022).
Yanai, N. & Kimizuka, N. New triplet sensitization routes for photon upconversion: thermally activated delayed fluorescence molecules, inorganic nanocrystals, and singlet-to-triplet absorption. Acc. Chem. Res. 50, 2487–2495 (2017).
Sasaki, Y. et al. Near-infrared optogenetic genome engineering based on photon-upconversion hydrogels. Angew. Chem. Int. Ed. 58, 17827–17833 (2019).
Meir, R. et al. Photon upconversion hydrogels for 3d optogenetics. Adv. Funct. Mater. 31, 2010907 (2021).
Atala, A. & Yoo, J. J. Essential of 3D Biofabrication and Translation (eds. Atala, A. & Yoo, J. J.) 89–121 (Academic, 2015).
Geng, Q., Wang, D., Chen, P. & Chen, S.-C. Ultrafast multi-focus 3-D nano-fabrication based on two-photon polymerization. Nat. Commun. 10, 2179 (2019).
Somers, P. et al. Rapid, continuous projection multi-photon 3D printing enabled by spatiotemporal focusing of femtosecond pulses. Light. Sci. Appl. 10, 199 (2021).
Limberg, D. K., Kang, J.-H. & Hayward, R. C. Triplet–triplet annihilation photopolymerization for high-resolution 3D printing. J. Am. Chem. Soc. 144, 5226–5232 (2022).
Schloemer, T. et al. Nanoengineering triplet–triplet annihilation upconversion: from materials to real-world applications. ACS Nano 17, 3259–3288 (2023).
Wong, J. et al. Triplet fusion upconversion for photocuring 3D-printed particle-reinforced composite networks. Adv. Mater. 35, 2207673 (2023).
Guo, Q., Zhou, C., Ma, Z. & Yang, X. Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges. Adv. Mater. 31, e1901997 (2019).
Huang, L. et al. Designing next generation of photon upconversion: recent advances in organic triplet–triplet annihilation upconversion nanoparticles. Biomaterials 201, 77–86 (2019).
Hoffmann, M. R., Martin, S. T., Choi, W. & Bahnemann, D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995).
Kim, J.-H. & Kim, J.-H. Encapsulated triplet–triplet annihilation-based upconversion in the aqueous phase for sub-band-gap semiconductor photocatalysis. J. Am. Chem. Soc. 134, 17478–17481 (2012).
Kwon, O. S., Kim, J.-H., Cho, J. K. & Kim, J.-H. Triplet–triplet annihilation upconversion in CdS-decorated SiO2nanocapsules for sub-bandgap photocatalysis. ACS Appl. Mater. Interfaces 7, 318–325 (2015).
Kim, H.-i, Kwon, O. S., Kim, S., Choi, W. & Kim, J.-H. Harnessing low energy photons (635 nm) for the production of H2O2using upconversion nanohybrid photocatalysts. Energy Environ. Sci. 9, 1063–1073 (2016).
Rahman, M. Z., Edvinsson, T. & Gascon, J. Hole utilization in solar hydrogen production. Nat. Rev. Chem. 6, 243–258 (2022).
Badea, G., Naghiu, G. S., Giurca, I., Aşchilean, I. & Megyesi, E. Hydrogen production using solar energy — technical analysis. Energy Procedia 112, 418–425 (2017).
Börjesson, K., Dzebo, D., Albinsson, B. & Moth-Poulsen, K. Photon upconversion facilitated molecular solar energy storage. J. Mater. Chem. A 1, 8521–8524 (2013).
Yu, T. et al. Triplet–triplet annihilation upconversion for photocatalytic hydrogen evolution. Chem. Eur. J. 25, 16270–16276 (2019).
Cao, X., Hu, B., Ding, R. & Zhang, P. Plasmon-enhanced homogeneous and heterogeneous triplet–triplet annihilation by gold nanoparticles. Phys. Chem. Chem. Phys. 17, 14479–14483 (2015).
Westbrook, E. G. & Zhang, P. Plasmon-enhanced triplet–triplet annihilation upconversion of post-modified polymeric acceptors. Dalton Trans. 47, 8638–8645 (2018).
Fang, J. et al. Highly efficient photocatalytic hydrogen generation of g-C3N4-CdS sheets based on plasmon-enhanced triplet–triplet annihilation upconversion. Appl. Catal. B 258, 117762 (2019).
Fang, J. et al. Enhanced triplet–triplet annihilation upconversion by photonic crystals and Au plasma resonance for efficient photocatalysis. Catal. Sci. Technol. 10, 8325–8331 (2020).
Fang, J. J. et al. Low-energy photons dual harvest for photocatalytic hydrogen evolution: bimodal surface plasma resonance related synergism of upconversion and pyroelectricity. Small 19, 2207467 (2023).
Choi, D., Nam, S. K., Kim, K. & Moon, J. H. Enhanced photoelectrochemical water splitting through bismuth vanadate with a photon upconversion luminescent reflector. Angew. Chem. Int. Ed. 58, 6891–6895 (2019).
Wang, C. S., Dixneuf, P. H. & Soulé, J. F. Photoredox catalysis for building C-C bonds from C(sp(2))-H bonds. Chem. Rev. 118, 7532–7585 (2018).
Majek, M., Faltermeier, U., Dick, B., Pérez-Ruiz, R. & Jacobi von Wangelin, A. Application of visible-to-UV photon upconversion to photoredox catalysis: the activation of aryl bromides. Chem. Eur. J. 21, 15496–15501 (2015).
Kerzig, C. & Wenger, O. S. Sensitized triplet–triplet annihilation upconversion in water and its application to photochemical transformations. Chem. Sci. 9, 6670–6678 (2018).
Raza, M. K., Noor, A. & Samantaray, P. K. Ir(III) and Ru(II) complexes in photoredox catalysis and photodynamic therapy: a new paradigm towards anticancer applications. ChemBioChem 22, 3270–3272 (2021).
Pfund, B. et al. UV light generation and challenging photoreactions enabled by upconversion in water. J. Am. Chem. Soc. 142, 10468–10476 (2020).
Häring, M., Pérez-Ruiz, R., Jacobi von Wangelin, A. & Díaz, D. D. Intragel photoreduction of aryl halides by green-to-blue upconversion under aerobic conditions. Chem. Comm. 51, 16848–16851 (2015).
López-Calixto, C., Liras, M., de la Peña O’Shea, V. & Perez‐Ruiz, R. Synchronized biphotonic process triggering CC coupling catalytic reactions. Appl. Catal. B 237, 18–23 (2018).
Zeng, L., Huang, L., Lin, W., Jiang, L.-H. & Han, G. Red light-driven electron sacrificial agents-free photoreduction of inert aryl halides via triplet–triplet annihilation. Nat. Commun. 14, 1102 (2023).
Aydogan, C., Yilmaz, G. & Yagci, Y. Synthesis of hyperbranched polymers by photoinduced metal-free ATRP. Macromolecules 50, 9115–9120 (2017).
Huang, Z., Luo, N., Zhang, C. & Wang, F. Radical generation and fate control for photocatalytic biomass conversion. Nat. Rev. Chem. 6, 197–214 (2022).
Glaser, F. & Wenger, O. S. Red light-based dual photoredox strategy resembling the Z-scheme of natural photosynthesis. JACS Au 2, 1488–1503 (2022).
Glaser, F. & Wenger, O. S. Sensitizer-controlled photochemical reactivity via upconversion of red light. Chem. Sci. 14, 149–161 (2023).
Xu, Z., Huang, Z., Jin, T., Lian, T. & Tang, M. L. Mechanistic understanding and rational design of quantum dot/mediator interfaces for efficient photon upconversion. Acc. Chem. Res. 54, 70–80 (2021).
Liang, W. et al. Near-infrared photon upconversion and solar synthesis using lead-free nanocrystals. Nat. Photon. 17, 346–353 (2023).
Liu, J., Kang, W. & Wang, W. Photocleavage-based photoresponsive drug delivery. Photochem. Photobiol. 98, 288–302 (2022).
Schulte, A. M., Alachouzos, G., Szymański, W. & Feringa, B. L. Strategy for engineering high photolysis efficiency of photocleavable protecting groups through cation stabilization. J. Am. Chem. Soc. 144, 12421–12430 (2022).
Atta, S., Ikbal, M., Boda, N., Gauri, S. S. & Singh, N. D. P. Photoremovable protecting groups as controlled-release device for sex pheromone. Photochem. Photobiol. Sci. 12, 393–403 (2013).
Jana, A., Ikbal, M. & Singh, N. D. P. Perylen-3-ylmethyl: fluorescent photoremovable protecting group (FPRPG) for carboxylic acids and alcohols. Tetrahedron 68, 1128–1136 (2012).
Singh, P. K., Majumdar, P. & Singh, S. P. Advances in BODIPY photocleavable protecting groups. Coord. Chem. Rev. 449, 214193 (2021).
Liu, Q. et al. A general strategy for biocompatible, high-effective upconversion nanocapsules based on triplet–triplet annihilation. J. Am. Chem. Soc. 135, 5029–5037 (2013).
Zhao, J., Xu, K., Yang, W., Wang, Z. & Zhong, F. The triplet excited state of Bodipy: formation, modulation and application. Chem. Soc. Rev. 44, 8904–8939 (2015).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Greene, F. D., Misrock, S. L. & Wolfe, J. R. Jr The structure of anthracene photodimers. J. Am. Chem. Soc. 77, 3852–3855 (1955).
Zheng, Y. et al. PEG-based hydrogel synthesis via the photodimerization of anthracene groups. Macromolecules 35, 5228–5234 (2002).
Islangulov, R. R. & Castellano, F. N. Photochemical upconversion: anthracene dimerization sensitized to visible light by a ruii chromophore. Angew. Chem. Int. Ed. 45, 5957–5959 (2006).
Ishida, Y. et al. Tunable chiral reaction media based on two-component liquid crystals: regio-, diastereo-, and enantiocontrolled photodimerization of anthracenecarboxylic acids. J. Am. Chem. Soc. 132, 17435–17446 (2010).
Rao, M. et al. Photocatalytic supramolecular enantiodifferentiating dimerization of 2-anthracenecarboxylic acid through triplet–triplet annihilation. Org. Lett. 20, 1680–1683 (2018).
Soto, F. et al. Smart materials for microrobots. Chem. Rev. 122, 5365–5403 (2022).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Borowiak, M. et al. Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 162, 403–411 (2015).
Liu, J. et al. Janus photochemical/photothermal azobenzene inverse opal actuator with shape self-recovery toward sophisticated motion. ACS Appl. Mater. Interfaces 14, 1727–1739 (2022).
Merritt, I. C. D., Jacquemin, D. & Vacher, M. Cis → trans photoisomerisation of azobenzene: a fresh theoretical look. Phys. Chem. Chem. Phys. 23, 19155–19165 (2021).
Yang, Y., Hughes, R. P. & Aprahamian, I. Visible light switching of a BF2-coordinated Azo compound. J. Am. Chem. Soc. 134, 15221–15224 (2012).
Jiang, Z., Xu, M., Li, F. & Yu, Y. Red-light-controllable liquid-crystal soft actuators via low-power excited upconversion based on triplet–triplet annihilation. J. Am. Chem. Soc. 135, 16446–16453 (2013).
Wu, W. et al. NIR-light-induced deformation of cross-linked liquid-crystal polymers using upconversion nanophosphors. J. Am. Chem. Soc. 133, 15810–15813 (2011).
Tokunaga, A. et al. Photochromic reaction by red light via triplet fusion upconversion. J. Am. Chem. Soc. 141, 17744–17753 (2019).
Sugunan, S. K., Tripathy, U., Brunet, S. M., Paige, M. F. & Steer, R. P. Mechanisms of low-power noncoherent photon upconversion in metalloporphyrin-organic blue emitter systems in solution. J. Phys. Chem. A 113, 8548–8556 (2009).
Cui, X., Zhao, J., Yang, P. & Sun, J. Zinc(ii) tetraphenyltetrabenzoporphyrin complex as triplet photosensitizer for triplet–triplet annihilation upconversion. Chem. Commun. 49, 10221–10223 (2013).
Zhang, Y. et al. Delayed fluorescence from a zirconium(iv) photosensitizer with ligand-to-metal charge-transfer excited states. Nat. Chem. 12, 345–352 (2020).
Zhao, J., Wu, W., Sun, J. & Guo, S. Triplet photosensitizers: from molecular design to applications. Chem. Soc. Rev. 42, 5323–5351 (2013).
Liang, H. et al. Heavy atom-free triplet photosensitizer based on thermally activated delayed fluorescence material for NIR-to-blue triplet–triplet annihilation upconversion. Chin. Chem. Lett. 34, 107515 (2023).
Nguyen, V.-N., Yan, Y., Zhao, J. & Yoon, J. Heavy-atom-free photosensitizers: from molecular design to applications in the photodynamic therapy of cancer. Acc. Chem. Res. 54, 207–220 (2021).
Alves, J., Feng, J., Nienhaus, L. & Schmidt, T. W. Challenges, progress and prospects in solid state triplet fusion upconversion. J. Mater. Chem. C 10, 7783–7798 (2022).
Carrod, A. J., Gray, V. & Börjesson, K. Recent advances in triplet–triplet annihilation upconversion and singlet fission, towards solar energy applications. Energy Environ. Sci. 15, 4982–5016 (2022).
Han, Y., He, S. & Wu, K. Molecular triplet sensitization and photon upconversion using colloidal semiconductor nanocrystals. ACS Energy Lett. 6, 3151–3166 (2021).
Lai, R., Sang, Y., Zhao, Y. & Wu, K. Triplet sensitization and photon upconversion using InP-based quantum dots. J. Am. Chem. Soc. 142, 19825–19829 (2020).
Liu, Q. et al. Highly photostable near-IR-excitation upconversion nanocapsules based on triplet–triplet annihilation for in vivo bioimaging application. ACS Appl. Mater. Interfaces 10, 9883–9888 (2018).
Bennison, M. J., Collins, A. R., Zhang, B. & Evans, R. C. Organic polymer hosts for triplet–triplet annihilation upconversion systems. Macromolecules 54, 5287–5303 (2021).
Marsico, F. et al. Hyperbranched unsaturated polyphosphates as a protective matrix for long-term photon upconversion in air. J. Am. Chem. Soc. 136, 11057–11064 (2014).
Huang, L., Le, T., Huang, K. & Han, G. Enzymatic enhancing of triplet–triplet annihilation upconversion by breaking oxygen quenching for background-free biological sensing. Nat. Commun. 12, 1898 (2021).
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5, 951–967 (2021).
Liu, R., Zuo, R. & Hudalla, G. A. Harnessing molecular recognition for localized drug delivery. Adv. Drug. Deliv. Rev. 170, 238–260 (2021).
Cheung, K. P. S., Sarkar, S. & Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 122, 1543–1625 (2022).
Dong, F. et al. Improving wastewater treatment by triboelectric–photo/electric coupling effect. ACS Nano 16, 3449–3475 (2022).
Shrestha, P. et al. Efficient far-red/near-IR absorbing BODIPY photocages by blocking unproductive conical intersections. J. Am. Chem. Soc. 142, 15505–15512 (2020).
Baluschev, S., Katta, K., Avlasevich, Y. & Landfester, K. Annihilation upconversion in nanoconfinement: solving the oxygen quenching problem. Mater. Horiz. 3, 478–486 (2016).
Filatov, M. A., Baluschev, S. & Landfester, K. Protection of densely populated excited triplet state ensembles against deactivation by molecular oxygen. Chem. Soc. Rev. 45, 4668–4689 (2016).
Xiong, H. et al. Photo-controllable biochemistry: exploiting the photocages in phototherapeutic window. Chem https://doi.org/10.1016/j.chempr.2022.11.007 (2022).
Liu, X. et al. Near-infrared manipulation of multiple neuronal populations via trichromatic upconversion. Nat. Commun. 12, 5662 (2021).
Author information
Authors and Affiliations
Contributions
L.H. and G.H. conceived the idea and drafted the proposal. L.H. wrote all the content and completed all graphic tasks. G.H. edited and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests for all the authors.
Peer review
Peer review information
Nature Reviews Chemistry thanks Yanai Nobuhiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, L., Han, G. Triplet–triplet annihilation photon upconversion-mediated photochemical reactions. Nat Rev Chem 8, 238–255 (2024). https://doi.org/10.1038/s41570-024-00585-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-024-00585-3