Abstract
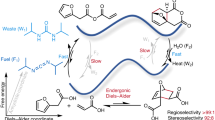
Synthetic chemistry has traditionally relied on reactions between reactants of high chemical potential and transformations that proceed energetically downhill to either a global or local minimum (thermodynamic or kinetic control). Catalysts can be used to manipulate kinetic control, lowering activation energies to influence reaction outcomes. However, such chemistry is still constrained by the shape of one-dimensional reaction coordinates. Coupling synthesis to an orthogonal energy input can allow ratcheting of chemical reaction outcomes, reminiscent of the ways that molecular machines ratchet random thermal motion to bias conformational dynamics. This fundamentally distinct approach to synthesis allows multi-dimensional potential energy surfaces to be navigated, enabling reaction outcomes that cannot be achieved under conventional kinetic or thermodynamic control. In this Review, we discuss how ratcheted synthesis is ubiquitous throughout biology and consider how chemists might harness ratchet mechanisms to accelerate catalysis, drive chemical reactions uphill and programme complex reaction sequences.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lin, K.-C. Understanding product optimization: kinetic versus thermodynamic control. J. Chem. Ed. 65, 857–860 (1988).
Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives (VCH, 1995).
Numata, M., Yagai, S. & Hamura, T. (eds) Kinetic Control in Synthesis and Self-Assembly (Academic, 2018).
Chorkendorff, I. & Niemantsverdriet, J. W. Concepts of Modern Catalysis and Kinetics (Wiley, 2017).
Kay, E. R., Leigh, D. A. & Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 46, 72–191 (2007). This seminal review provided an explanation of ratchet mechanisms for chemistry. It dispelled the view, prevalent in the artificial molecular machines community at the time, that molecular switches were molecular motors and provided a framework for translating the physics literature on ratchets to chemical systems.
Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotechnol. 13, 882–889 (2018). This perspective provides a detailed analysis of the energy consumption in chemical fuel-driven self-assembly and introduces the term ‘ratcheting constant’ for Astumian’s directionality, r0, that quantifies the tendency of a system to shift one way or the other when driven away from equilibrium by input energy.
Das, K., Gabrielli, L. & Prins, L. J. Chemically fueled self‐assembly in biology and chemistry. Angew. Chem. Int. Ed. 60, 20120–20143 (2021).
Astumian, R. D. Design principles for Brownian molecular machines: how to swim in molasses and walk in a hurricane. Phys. Chem. Chem. Phys. 9, 5067–5083 (2007).
Feng, Y. et al. Molecular pumps and motors. J. Am. Chem. Soc. 143, 5569–5591 (2021).
Lau, B., Kedem, O., Schwabacher, J., Kwasnieski, D. & Weiss, E. A. An introduction to ratchets in chemistry and biology. Mater. Horiz. 4, 310–318 (2017).
Bier, M. Brownian ratchets in physics and biology. Contemp. Phys. 38, 371–379 (1997).
Branscomb, E., Biancalani, T., Goldenfeld, N. & Russell, M. Escapement mechanisms and the conversion of disequilibria; the engines of creation. Phys. Rep. 667, 1–60 (2017).
Krishna, P. E. S., Dev, V. V., Ramakrishnan, R. & Hariharan, M. Retaining Hückel aromaticity in the triplet excited state of azobenzene. ChemPhysChem 23, e202200045 (2022).
Borsley, S., Leigh, D. A. & Roberts, B. M. W. Chemical fuels for molecular machinery. Nat. Chem. 14, 728–738 (2022). This review explains what a chemical fuel is and the features it should possess and discusses how energy is transduced from the fuel-to-waste reaction by molecular machines.
Zhang, L. et al. An electric molecular motor. Nature 613, 280–286 (2023).
Hein, J. E., Huynh Cao, B., Viedma, C., Kellogg, R. M. & Blackmond, D. G. Pasteur’s tweezers revisited: on the mechanism of attrition-enhanced deracemization and resolution of chiral conglomerate solids. J. Am. Chem. Soc. 134, 12629–12636 (2012).
Astumian, R. D. & Robertson, B. Imposed oscillations of kinetic barriers can cause an enzyme to drive a chemical reaction away from equilibrium. J. Am. Chem. Soc. 115, 11063–11068 (1993). This seminal paper pointed out that the addition of a catalyst to a chemical reaction initially at equilibrium but exposed to an oscillating field could, in principle, cause the reaction to proceed away from equilibrium.
von Delius, M., Geertsema, E. M., Leigh, D. A. & Tang, D.-T. D. Design, synthesis, and operation of small molecules that walk along tracks. J. Am. Chem. Soc. 132, 16134–16145 (2010).
Amano, S., Borsley, S., Leigh, D. A. & Sun, Z. Chemical engines: driving systems away from equilibrium through catalyst reaction cycles. Nat. Nanotechnol. 16, 1057–1067 (2021). This perspective describes the chemical engine cycle as a key element of nonequilibrium systems, in which a fuel-to-waste reaction is coupled to an orthogonal dynamic process.
Stroock, D. W. An Introduction to Markov Processes (Springer, 2013).
Parrondo, J. M. R., Horowitz, J. M. & Sagawa, T. Thermodynamics of information. Nat. Phys. 11, 131–139 (2015).
Astumian, R. D., Mukherjee, S. & Warshel, A. The physics and physical chemistry of molecular machines. ChemPhysChem 17, 1719–1741 (2016). This paper describes the physics and physical chemistry of molecular machines, specifically describing synthesis of ATP by ATP synthase, a strongly coupled ratchet.
Astumian, R. D. Thermodynamics and kinetics of a Brownian motor. Science 276, 917–922 (1997).
Astumian, R. D. Kinetic asymmetry allows macromolecular catalysts to drive an information ratchet. Nat. Commun. 10, 3837 (2019). This paper describes the kinetics of molecular motors through a framework that has come to be known as trajectory thermodynamics, useful for the understanding of ratchets.
Wilson, M. R. et al. An autonomous chemically fuelled small-molecule motor. Nature 534, 235–240 (2016).
Erbas-Cakmak, S. et al. Rotary and linear molecular motors driven by pulses of a chemical fuel. Science 358, 340–343 (2017).
Borsley, S., Kreidt, E., Leigh, D. A. & Roberts, B. M. W. Autonomous fuelled directional rotation about a covalent single bond. Nature 604, 80–85 (2022). This paper reports an autonomous single-bond rotary motor driven by catalysis and reveals an implicit connection between motor operation and faster catalysis.
Hernández, J. V., Kay, E. R. & Leigh, D. A. A reversible synthetic rotary molecular motor. Science 306, 1532–1537 (2004).
Leigh, D. A., Wong, J. K. Y., Dehez, F. & Zerbetto, F. Unidirectional rotation in a mechanically interlocked molecular rotor. Nature 424, 174–179 (2003).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Geertsema, E. M., van der Molen, S. J., Martens, M. & Feringa, B. L. Optimizing rotary processes in synthetic molecular motors. Proc. Natl Acad. Sci. USA 106, 16919–16924 (2009).
Pumm, A.-K. et al. A DNA origami rotary ratchet motor. Nature 607, 492–498 (2022).
Ragazzon, G., Baroncini, M., Silvi, S., Venturi, M. & Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nat. Nanotechnol. 10, 70–75 (2015).
Shi, X. et al. Sustained unidirectional rotation of a self-organized DNA rotor on a nanopore. Nat. Phys. 18, 1105–1111 (2022).
Serreli, V., Lee, C.-F., Kay, E. R. & Leigh, D. A. A molecular information ratchet. Nature 445, 523–527 (2007).
Amano, S., Fielden, S. D. P. & Leigh, D. A. A catalysis-driven artificial molecular pump. Nature 594, 529–534 (2021).
Borsley, S., Leigh, D. A. & Roberts, B. M. W. A doubly kinetically-gated information ratchet autonomously driven by carbodiimide hydration. J. Am. Chem. Soc. 143, 4414–4420 (2021).
Borsley, S., Leigh, D. A., Roberts, B. M. W. & Vitorica-Yrezabal, I. J. Tuning the force, speed, and efficiency of an autonomous chemically fueled information ratchet. J. Am. Chem. Soc. 144, 17241–17248 (2022).
Cheng, C. et al. An artificial molecular pump. Nat. Nanotechnol. 10, 547–553 (2015).
Qui, Y. et al. A precise polyrotaxane synthesizer. Science 368, 1247–1253 (2020).
Feng, L. et al. Active mechanisorption driven by pumping cassettes. Science 374, 1215–1221 (2021).
Thomas, D. et al. Pumping between phases with a pulsed-fuel molecular ratchet. Nat. Nanotechnol. 17, 701–707 (2022).
Liu, E. et al. A molecular information ratchet using a cone-shaped macrocycle. Chem https://doi.org/10.1016/j.chempr.2022.12.017 (2023).
Binks, L. et al. The role of kinetic asymmetry and power strokes in an information ratchet. Chem https://doi.org/10.1016/j.chempr.2023.05.035 (2023).
Parrondo, J. M. R. & Dinís, L. Brownian motion and gambling: from ratchets to paradoxical games. Contemp. Phys. 45, 147–157 (2004).
Kurtz, T. G. The relationship between stochastic and deterministic models for chemical reactions. J. Chem. Phys. 57, 2976–2978 (1972).
Anderson, D. F. & Kurtz, T. G. in Design and Analysis of Biomolecular Circuits: Engineering Approaches to Systems and Synthetic Biology (eds Koeppl, H. et al.) 3–42 (Springer, 2011). This book chapter describes chemical reactions as stochastic Brownian processes, describing reaction networks through continuous-time Markov chain models.
Carrillo, L., Escobar, J. A., Clempner, J. B. & Poznyak, A. S. Optimization problems in chemical reactions using continuous-time Markov chains. J. Math. Chem. 54, 1233–1254 (2016).
Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003).
Nelson, N. & Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 5, 971–982 (2004).
Walsh, C. T., Tu, B. P. & Tang, Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 118, 1460–1494 (2018).
Chen, L. B. Mitochondrial membrane potential in living cells. Ann. Rev. Cell Biol. 4, 155–181 (1988).
Khatter, H., Myasnikov, A. G., Natchiar, S. K. & Klaholz, B. P. Structure of the human 80S ribosome. Nature 520, 640–645 (2015).
Spirin, A. S. & Finkelstein, A. V. in Molecular Machines in Biology: Workshop of the Cell (ed. Frank J.) 158–190 (Cambridge Univ. Press, 2012). This book chapter describes the operation of a ribosome, relating its directional motion and sequence-specific peptide synthesis to a Brownian ratchet mechanism.
Beese, L. S., Derbyshire, V. & Steitz, T. A. Structure of DNA polymerase I Klenow fragment bound to duplex DNA. Science 260, 352–355 (1993).
Bar-Nahum, G. et al. A ratchet mechanism of transcription elongation and its control. Cell 120, 183–193 (2005).
Vassylyev, D. G. et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417, 712–719 (2002).
Galburt, E. A., Parrondo, J. M. R. & Grill, S. W. RNA polymerase pushing. Biophys. Chem. 157, 43–47 (2011).
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
Anselmi, C., Grininger, M., Gipson, P. & Faraldo-Gómez, J. D. Mechanism of substrate shuttling by the acyl-carrier protein within the fatty acid mega-synthase. J. Am. Chem. Soc. 132, 12357–12364 (2010).
Guo, H., Suzuki, T. & Rubinstein, J. L. Structure of a bacterial ATP synthase. eLife 8, e43128 (2019).
Buttgereit, F. & Brand, M. D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 312, 163–167 (1995).
Russell, J. B. & Cook, G. M. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59, 48–62 (1995).
Penocchio, E., Rao, R. & Esposito, M. Nonequilibrium thermodynamics of light-induced reactions. J. Chem. Phys. 155, 114101 (2021).
Weißenfels, M., Gemen, J. & Klajn, R. Dissipative self-assembly: fueling with chemicals versus light. Chem 7, 23–37 (2021).
Amano, S. et al. Using catalysis to drive chemistry away from equilibrium: relating kinetic asymmetry, power strokes, and the Curtin-Hammett principle in Brownian ratchets. J. Am. Chem. Soc. 144, 20153–20164 (2022).
Penocchio, E. & Ragazzon, G. Kinetic barrier diagrams to visualize and engineer molecular nonequilibrium systems. Small 19, 2206188 (2023).
Amano, S. et al. Insights from an information thermodynamics analysis of a synthetic molecular motor. Nat. Chem. 14, 530–537 (2022).
Ehrich, J. & Sivak, D. A. Energy and information flows in autonomous systems. Front. Phys. 11, 1108357 (2023).
Rao, R. & Esposito, M. Nonequilibrium thermodynamics of chemical reaction networks: wisdom from stochastic thermodynamics. Phys. Rev. X 6, 041064 (2016).
Wyman, J. The turning wheel: a study in steady states. Proc. Natl Acad. Sci. USA 72, 3983–3987 (1975).
Rozenbaum, V. M., Yang, D.-Y., Lin, S. H. & Tsong, T. Y. Catalytic wheel as a Brownian motor. J. Phys. Chem. B 108, 15880–15889 (2004).
Herges, R. Molecular assemblers: molecular machines performing chemical synthesis. Chem. Sci. 11, 9048–9055 (2020).
van Dijk, L. et al. Molecular machines for catalysis. Nat. Rev. Chem. 2, 0117 (2018).
Heard, A. W., Suárez, J. M. & Goldup, S. M. Controlling catalyst activity, chemoselectivity and stereoselectivity with the mechanical bond. Nat. Rev. Chem. 6, 182–196 (2022).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013). This paper reports an artificial small-molecule machine that transfers sequence information, in the form of building blocks, from a strand in order to synthesize sequence-specific oligomers.
De, Bo,G. et al. Efficient assembly of threaded molecular machines for sequence-specific synthesis. J. Am. Chem. Soc. 136, 5811–5814 (2014).
De Bo, G. et al. Sequence-specific β-peptide synthesis by a rotaxane-based molecular machine. J. Am. Chem. Soc. 139, 10875–10879 (2017).
De Bo, G. et al. An artificial molecular machine that builds an asymmetric catalyst. Nat. Nanotechnol. 13, 381–385 (2018).
McTernan, C. T., De Bo, G. & Leigh, D. A. A track-based molecular synthesizer that builds a single-sequence oligomer through iterative carbon-carbon bond formation. Chem 6, 2964–2973 (2020).
Echavarren, J. et al. Sequence-selective decapeptide synthesis by the parallel operation of two artificial molecular machines. J. Am. Chem. Soc. 143, 5158–5165 (2021).
Kassem, S. et al. Stereodivergent synthesis with a programmable molecular machine. Nature 549, 374–378 (2017). This paper describes a molecular machine that can synthesize any of four diastereomeric products in a programmable manner by physically manipulating the substrate to be proximal to the desired active site.
Sell, H. et al. Towards a light driven molecular assembler. Commun. Chem. 2, 62 (2019). This paper describes a molecular machine designed to perform endergonic synthesis of a tetramer by physically bringing the monomers into close proximity.
Meng, W. et al. An autonomous molecular assembler for programmable chemical synthesis. Nat. Chem. 8, 542–548 (2016). This paper describes an oligonucleotide-based machine capable of synthesizing a sequence-specific oligomer in a programmable manner.
Bartel, D. P. & Szostak, J. W. Isolation of new ribozymes from a large pool of random sequences. Science 261, 1411–1418 (1993).
Ekland, E. H., Szostak, J. W. & Bartel, D. P. Structurally complex and highly active RNA ligases derived from random RNA sequences. Science 269, 364–370 (1995).
Ekland, E. H. & Bartel, D. P. RNA-catalysed RNA polymerization using nucleoside triphosphates. Nature 382, 373–376 (1996).
Johnston, W. K., Unrau, P. J., Lawrence, M. S., Glasner, M. E. & Bartel, D. P. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science 292, 1319–1325 (2001). This paper describes a ribozyme capable of autonomously synthesizing a complementary ribonucleotide.
Paul, N. & Joyce, G. F. A self-replicating ligase ribozyme. Proc. Natl Acad. Sci. USA 99, 12733–12740 (2002).
Shechner, D. M. et al. Crystal structure of the catalytic core of an RNA-polymerase ribozyme. Science 326, 1271–1275 (2009).
Wochner, A., Attwater, J., Coulson, A. & Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 332, 209–212 (2011).
Attwater, J., Wochner, A. & Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 5, 1011–1018 (2013).
Mutschler, H., Wochner, A. & Holliger, P. Freeze–thaw cycles as drivers of complex ribozyme assembly. Nat. Chem. 7, 502–508 (2015).
Attwater, J., Raguram, A., Morgunov, A. S., Gianni, E. & Holliger, P. Ribozyme-catalysed RNA synthesis using triplet building blocks. eLife 7, e35255 (2018).
McRae, E. K. S. et al. Cryo-EM structure and functional landscape of an RNA polymerase ribozyme. Preprint at https://doi.org/10.1101/2022.08.23.504927 (2022).
Ardagh, M. A., Birol, T., Zhang, Q., Abdelrahman, O. A. & Dauenhauer, P. J. Catalytic resonance theory: super volcanoes, catalytic molecular pumps, and oscillatory steady state. Catal. Sci. Technol. 9, 5058–5076 (2019). This seminal work describes the concept of catalytic resonance, in which alternating the potential applied across a surface during a reaction allows the Sabatier limit to be exceeded.
Ardagh, M. A., Abdelrahman, O. A. & Dauenhauer, P. J. Principles of dynamic heterogeneous catalysis: surface resonance and turnover frequency response. ACS Catal. 9, 6929–6937 (2019).
Shetty, M. et al. The catalytic mechanics of dynamic surfaces: stimulating methods for promoting catalytic resonance. ACS Catal. 10, 12666–12695 (2020).
Ardagh, M. A. et al. Catalytic resonance theory: parallel reaction pathway control. Chem. Sci. 11, 3501–3510 (2020).
Qi, J. et al. Dynamic control of elementary step energetics via pulsed illumination enhances photocatalysis on metal nanoparticles. ACS Energy Lett. 5, 3518–3525 (2020).
Gopeesingh, J. et al. Resonance-promoted formic acid oxidation via dynamic electrocatalytic modulation. ACS Catal. 10, 9932–9942 (2020).
Wittreich, G. R., Liu, S., Dauenhauer, P. J. & Vlachos, D. G. Catalytic resonance of ammonia synthesis by simulated dynamic ruthenium crystal strain. Sci. Adv. 8, eabl6576 (2022).
Gathmann, S. R., Ardagh, M. A. & Dauenhauer, P. J. Catalytic resonance theory: negative dynamic surfaces for programmable catalysts. Chem. Catal. 2, 140–163 (2022).
Baz, A., Lyons, M. & Holewinski, A. Dynamic electrocatalysis: examining resonant catalytic rate enhancement under oscillating electrochemical potential. Chem. Catal. 2, 3497–3516 (2022).
Dong, Q. et al. Programmable heating and quenching for efficient thermochemical synthesis. Nature 605, 470–476 (2022).
Psarellis, Y. M., Kavousanakis, M., Dauenhauer, P. J. & Kevrekidis, I. G. Writing the programs of programmable catalysis. ACS Catal. 13, 7457–7471 (2023).
Abdelrahman, O. A. & Dauenhauer, P. J. Energy flows in static and programmable catalysts. ACS Energy Lett. 8, 2292–2299 (2023).
Welin, E. R., Le, C., Arias-Rotondo, D. M., McCusker, J. K. & MacMillan, D. W. C. Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(II). Science 355, 380–385 (2017). This seminal work describes how an unfavourable step in a catalytic cycle is overcome by accessing a photochemically generated excited state.
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Kornfilt, D. J. P. & MacMillan, D. W. C. Copper-catalyzed trifluoromethylation of alkyl bromides. J. Am. Chem. Soc. 141, 6853–6858 (2019).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism, and applications. J. Am. Chem. Soc. 141, 6392–6402 (2019).
Kawamata, Y. et al. Chemoselective electrosynthesis using rapid alternating polarity. J. Am. Chem. Soc. 143, 16580–16588 (2021).
Zhang, L., Israel, E. M., Yan, J. & Ritter, T. Copper-mediated etherification via aryl radicals generated from triplet states. Nat. Synth. 1, 376–381 (2022).
Wang, H., Tian, Y.-M. & König, B. Energy- and atom-efficient chemical synthesis with endergonic photocatalysis. Nat. Rev. Chem. 6, 745–755 (2022). This review describes a range of photochemically driven endergonic syntheses, quantifying the work done.
Singh, K., Staig, S. J. & Weaver, J. D. Facile synthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 136, 5275–5278 (2014).
West, J. G., Huang, D. & Sorensen, E. J. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 6, 10093 (2015).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727–730 (2017).
Ota, E., Wang, H., Frye, N. L. & Knowles, R. R. A redox strategy for light-driven, out-of-equilibrium isomerizations and application to catalytic C–C bond cleavage reactions. J. Am. Chem. Soc. 141, 1457–1462 (2019).
Molloy, J. J., Metternich, J. B., Daniliuc, C. G., Watson, A. J. B. & Gilmour, R. Contra‐thermodynamic, photocatalytic E→Z isomerization of styrenyl boron species: vectors to facilitate exploration of two‐dimensional chemical space. Angew. Chem. Int. Ed. 57, 3168–3172 (2018).
Molloy, J. J. et al. Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis. Science 369, 302–306 (2020).
Zhao, K. & Knowles, R. R. Contra-thermodynamic positional isomerization of olefins. J. Am. Chem. Soc. 144, 137–144 (2021).
Neveselý, T. et al. Leveraging the n→π* interaction in alkene isomerization by selective energy transfer catalysis. Angew. Chem. Int. Ed. 61, e202113600 (2022).
DeHovitz, J. S. & Hyster, T. K. Photoinduced dynamic radical processes for isomerizations, deracemizations, and dynamic kinetic resolutions. ACS Catal. 12, 8911–8924 (2022).
Wang, P. Z., Xiao, W. J. & Chen, J. R. Light-empowered contra-thermodynamic stereochemical editing. Nat. Rev. Chem. 7, 35–50 (2023).
Alexeeva, M., Enright, A., Dawson, M. J., Mahmoudian, M. & Turner, N. J. Deracemization of α-methylbenzylamine using an enzyme obtained by in vitro evolution. Angew. Chem. Int. Ed. 41, 3177–3180 (2002).
Lackner, A. D., Samant, A. V. & Toste, F. D. Single-operation deracemization of 3H-indolines and tetrahydroquinolines enabled by phase separation. J. Am. Chem. Soc. 135, 14090–14093 (2013).
Ji, Y., Shi, L., Chen, M.-W., Feng, G.-S. & Zhou, Y.-G. Concise redox deracemization of secondary and tertiary amines with a tetrahydroisoquinoline core via a nonenzymatic process. J. Am. Chem. Soc. 137, 10496–10499 (2015).
Hölzl-Hobmeier, A. et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564, 240–243 (2018).
Shin, N. Y., Ryss, J. M., Zhang, X., Miller, S. J. & Knowles, R. R. Light-driven deracemization enabled by excited-state electron transfer. Science 366, 364–369 (2019). This paper is an important representative example of photochemically driven deracemization reminiscent of a ratchet mechanism.
Lautens, M. & Loup, J. α-Deracemization of pyridylketones by photoredox catalysis. Synfacts 17, 1228 (2021).
Zhang, Z. & Hu, X. Visible-light-driven catalytic deracemization of secondary alcohols. Angew. Chem. Int. Ed. 60, 22833–22838 (2021).
Zhang, C. et al. Catalytic α-deracemization of ketones enabled by photoredox deprotonation and enantioselective protonation. J. Am. Chem. Soc. 143, 13393–13400 (2021).
Wingstrand, E., Laurell, A., Fransson, L., Hult, K. & Moberg, C. Minor enantiomer recycling: metal catalyst, organocatalyst and biocatalyst working in concert. Chem. Eur. J. 15, 12107–12113 (2009).
Laurell, A. & Moberg, C. Opposite enantiomers from minor enantiomer recycling and dynamic kinetic resolution using a single biocatalyst. Eur. J. Org. Chem. 2011, 3980–3984 (2011).
Wen, Y.-Q., Hertzberg, R., Gonzalez, I. & Moberg, C. Minor enantiomer recycling: application to enantioselective syntheses of beta blockers. Chem. Eur. J. 20, 3806–3812 (2014).
Laurell Nash, A., Widyan, K. & Moberg, C. Recycling powered by release of carbon dioxide. ChemCatChem 6, 3314–3317 (2014).
Moberg, C. Recycling in asymmetric catalysis. Acc. Chem. Res. 49, 2736–2745 (2016). This account summarizes the Moberg group’s work on minor enantiomer recycling, in which stereoselective reactions are improved by expanding energy to kinetically recycle the incorrect enantiomer.
Gust, D., Moore, T. A. & Moore, A. L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 42, 1890–1898 (2009).
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011).
Surendranath, Y., Bediako, D. K. & Nocera, D. G. Interplay of oxygen evolution kinetics and photovoltaic power curves on the construction of artificial leaves. Proc. Natl Acad. Sci. USA 109, 15617–15621 (2012).
Nocera, D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012). This account summarizes the Nocera group’s work on artificial photosynthesis, using photovoltaics to achieve biomimetic water splitting.
Concepcion, J. J., House, R. L., Papanikolas, J. M. & Meyer, T. J. Chemical approaches to artificial photosynthesis. Proc. Natl Acad. Sci. USA 109, 15560–15564 (2012).
Berardi, S. et al. Molecular artificial photosynthesis. Chem. Soc. Rev. 43, 7501–7519 (2014).
Dogutan, D. K. & Nocera, D. G. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis. Acc. Chem. Res. 52, 3143–3148 (2019).
Keijer, T., Bouwens, T., Hessels, J. & Reek, J. N. H. Supramolecular strategies in artificial photosynthesis. Chem. Sci. 12, 50–70 (2021).
Teitsworth, T. S. et al. Water splitting with silicon p–i–n superlattices suspended in solution. Nature 614, 270–274 (2023).
Sabatier, P. La Catalyse en Chimie Organique (C. Béranger, 1920).
Balandin, A. A. Modern state of the multiplet theory of heterogeneous catalysis. Adv. Catal. 19, 1–210 (1969).
Foley, B. & Razdan, N. Dynamic catalysis fundamentals: I. Fast calculation of limit cycles in dynamic catalysis. Preprint at https://doi.org/10.26434/chemrxiv-2021-10hk4 (2022).
Foley, B. & Razdan, N. Dynamic catalysis fundamentals: II. Consequences of scaling relationships on mechanisms and kinetics. Preprint at https://doi.org/10.26434/chemrxiv-2022-sgc5w (2022).
Boerner, L. K. Industrial ammonia production emits more CO2 than any other chemical-making reaction. Chemists want to change that. Chem. Eng. News 97, 1–9 (2019).
Ritter, S. K. The Haber-Bosch reaction: an early chemical impact on sustainability. Chem. Eng. N. 86, 53 (2008).
Fu, X. et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023).
Busch, M., Wodrich, M. D. & Corminboeuf, C. Linear scaling relationships and volcano plots in homogeneous catalysis — revisiting the Suzuki reaction. Chem. Sci. 6, 6754–6761 (2015).
Wodrich, M. D., Busch, M. & Corminboeuf, C. Accessing and predicting the kinetic profiles of homogeneous catalysts from volcano plots. Chem. Sci. 7, 5723–5735 (2016).
Wodrich, M. D., Sawatlon, B., Busch, M. & Corminboeuf, C. The genesis of molecular volcano plots. Acc. Chem. Res. 54, 1107–1117 (2021).
Ragazzon, G. et al. Autonomous non-equilibrium self-assembly and molecular movements powered by electrical energy. Angew. Chem. Int. Ed. 62, e202214265 (2023).
Borsley, S. et al. Electrostatic forces in field-perturbed equilibria: nanopore analysis of cage complexes. Chem 5, 1275–1292 (2019).
Kedema, O., Lau, B., Ratner, M. A. & Weiss, E. A. Light-responsive organic flashing electron ratchet. Proc. Natl Acad. Sci. USA 114, 8698–8703 (2017).
Kedem, O., Lau, B. & Weiss, E. A. How to drive a flashing electron ratchet to maximize current. Nano Lett. 17, 5848–5854 (2017).
Radzicka, A. & Wolfenden, R. A proficient enzyme. Science 267, 90–93 (1995).
Wolfenden, R. & Snider, M. J. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 34, 938–945 (2001).
Pauling, L. Nature of forces between large molecules of biological interest. Nature 161, 707–709 (1948).
Pauling, L. Molecular architecture and biological reactions. Chem. Eng. N. 24, 1375–1377 (1946).
Gurd, F. R. N. & Rothges, T. M. Motions in proteins. Adv. Protein Chem. 33, 73–165 (1979).
Koshland, D. E. Jr & Neet, K. E. The catalytic and regulatory properties of enzymes. Ann. Rev. Biochem. 37, 359–411 (1968).
Straub, F. B. Formation of the secondary and tertiary structure of enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 26, 89–114 (1964).
Citri, N. Conformational adaptability in enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 37, 397–648 (1973).
Yon, J. M., Perahia, D. & Ghelis, C. Conformational dynamics and enzyme activity. Biochimie 80, 33–42 (1998).
Eisenmesser, E. Z., Bosco, D. A., Akke, M. & Kern, D. Enzyme dynamics during catalysis. Science 295, 1570–1573 (2002).
Hammes, G. G. Multiple conformational changes in enzyme catalysis. Biochemistry 41, 8221–8228 (2002).
Eisenmesser, E. Z. et al. Intrinsic dynamics of an enzyme underlies catalysis. Nature 438, 117–121 (2005).
Hammes-Schiffer, S. & Benkovic, S. J. Relating protein motion to catalysis. Annu. Rev. Biochem. 75, 519–541 (2006).
Boehr, D. D., McElheny, D., Dyson, H. J. & Wright, P. E. The dynamic energy landscape of dihydrofolate reductase catalysis. Science 313, 1638–1642 (2006).
Henzler-Wildman, K. & Kern, D. Dynamic personalities of proteins. Nature 450, 964–972 (2007).
Hanson, J. A. et al. Illuminating the mechanistic roles of enzyme conformational dynamics. Proc. Natl Acad. Sci. USA 104, 18055–18060 (2007).
Fraser, J. S. et al. Hidden alternative structures of proline isomerase essential for catalysis. Nature 462, 669–673 (2009).
Ma, B. & Nussinov, R. Enzyme dynamics point to stepwise conformational selection in catalysis. Curr. Opin. Chem. Biol. 14, 652–659 (2010). This key work describes the theory of general conformational selection for enzymatic rate enhancement through dynamics.
Nashine, V. C., Hammes-Schiffer, S. & Benkovic, S. J. Coupled motions in enzyme catalysis. Curr. Opin. Chem. Biol. 14, 644–651 (2010).
Ma, B. & Nussinov, R. Conformational footprints. Nat. Chem. Biol. 12, 890–891 (2016).
Warshel, A. Electrostatic origin of the catalytic power of enzymes and the role of preorganized active sites. J. Biol. Chem. 273, 27035–27038 (1998).
Warshel, A. et al. Electrostatic basis for enzyme catalysis. Chem. Rev. 106, 3210–3235 (2006).
Kamerlin, S. C. L. & Warshel, A. At the dawn of the 21st century: is dynamics the missing link for understanding enzyme catalysis? Proteins 78, 1339–1375 (2010). This paper argues against a causal link between enzyme conformational dynamics and rate accelerations in enzyme-catalysed reactions.
Kohen, A. Role of dynamics in enzyme catalysis: substantial versus semantic controversies. Acc. Chem. Res. 48, 466–473 (2015).
Warshel, A. & Bora, R. P. Defining and quantifying the role of dynamics in enzyme catalysis. J. Chem. Phys. 144, 180901 (2016).
Otten, R. et al. How directed evolution reshapes the energy landscape in an enzyme to boost catalysis. Science 370, 1442–1446 (2020).
Hanoian, P., Liu, C. T., Hammes-Schiffer, S. & Benkovic, S. Perspectives on electrostatics and conformational motions in enzyme catalysis. Acc. Chem. Res. 48, 482–489 (2015).
Závodszky, P., Kardos, J., Svingor, Á. & Petsko, G. A. Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc. Natl Acad. Sci. USA 95, 7406–7411 (1998).
Wolf-Watz, M. et al. Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat. Struct. Mol. Biol. 11, 945–949 (2004).
Bhabha, G. et al. A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science 332, 234–238 (2011).
Otten, R. et al. Rescue of conformational dynamics in enzyme catalysis by directed evolution. Nat. Commun. 9, 1314 (2018).
Singh, P. et al. Evolution of the chemical step in enzyme catalysis. ACS Catal. 11, 6726–6732 (2021).
Corey, E. J. & Ishihara, K. Highly enantioselective catalytic Diels–Alder addition promoted by a chiral bis(oxazoline)-magnesium complex. Tetrahedron Lett. 33, 6807–6810 (1992).
Ahrendt, K. A., Borths, C. J. & MacMillan, D. W. C. New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels–Alder reaction. J. Am. Chem. Soc. 122, 4243–4244 (2000).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki–Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Čorić, I. & List, B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315–319 (2012).
Crawford, J. M. & Sigman, M. S. Conformational dynamics in asymmetric catalysis: is catalyst flexibility a design element? Synthesis 51, 1021–1036 (2019). This paper explores the benefits of conformational dynamics in asymmetric catalysis and argues that conformational dynamics may affect rate.
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Fanourakis, A., Docherty, P. J., Chuentragool, P. & Phipps, R. J. Recent developments in enantioselective transition metal catalysis featuring attractive noncovalent interactions between ligand and substrate. ACS Catal. 10, 10672–10714 (2020).
Fierman, M. B., O’Leary, D. J., Steinmetz, W. E. & Miller, S. J. Structure-selectivity relationships and structure for a peptide-based enantioselective acylation catalyst. J. Am. Chem. Soc. 126, 6967–6971 (2004).
Sohtome, Y., Tanaka, S., Takada, K., Yamaguchi, T. & Nagasawa, K. Solvent‐dependent enantiodivergent Mannich‐type reaction: utilizing a conformationally flexible guanidine/bisthiourea organocatalyst. Angew. Chem. Int. Ed. 49, 9254–9257 (2010).
Bellini, R. et al. Conformational preferences of a tropos biphenyl phosphinooxazoline — a ligand with wide substrate scope. ACS Catal. 6, 1701–1712 (2016).
Crawford, J. M., Stone, E. A., Metrano, A. J., Miller, S. J. & Sigman, M. S. Parameterization and analysis of peptide-based catalysts for the atroposelective bromination of 3-arylquinazolin-4(3H)-ones. J. Am. Chem. Soc. 140, 868–871 (2018).
Stone, E. A. et al. Isolating conformers to assess dynamics of peptidic catalysts using computationally designed macrocyclic peptides. ACS Catal. 11, 4395–4400 (2021).
Shao, H., Chakrabarty, S., Qi, X., Takacs, J. M. & Liu, P. Ligand conformational flexibility enables enantioselective tertiary C–B bond formation in the phosphonate-directed catalytic asymmetric alkene hydroboration. J. Am. Chem. Soc. 143, 4801–4808 (2021).
Jarvo, E. R., Copeland, G. T., Papaioannou, N., Bonitatebus, P. J. & Miller, S. J. A biomimetic approach to asymmetric acyl transfer catalysis. J. Am. Chem. Soc. 121, 11638–11643 (1999).
Kawabata, T., Nagato, M., Takasu, K. & Fuji, K. Nonenzymatic kinetic resolution of racemic alcohols through an “induced fit” process. J. Am. Chem. Soc. 119, 3169–3170 (1997).
Yamada, S., Misono, T. & Iwai, Y. Kinetic resolution of sec-alcohols by a new class of pyridine catalysts having a conformation switch system. Tetrahedron Lett. 46, 2239–2242 (2005).
Horvath, S., Fernandez, L. E., Soudackov, A. V. & Hammes-Schiffer, S. Insights into proton-coupled electron transfer mechanisms of electrocatalytic H2 oxidation and production. Proc. Natl Acad. Sci. USA 109, 15663–15668 (2012).
Biswas, P. K., Saha, S., Paululat, T. & Schmittel, M. Rotating catalysts are superior: suppressing product inhibition by anchimeric assistance in four-component catalytic machinery. J. Am. Chem. Soc. 140, 9038–9041 (2018).
Orsino, A. F., Gutiérrez del Campo, M., Lutz, M. & Moret, M.-E. Enhanced catalytic activity of nickel complexes of an adaptive diphosphine–benzophenone ligand in alkyne cyclotrimerization. ACS Catal. 9, 2458–2481 (2019).
Elramadi, E. et al. Catalytic machinery in motion: controlling catalysis via speed. Chem. Commun. 58, 8073–8076 (2022).
Veth, L. & Dydio, P. Shapeshifting xantphos. Nat. Chem. 14, 1088 (2022).
Newman-Stonebraker, S. H., Wang, J. Y., Jeffrey, P. D. & Doyle, A. G. Structure–reactivity relationships of Buchwald-type phosphines in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 144, 19635–19648 (2022).
Adams, G. M. & Weller, A. S. POP-type ligands: variable coordination and hemilabile behaviour. Coord. Chem. Rev. 355, 150–172 (2018).
Adamski, P. et al. From self-replication to replicator systems en route to de novo life. Nat. Rev. Chem. 4, 386–403 (2020).
Frenkel-Pinter, M. et al. Adaptation and exaptation: from small molecules to feathers. J. Mol. Evol. 90, 166–175 (2022).
Tokuriki, N. & Tawfik, D. S. Protein dynamism and evolvability. Science 324, 203–207 (2009).
Campbell, E. et al. The role of protein dynamics in the evolution of new enzyme function. Nat. Chem. Biol. 12, 944–950 (2016).
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry (Oxford Univ. Press, 2012).
Efremov, A. & Wang, Z. Universal optimal working cycles of molecular motors. Phys. Chem. Chem. Phys. 13, 6223–6233 (2011).
Steinberg-Yfrach, G. et al. Conversion of light energy to proton potential in liposomes by artificial photosynthetic reaction centres. Nature 385, 239–241 (1997).
Steinberg-Yfrach, G. et al. Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. Nature 392, 479–482 (1998). This paper couples a light-driven transmembrane proton pump to ATP synthase to enable light-driven ATP synthesis.
Liu, F. & Morokuma, K. Computational study on the working mechanism of a stilbene light-driven molecular rotary motor: sloped minimal energy path and unidirectional nonadiabatic photoisomerization. J. Am. Chem. Soc. 134, 4864–4876 (2012).
Rosing, J. & Slater, E. C. The value of ΔG° for the hydrolysis of ATP. Biochim. Biophys. Acta Bioenerg. 267, 275–290 (1972).
Yan, J., Magnasco, M. O. & Marko, J. F. A kinetic proofreading mechanism for disentanglement of DNA by topoisomerases. Nature 401, 932–935 (1999).
Hopfield, J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl Acad. Sci. USA 71, 4135–4139 (1974). This seminal paper identifies and proposes a mechanism of kinetic proofreading, addressing longstanding questions about how biology achieves such high sequence fidelity in replication.
Eigen, M. Self organization of matter and evolution of biological macromolecules. Naturwissenschaften 58, 465–523 (1971).
Orgel, L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl Acad. Sci. USA 49, 517–521 (1963).
Blackmond, D. G. “If pigs could fly” chemistry: a tutorial on the principle of microscopic reversibility. Angew. Chem. Int. Ed. 48, 2648–2654 (2009).
Smith, J. M. The concept of information in biology. Philos. Sci. 67, 177–194 (2000).
Bennett, C. H. The thermodynamics of computation — a review. Int. J. Theor. Phys. 21, 905–940 (1982).
Heil, C. S., Wehrheim, S. S., Paithankar, K. S. & Grininger, M. Fatty acid biosynthesis: chain‐length regulation and control. ChemBioChem 20, 2298–2321 (2019).
Kassem, S., Lee, A. T. L., Leigh, D. A., Markevicius, A. & Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 8, 138–143 (2016).
Barrell, M. J., Campaña, A. G., von Delius, M., Geertsema, E. M. & Leigh, D. A. Light‐driven transport of a molecular walker in either direction along a molecular track. Angew. Chem. Int. Ed. 50, 285–290 (2011).
Rutten, M. G. T. A., Vaandrager, F. W., Elemans, J. A. A. W. & Nolte, R. J. M. Encoding information into polymers. Nat. Rev. Chem. 2, 365–381 (2018).
Crowley, J. D., Goldup, S. M., Lee, A. L., Leigh, D. A. & McBurney, R. T. Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem. Soc. Rev. 38, 1530–1541 (2009).
Dawson, P. E., Muir, T. W., Clark-Lewis, I. & Kent, S. B. H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. Epoxidation of polybutadiene by a topologically linked catalyst. Nature 424, 915–918 (2003).
van Dongen, S. F. M., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Processive catalysis. Angew. Chem. Int. Ed. Engl. 53, 11420–11428 (2014).
Ren, Y., Jamagne, R., Tetlow, D. J. & Leigh, D. A. A tape-reading molecular ratchet. Nature 612, 78–82 (2022).
Deamer, D., Akeson, M. & Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 34, 518–524 (2016).
Ashkenasy, G., Hermans, T. M., Otto, S. & Taylor, A. F. Systems chemistry. Chem. Soc. Rev. 46, 2543–2554 (2017).
Ramezani, H. & Dietz, H. Building machines with DNA molecules. Nat. Rev. Genet. 21, 5–26 (2020).
Hong, F., Zhang, F., Liu, Y. & Yan, H. DNA origami: scaffolds for creating higher order structures. Chem. Rev. 117, 12584–12640 (2017).
Pan, J., Li, F., Cha, T.-G., Chen, H. & Choi, J. H. Recent progress on DNA based walkers. Curr. Opin. Biotechnol. 34, 56–64 (2015).
Paun, G., Rozenberg, G. & Salomaa, A. in DNA Computing: New Computing Paradigms (Springer, 2005).
Srinivas, N. et al. On the biophysics and kinetics of toehold-mediated DNA strand displacement. Nucleic Acids Res. 41, 10641–10658 (2013).
Gilbert, W. Origin of life: the RNA world. Nature 319, 618 (1986).
Cech, T. R. Crawling out of the RNA world. Cell 136, 599–602 (2009).
Feynman, R. in Feynman and Computation 63–76 (CRC, 2018).
Drexler, K. E. Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc. Natl Acad. Sci. USA 78, 5275–5278 (1981).
Kelly, T. R., Tellitu, I. & Sestelo, J. P. In search of molecular ratchets. Angew. Chem. Int. Ed. Engl. 36, 1866–1868 (1997).
Kelly, T. R., De Silva, H. & Silva, R. A. Unidirectional rotary motion in a molecular system. Nature 401, 150–152 (1999).
Feringa, B. L. The art of building small: from molecular switches to motors (Nobel lecture). Angew. Chem. Int. Ed. 56, 11060–11078 (2017).
Marchetti, T., Frezzato, D., Gabrielli, L. & Prins, L. J. ATP drives the formation of a catalytic hydrazone through an energy ratchet mechanism. Angew. Chem. Int. Ed. 62, e202307530 (2023).
Gallagher, J. M., Olivieri, E., Mrad, T. W., Betts, A. & Leigh, D. A. Endergonic synthesis driven by chemical fuelling. Preprint at https://doi.org/10.26434/chemrxiv-2023-6nqn3 (2023).
Leigh, D. A. Conformational selection accelerates catalysis by an organocatalytic molecular motor. Chem 10, 1–12 (2024).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC; grant number EP/P027067/1) and the European Research Council (ERC; advanced grant number 786630) for the funding. D.A.L. is a Royal Society Research Professor.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks R. Astumian, C. Moberg, P. Dauenhauer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Brownian ratchet
-
A mechanism for rectifying stochastic motion on an asymmetric potential energy surface in response to an energy input.
- Catalytic resonance
-
The exploitation of periodic changes in binding energy to enhance the rate and control the outcome of heterogeneous catalysed reactions.
- Catalyst dynamics
-
Consequential conformational changes in a catalyst that occur during the catalytic cycle.
- Chemical engine cycle
-
Catalytic cycle for the fuel-to-waste reaction encompassing different chemical and orthogonal dynamic (for example, mechanical) states of a molecular ratchet.
- Chemical fuels
-
The reactants in a fuel-to-waste reaction that release free energy that is transduced to drive an orthogonal nonequilibrium process, either continuously (via an information ratchet mechanism) or through pulsed or sequential operations (often via an energy ratchet mechanism). We find it helpful to use the term ‘fuel’ (as explained here14) to differentiate such reactants from the more typical use of chemical reagents to react directly with functional groups in the transformation to be powered.
- Endergonic synthesis
-
The synthesis of a molecule that, under the reaction conditions, has a more positive free-energy (higher chemical potential) than the starting materials.
- Energy ratchets
-
Brownian ratchets that transition between two (or more) potential energy surfaces allowing a particle to relax directionally to a local minimum, driving the system away from the global equilibrium.
- Fuel-to-waste reaction
-
The exergonic (that is, free-energy-releasing) conversion of chemical fuel into waste products that provides the chemical potential gradient necessary to drive chemical systems away from equilibrium.
- Information ratchets
-
Brownian ratchets in which differences in the rate of an energy-dissipating process dependant on a stochastic process (for example, dynamics) kinetically drive the system out of equilibrium.
- Kinetic asymmetry
-
The overall kinetic bias in a chemical engine cycle, characterized by the ratcheting constant, Kr, which represents the number of forward cycles divided by the number of backward cycles.
- Kinetic gating
-
The kinetic bias in a process depending on the state of a ratchet, usually represented as a ratio of rates, corresponding to the relative activation energies.
- Kinetic proofreading
-
Kinetic selection of incorrect products for removal, resulting in selectivities exceeding the native thermodynamic preference.
- Markov process
-
A stochastic process in which the probability of an event does not depend on the history of events in the system.
- Programmable synthesis
-
The translation of external inputs into a controlled sequence of reactions.
- Ratcheted synthesis
-
The exploitation of a Brownian ratchet to control reaction pathways and outcomes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borsley, S., Gallagher, J.M., Leigh, D.A. et al. Ratcheting synthesis. Nat Rev Chem 8, 8–29 (2024). https://doi.org/10.1038/s41570-023-00558-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00558-y
This article is cited by
-
Artificial molecular pumps
Nature Reviews Methods Primers (2024)
-
Endergonic synthesis driven by chemical fuelling
Nature Synthesis (2024)