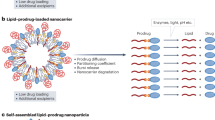
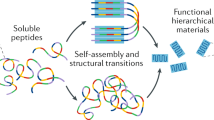
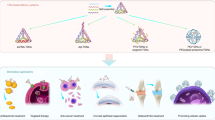
Abstract
Lipopeptides are amphiphilic peptides in which an aliphatic chain is attached to either the C or N terminus of peptides. Their self-assembly — into micelles, vesicles, nanotubes, fibres or nanobelts — leads to applications in nanotechnology, catalysis or medicinal chemistry. Self-organization of lipopeptides is dependent on both the length of the lipid tail and the amino acid sequence, in which the chirality of the peptide sequence can be transmitted into the supramolecular species. This Review describes the use of lipopeptides to design synthetic advanced dynamic supramolecular systems, nanostructured materials or self-responsive delivery systems in the area of medical biotechnology. We examine the influence of external stimuli, the ability of lipopeptide-derived structures to adapt over time and their application as medicinal agents with antibacterial, antifungal, antiviral or anticancer activities. Finally, we discuss the catalytic efficiency of lipopeptides, with the aim of building minimal synthetic enzymes, and recent efforts to incorporate metals into lipopeptide assemblies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Hamley, I. W. Self-assembly of amphiphilic peptides. Soft Matter 7, 4122–4138 (2011).
Löwik, D. W. P. M. & van Hest, J. C. M. Peptide based amphiphiles. Chem. Soc. Rev. 33, 234–245 (2004).
Cavalli, S., Albericio, F. & Kros, A. Amphiphilic peptides and their cross-disciplinary role as building blocks for nanoscience. Chem. Soc. Rev. 39, 241–263 (2010).
Hamley, I. W. Lipopeptides: from self-assembly to bioactivity. Chem. Commun. 51, 8574–8583 (2015).
Götze, S. & Stallforth, P. Structure elucidation of bacterial nonribosomal lipopeptides. Org. Biomol. Chem. 18, 1710–1727 (2020).
Hubrich, F. et al. Ribosomally derived lipopeptides containing distinct fatty acyl moieties. Proc. Natl Acad. Sci. USA 119, e2113120119 (2022).
Raaijmakers, J. M., De Bruijn, I., Nybroe, O. & Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34, 1037–1062 (2010).
Mnif, I. & Ghribi, D. Review lipopeptides biosurfactants: mean classes and new insights for industrial, biomedical, and environmental applications. Pept. Sci. 104, 129–147 (2015).
Adak, A., Ghosh, S., Gupta, V. & Ghosh, S. Biocompatible lipopeptide-based antibacterial hydrogel. Biomacromolecules 20, 1889–1898 (2019).
Abu Samah, N. H. & Heard, C. M. Topically applied KTTKS: a review. Int. J. Cosmet. Sci. 33, 483–490 (2011).
Castelletto, V. et al. Self-assembly of palmitoyl lipopeptides used in skin care products. Langmuir 29, 9149–9155 (2013).
Imoto, T. & Goto, M. Self-assembled palmitoyl–glycine–histidine as a permeation enhancer for transdermal delivery. Langmuir 37, 8971–8977 (2021).
Yu, H., Park, K. M. & Chang, P. S. Lipase-catalyzed synthesis of lauroyl tripeptide-KHA with multi-functionalities: its surface-active, antibacterial, and antioxidant properties. Food Chem. 319, 126533 (2020).
Roberts, K. D. et al. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant Gram-negative bacteria. ACS Infect. Dis. 1, 568–575 (2015).
Neubauer, D., Jaśkiewicz, M., Bauer, M., Gołacki, K. & Kamysz, W. Ultrashort cationic lipopeptides — effect of N-terminal amino acid and fatty acid type on antimicrobial activity and hemolysis. Molecules 25, 257 (2020).
Fa, K. et al. Acyl chain length tuning improves antimicrobial potency and biocompatibility of short designed lipopeptides. J. Colloid Interface Sci. 630, 911–923 (2023).
Mulligan, C. N., Sharma, S. K. & Mudhoo, A. (eds) Biosurfactants: Research Trends and Applications (CRC Press, 2014).
Martin, V. et al. Greening the synthesis of peptide therapeutics: an industrial perspective. RSC Adv. 10, 42457–42492 (2020).
Du, X., Zhou, J., Shi, J. & Xu, B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem. Rev. 115, 13165–13307 (2015).
Singh, N., Kumar, M., Miravet, J. F., Ulijn, R. V. & Escuder, B. Peptide-based molecular hydrogels as supramolecular protein mimics. Chem. A Eur. J. 23, 981–993 (2017).
Dehsorkhi, A., Castelletto, V. & Hamley, I. W. Self-assembling amphiphilic peptides. J. Pept. Sci. 20, 453–467 (2014).
Liu, M., Zhang, L. & Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 115, 7304–7397 (2015).
Lin, S., Li, Y., Li, B. & Yang, Y. Control of the handedness of self-assemblies of dipeptides by the chirality of phenylalanine and steric hindrance of phenylglycine. Langmuir 32, 7420–7426 (2016).
Hendricks, M. P., Sato, K., Palmer, L. C. & Stupp, S. I. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Res. 50, 2440–2448 (2017).
Sato, K., Hendricks, M. P., Palmer, L. C. & Stupp, S. I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 47, 7539–7551 (2018).
Singh, N., Tena-Solsona, M., Miravet, J. F. & Escuder, B. Towards supramolecular catalysis with small self-assembled peptides. Isr. J. Chem. 55, 711–723 (2015).
Nothling, M. D. et al. Synthetic catalysts inspired by hydrolytic enzymes. ACS Catal. 9, 168–187 (2019).
Lin, D. et al. Potent influenza A virus entry inhibitors targeting a conserved region of hemagglutinin. Biochem. Pharmacol. 144, 35–51 (2017).
Menacho-Melgar, R., Decker, J. S., Hennigan, J. N. & Lynch, M. D. A review of lipidation in the development of advanced protein and peptide therapeutics. J. Control. Rel. 295, 1–12 (2019).
Jiang, H. et al. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 118, 919–988 (2018).
Bordes, R. & Holmberg, K. Amino acid-based surfactants — do they deserve more attention? Adv. Colloid Interface Sci. 222, 79–91 (2015).
Albericio, F. & El-Faham, A. Choosing the right coupling reagent for peptides: a twenty-five-year journey. Org. Process Res. Dev. 22, 760–772 (2018).
Domalaon, R. et al. Structure–activity relationships in ultrashort cationic lipopeptides: the effects of amino acid ring constraint on antibacterial activity. Amino Acids 46, 2517–2530 (2014).
Dettori, L. et al. Molecular rules for selectivity in lipase-catalysed acylation of lysine. Process Biochem. 74, 50–60 (2018).
Dettori, L. et al. An aminoacylase activity from Streptomyces ambofaciens catalyzes the acylation of lysine on α-position and peptides on N-terminal position. Eng. Life Sci. 18, 589–599 (2018).
Guez, J. S., Vassaux, A., Larroche, C., Jacques, P. & Coutte, F. New continuous process for the production of lipopeptide biosurfactants in foam overflowing bioreactor. Front. Bioeng. Biotechnol. 9, 678469 (2021).
Tao, K., Levin, A., Jacoby, G., Beck, R. & Gazit, E. Entropic phase transitions with stable twisted intermediates of bio-inspired self-assembly. Chem. A Eur. J. 22, 15237–15241 (2016).
Baral, A. et al. Time-dependent gel to gel transformation of a peptide based supramolecular gelator. Soft Matter 11, 4944–4951 (2015).
Pashuck, E. T. & Stupp, S. I. Direct observation of morphological transformation from twisted ribbons into helical ribbons. J. Am. Chem. Soc. 132, 8819–8821 (2010).
Mabrouk, M. M., Hamed, N. A. & Mansour, F. R. Spectroscopic methods for determination of critical micelle concentrations of surfactants; a comprehensive review. Appl. Spectrosc. Rev. 58, 206–234 (2023).
Mabrouk, M. M., Hamed, N. A. & Mansour, F. R. Physicochemical and electrochemical methods for determination of critical micelle concentrations of surfactants: a comprehensive review. Monatsh. Chem. 153, 125–138 (2022).
Maji, S. K. & Haldar, S. Effect of peptide architecture on the self-assembly properties of tripeptide based anionic surfactants issued from two different peptide sequences: Ala-Ala-Val and Ala-Pro-Val in aqueous media (pH 7.4). Colloids Surf. A Physicochem. Eng. Asp. 414, 422–432 (2012).
Cui, H., Muraoka, T., Cheetham, A. G. & Stupp, S. I. Self-assembly of giant peptide nanobelts. Nano Lett. 9, 945–951 (2009).
Sato, K., Ji, W., Álvarez, Z., Palmer, L. C. & Stupp, S. I. Chiral recognition of lipid bilayer membranes by supramolecular assemblies of peptide amphiphiles. ACS Biomater. Sci. Eng. 5, 2786–2792 (2019).
Webber, M. J., Berns, E. J. & Stupp, S. I. Supramolecular nanofibers of peptide amphiphiles for medicine. Isr. J. Chem. 53, 530–554 (2013).
Cui, H., Webber, M. J. & Stupp, S. I. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Pept. Sci. 94, 1–18 (2010).
Rocco, D., Ross, J., Murray, P. & Caccetta, R. Acyl lipidation of a peptide: effects on activity and epidermal permeability in vitro. Drug Des. Dev. Ther. 10, 2203–2209 (2016).
Hutchinson, J. A. et al. Self-assembly of lipopeptides containing short peptide fragments derived from the gastrointestinal hormone PYY 3-36: from micelles to amyloid fibrils. J. Phys. Chem. B 123, 614–621 (2019).
Hamley, I. W. et al. Toll-like receptor agonist lipopeptides self-assemble into distinct nanostructures. Chem. Commun. 50, 15948–15951 (2014).
Hamley, I. W. Lipopeptides for vaccine development. Bioconjug. Chem. 32, 1472–1490 (2021).
Swiecicki, J. M. et al. Unsaturated acyl chains dramatically enhanced cellular uptake by direct translocation of a minimalist oligo-arginine lipopeptide. Chem. Commun. 51, 14656–14659 (2015).
Damen, M. et al. Delivery of DNA and siRNA by novel gemini-like amphiphilic peptides. J. Control. Rel. 145, 33–39 (2010).
Nasompag, S. et al. Effect of acyl chain length on therapeutic activity and mode of action of the CX-KYR-NH2 antimicrobial lipopeptide. Biochim. Biophys. Acta Biomembr. 1848, 2351–2364 (2015).
Steigenberger, J., Verleysen, Y., Geudens, N., Martins, J. C. & Heerklotz, H. The optimal lipid chain length of a membrane-permeabilizing lipopeptide results from the balance of membrane partitioning and local damage. Front. Microbiol. 12, 669709 (2021).
Fang, Y. et al. Tuning the antimicrobial pharmacophore to enable discovery of short lipopeptides with multiple modes of action. Eur. J. Med. Chem. 83, 36–44 (2014).
Guler, M. O. & Stupp, S. I. A self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc. 129, 12082–12083 (2007).
Tena-Solsona, M. et al. Emergent catalytic behavior of self-assembled low molecular weight peptide-based aggregates and hydrogels. Chem. A Eur. J. 22, 6687–6694 (2016).
Lin, S., Qin, J., Li, Y., Li, B. & Yang, Y. Chirality-driven parallel and antiparallel β-sheet secondary structures of Phe-Ala lipodipeptides. Langmuir 33, 8246–8252 (2017).
Mishra, N. K., Kumar, V. & Joshi, K. B. Thermoplasmonic effect of silver nanoparticles modulates peptide amphiphile fiber into nanowreath-like assembly. Nanoscale 7, 20238–20248 (2015).
Singh, R., Kumar Mishra, N., Kumar, V., Vinayak, V. & Ballabh Joshi, K. Transition metal ion-mediated tyrosine-based short-peptide amphiphile nanostructures inhibit bacterial growth. ChemBioChem 19, 1630–1637 (2018). This work shows a new approach to produce antibacterial lipopeptides by complexing them with transition metals, ultimately affecting cell homeostasis.
Bélières, M., Déjugnat, C. & Chouini-Lalanne, N. Histidine-based lipopeptides enhance cleavage of nucleic acids: interactions with DNA and hydrolytic properties. Bioconjug. Chem. 26, 2520–2529 (2015).
Tarwadi, Jazayeri, J. A., Prankerd, R. J. & Pouton, C. W. Preparation and in vitro evaluation of novel lipopeptide transfection agents for efficient gene delivery. Bioconjug. Chem. 19, 940–950 (2008).
Mishra, B., Lushnikova, T. & Wang, G. Small lipopeptides possess anti-biofilm capability comparable to daptomycin and vancomycin. RSC Adv. 5, 59758–59769 (2015).
Castelletto, V. et al. Self-assembly, tunable hydrogel properties, and selective anti-cancer activity of a carnosine-derived lipidated peptide. ACS Appl. Mater. Interfaces 11, 33573–33580 (2019).
Ravula, V., Lo, Y.-L., Wang, L.-F. & Patri, S. V. Gemini lipopeptide bearing an ultrashort peptide for enhanced transfection efficiency and cancer-cell-specific cytotoxicity. ACS Omega 6, 22955–22968 (2021).
Cheng, P.-N., Pham, J. D. & Nowick, J. S. The supramolecular chemistry of β-sheets. J. Am. Chem. Soc. 135, 5477–5492 (2013).
Qi, R. et al. Assembly and evolution of gemini-type peptide amphiphile with a di-lysine spacer. Langmuir 35, 6154–6160 (2019). A deep study showing the evolution of dynamic supramolecular structures, from vesicles, to fibrils, to ribbons and belts, using a lysine lipopeptide.
Zheng, C. et al. Bola-type Ala-Ala dipeptides: odd–even effect in molecular packing structures. Langmuir 35, 11406–11413 (2019). A representative study of the odd–even effect using Ala-Ala bolaamphiphiles that self-assemble into different morphologies.
Ohta, A., Shirai, M., Asakawa, T. & Miyagishi, S. Effect of stereochemistry on the molecular aggregation of phenylalanine dipeptide-type surfactants. J. Oleo Sci. 57, 659–667 (2008).
Tong, Q., Zhang, L., Li, Y., Li, B. & Yang, Y. Alignment of twisted nanoribbons formed by C17H35CO-Val-Ala sodium salts. Soft Matter 14, 6353–6359 (2018).
Guo, K. et al. A ‘center-determination’ phenomenon of C13H27CO-Gly-Ala-Ala lipotripeptides: relationship between the molecular chirality and handedness of organic self-assemblies. N. J. Chem. 43, 11503–11509 (2019).
Boettcher, C., Schade, B. & Fuhrhop, J. H. Comparative cryo-electron microscopy of noncovalent N-dodecanoyl-(d- and l-) serine assemblies in vitreous toluene and water. Langmuir 17, 873–877 (2001).
Li, Y., Li, B., Fu, Y., Lin, S. & Yang, Y. Solvent-induced handedness inversion of dipeptide sodium salts derived from alanine. Langmuir 29, 9721–9726 (2013). A work that highlights the solvent effect on promoting opposite ribbon handedness using THF or water.
Otsuka, T., Maeda, T. & Hotta, A. Effects of salt concentrations of the aqueous peptide-amphiphile solutions on the sol–gel transitions, the gelation speed, and the gel characteristics. J. Phys. Chem. B 118, 11537–11545 (2014).
Stendahl, J. C., Rao, M. S., Guler, M. O. & Stupp, S. I. Intermolecular forces in the self-assembly of peptide amphiphile nanofibers. Adv. Funct. Mater. 16, 499–508 (2006).
Mnif, I., Rajhi, H., Bouallegue, A., Trabelsi, N. & Ghribi, D. Characterization of lipopeptides biosurfactants produced by a newly isolated strain Bacillus subtilis ZNI5: potential environmental application. J. Polym. Environ. 30, 2378–2391 (2022).
Kar, T., Debnath, S., Das, D., Shome, A. & Das, P. K. Organogelation and hydrogelation of low-molecular-weight amphiphilic dipeptides: pH responsiveness in phase-selective gelation and dye removal. Langmuir 25, 8639–8648 (2009).
Duan, P., Qin, L., Zhu, X. & Liu, M. Hierarchical self-assembly of amphiphilic peptide dendrons: evolution of diverse chiral nanostructures through hydrogel formation over a wide pH range. Chem. A Eur. J. 17, 6389–6395 (2011).
Wang, D. et al. Light- and pH-controlled hierarchical coassembly of peptide amphiphiles. Langmuir 35, 9841–9847 (2019).
Hatip Koc, M. et al. Hierarchical self-assembly of histidine-functionalized peptide amphiphiles into supramolecular chiral nanostructures. Langmuir 33, 7947–7956 (2017).
Billiot, F. H. et al. Comparison of the aggregation behavior of 15 polymeric and monomeric dipeptide surfactants in aqueous solution. Langmuir 18, 2993–2997 (2002).
Zaldivar, G., Vemulapalli, S., Udumula, V., Conda-Sheridan, M. & Tagliazucchi, M. Self-assembled nanostructures of peptide amphiphiles: charge regulation by size regulation. J. Phys. Chem. C 123, 17606–17615 (2019).
Novelli, F. et al. Polymorphic self-organization of lauroyl peptide in response to pH and concentration. Langmuir 36, 3941–3951 (2020).
Zhang, H. et al. Modulating self-assembly behavior of a salt-free peptide amphiphile (PA) and Zwitterionic surfactant mixed system. J. Colloid Interface Sci. 467, 43–50 (2016).
Zhang, H. et al. Modulating hierarchical self-assembly behavior of a peptide amphiphile/nonionic surfactant mixed system. RSC Adv. 6, 9186–9193 (2016).
Basak, S., Nanda, J. & Banerjee, A. A new aromatic amino acid based organogel for oil spill recovery. J. Mater. Chem. 22, 11658–11664 (2012).
Sharma, A., Gupta, A., Tiwari, P., Basu, A. & Duttkonar, A. 12-Hydroxy stearic acid appended new amphiphilic scaffolds for selective capture of hydrogen halides through supramolecular hydrogelation. N. J. Chem. 44, 3828–3832 (2020).
Asl, H. F. et al. Experimental investigation into l-Arg and l-Cys eco-friendly surfactants in enhanced oil recovery by considering IFT reduction and wettability alteration. Pet. Sci. 17, 105–117 (2020).
Asl, H. F. et al. Effect of SiO2 nanoparticles on the performance of L-Arg and L-Cys surfactants for enhanced oil recovery in carbonate porous media. J. Mol. Liq. 300, 112290 (2020).
Nandi, M., Maiti, B., Banerjee, S. & De, P. Hydrogen bonding driven self-assembly of side-chain amino acid and fatty acid appended poly(methacrylate)s: gelation and application in oil spill recovery. J. Polym. Sci. Part A Polym. Chem. 57, 511–521 (2019).
Zhu, Z. et al. A critical review on the environmental application of lipopeptide micelles. Bioresour. Technol. 339, 125602 (2021).
Basak, S., Nandi, N., Paul, S., Hamley, I. W. & Banerjee, A. A tripeptide-based self-shrinking hydrogel for waste-water treatment: removal of toxic organic dyes and lead (Pb2+) ions. Chem. Commun. 53, 5910–5913 (2017).
Veliscek-Carolan, J., Hanley, T. L. & Jolliffe, K. A. The impact of structural variation in simple lanthanide binding peptides. RSC Adv. 6, 75336–75346 (2016).
Koda, D., Maruyama, T., Minakuchi, N., Nakashima, K. & Goto, M. Proteinase-mediated drastic morphological change of peptide-amphiphile to induce supramolecular hydrogelation. Chem. Commun. 46, 979–981 (2010). An innovative tool for disease diagnosis that correlates a lipopeptide morphological change (gelation) with an enzyme activity.
Shamsi, S. A., Valle, B. C., Billiot, F. & Warner, I. M. Polysodium N-undecanoyl-l-leucylvalinate: a versatile chiral selector for micellar electrokinetic chromatography. Anal. Chem. 75, 379–387 (2003).
Ji, Q., Iwaura, R. & Shimizu, T. Regulation of silica nanotube diameters: sol−gel transcription using solvent-sensitive morphological change of peptidic lipid nanotubes as templates. Chem. Mater. 19, 1329–1334 (2007).
Ji, Q. & Shimizu, T. Chemical synthesis of transition metal oxide nanotubes in water using an iced lipid nanotube as a template. Chem. Commun. 4411–4413 (2005).
Mishra, N. K., Joshi, K. B. & Verma, S. Modulating peptide amphiphile morphology by gold nanocolloids. J. Colloid Interface Sci. 455, 145–153 (2015).
Kumar, V., Mishra, N. K., Gupta, S. & Joshi, K. B. Short peptide amphiphile cage facilitate engineering of gold nanoparticles under the laser field. ChemistrySelect 2, 211–218 (2017).
Mandal, S. K., Kar, T., Das, D. & Das, P. K. The striking influence of SWNT–COOH on self-assembled gelation. Chem. Commun. 48, 1814–1816 (2012).
Kar, T., Mandal, S. K. & Das, P. K. Influence of pristine SWNTs in supramolecular hydrogelation: scaffold for superior peroxidase activity of cytochrome c. Chem. Commun. 48, 8389–8391 (2012).
Zeng, F. et al. A pore-forming tripeptide as an extraordinarily active anion channel. Org. Lett. 21, 4826–4830 (2019). An outstanding example of gemini lipopeptide that self-assembles into rod-like structures, generating small pores within a membrane, allowing transport of anions.
Ren, C. et al. Pore-forming monopeptides as exceptionally active anion channels. J. Am. Chem. Soc. 140, 8817–8826 (2018).
Ren, C., Shen, J. & Zeng, H. Combinatorial evolution of fast-conducting highly selective K+-channels via modularly tunable directional assembly of crown ethers. J. Am. Chem. Soc. 139, 12338–12341 (2017).
Zeng, L. Z., Zhang, H., Wang, T. & Li, T. Enhancing K+ transport activity and selectivity of synthetic K+ channels: via electron-donating effects. Chem. Commun. 56, 1211–1214 (2020).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294, 1684–1689 (2001). A fascinating example of tailored lipopeptide reversibly crosslinking into a fibrous scaffold that is able to direct hydroxyapatite mineralization.
Guler, M. O., Soukasene, S., Hulvat, J. F. & Stupp, S. I. Presentation and recognition of biotin on nanofibers formed by branched peptide amphiphiles. Nano Lett. 5, 249–252 (2005).
Tovar, J. D., Rabatic, B. M. & Stupp, S. I. Conducting polymers confined within bioactive peptide amphiphile nanostructures. Small 3, 2024–2028 (2007).
Tovar, J. D., Claussen, R. C. & Stupp, S. I. Probing the interior of peptide amphiphile supramolecular aggregates. J. Am. Chem. Soc. 127, 7337–7345 (2005).
Capito, R. M., Azevedo, H. S., Velichko, Y. S., Mata, A. & Stupp, S. I. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 319, 1812–1816 (2008).
Sato, K. et al. Programmable assembly of peptide amphiphile via noncovalent-to-covalent bond conversion. J. Am. Chem. Soc. 139, 8995–9000 (2017).
Cui, H., Cheetham, A. G., Pashuck, E. T. & Stupp, S. I. Amino acid sequence in constitutionally isomeric tetrapeptide amphiphiles dictates architecture of one-dimensional nanostructures. J. Am. Chem. Soc. 136, 12461–12468 (2014).
Yu, Z., Tantakitti, F., Palmer, L. C. & Stupp, S. I. Asymmetric peptide nanoribbons. Nano Lett. 16, 6967–6974 (2016).
Yu, Z. et al. Simultaneous covalent and noncovalent hybrid polymerizations. Science 351, 497–502 (2016).
Gao, C. et al. Electrostatic control of polymorphism in charged amphiphile assemblies. J. Phys. Chem. B 121, 1623–1628 (2017).
Freeman, R. et al. Reversible self-assembly of superstructured networks. Science 362, 808–813 (2018). A sophisticated example of a lipopeptide hydrogel that interacts with DNA strands, mimicking extracellular matrix in neural cells growth.
Preslar, A. T. et al. 19F magnetic resonance imaging signals from peptide amphiphile nanostructures are strongly affected by their shape. ACS Nano 10, 7376–7384 (2016). An example of pH-sensitive fluorinated lipopeptide with potential application in 19F MRI.
Krishnan, V., Pham, W. N., Messer, W. S. & Peseckis, S. M. First fatty acylated dipeptides to affect muscarinic receptor ligand binding. Bioorg. Med. Chem. Lett. 9, 3363–3368 (1999).
Todorovic, A. et al. N-terminal fatty acylated His-DPhe-Arg-Trp-NH2 tetrapeptides: influence of fatty acid chain length on potency and selectivity at the mouse melanocortin receptors and human melanocytes. J. Med. Chem. 48, 3328–3336 (2005).
Lopes, S. C. D. N. et al. Conformational and orientational guidance of the analgesic dipeptide kyotorphin induced by lipidic membranes: putative correlation toward receptor docking. J. Phys. Chem. B 110, 3385–3394 (2006).
Yao, L. Y. et al. Synthesis of lipoamino acids and their activity against cerebral ischemic injury. Molecules 14, 4051–4064 (2009).
Cui, H. J., Liu, S., Yang, R., Fu, G. H. & Lu, Y. N-stearoyltyrosine protects primary cortical neurons against oxygen-glucose deprivation-induced apoptosis through inhibiting anandamide inactivation system. Neurosci. Res. 123, 8–18 (2017).
Cui, H., Yang, R., Liu, S., Fu, G. & Lu, Y. N-stearoyltyrosine protects primary cortical neurons against Aβ(1–40)-induced injury through inhibiting endocannabinoid degradation. Life Sci. 124, 91–100 (2015).
Yang, Z. H. et al. N-stearoyltyrosine protects primary neurons from Aβ-induced apoptosis through modulating mitogen-activated protein kinase activity. Neuroscience 169, 1840–1847 (2010).
Guarise, C. et al. Amphiphilic peptide-based MMP3 inhibitors for intra-articular treatment of knee OA. Bioorg. Med. Chem. 38, 116132 (2021).
Biesalski, M. A., Knaebel, A., Tu, R. & Tirrell, M. Cell adhesion on a polymerized peptide-amphiphile monolayer. Biomaterials 27, 1259–1269 (2006).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Álvarez, Z. et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021). This work reports a novel fibrillar lipopeptide that promotes nerve regeneration in a paralysing human spinal cord injury mouse model.
Zhao, P. et al. Fungi-derived lipopeptide antibiotics developed since 2000. Peptides 113, 52–65 (2019).
McPhee, J., Scott, M. & Hancock, R. Design of host defence peptides for antimicrobial and immunity enhancing activities. Comb. Chem. High. Throughput Screen. 8, 257–272 (2005).
Wood, T. M. & Martin, N. I. The calcium-dependent lipopeptide antibiotics: structure, mechanism, & medicinal chemistry. MedChemComm 10, 634–646 (2019).
Wang, Z., Koirala, B., Hernandez, Y., Zimmerman, M. & Brady, S. F. Bioinformatic prospecting and synthesis of a bifunctional lipopeptide antibiotic that evades resistance. Science 376, 991–996 (2022).
Wang, Z. et al. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 601, 606–611 (2022).
Nandi, N. et al. Amphiphilic peptide-based supramolecular, noncytotoxic, stimuli-responsive hydrogels with antibacterial activity. Biomacromolecules 18, 3621–3629 (2017).
Vicente-García, C. & Colomer, I. New antimicrobial self-assembling short lipopeptides. Org. Biomol. Chem. 19, 6797–6803 (2021).
Ahn, M. et al. Discovery of novel histidine-derived lipo-amino acids: applied in the synthesis of ultra-short antimicrobial peptidomimetics having potent antimicrobial activity, salt resistance and protease stability. Eur. J. Med. Chem. 68, 10–18 (2013).
Greber, K. E., Dawgul, M., Kamysz, W. & Sawicki, W. Cationic net charge and counter ion type as antimicrobial activity determinant factors of short lipopeptides. Front. Microbiol. 8, 1–10 (2017).
Greber, K. E. et al. Are the short cationic lipopeptides bacterial membrane disruptors? Structure–activity relationship and molecular dynamic evaluation. Biochim. Biophys. Acta Biomembr. 1861, 93–99 (2019).
Domalaon, R. et al. Dilipid ultrashort cationic lipopeptides as adjuvants for chloramphenicol and other conventional antibiotics against Gram-negative bacteria. Amino Acids 51, 383–393 (2019).
Paduszynska, M. A. et al. Influence of short cationic lipopeptides with fatty acids of different chain lengths on bacterial biofilms formed on polystyrene and hydrogel surfaces. Pharmaceutics 11, 506 (2019).
Pazos, E. et al. Nucleation and growth of ordered arrays of silver nanoparticles on peptide nanofibers: hybrid nanostructures with antimicrobial properties. J. Am. Chem. Soc. 138, 5507–5510 (2016).
Rofeal, M. & El-Malek, F. A. Valorization of lipopeptides biosurfactants as anticancer agents. Int. J. Pept. Res. Ther. 27, 447–455 (2021).
Domalaon, R., Findlay, B., Ogunsina, M., Arthur, G. & Schweizer, F. Ultrashort cationic lipopeptides and lipopeptoids: evaluation and mechanistic insights against epithelial cancer cells. Peptides 84, 58–67 (2016).
Abdel-Malek, Z. A. et al. Melanoma prevention strategy based on using tetrapeptide α‐MSH analogs that protect human melanocytes from UV‐induced DNA damage and cytotoxicity. FASEB J. 20, 1561–1563 (2006).
Standley, S. M. et al. Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 70, 3020–3026 (2010). A neat study in which lipidation of cytotoxic small peptide enhances cell permeation, intracellular toxicity and tumour cell selectivity.
Makovitzki, A., Avrahami, D. & Shai, Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl Acad. Sci. USA 103, 15997–16002 (2006). A comprehensive study showing antibacterial and antifungal activity of short lipopeptide, via a membrane-permeation mechanism.
Nagarajan, S. R. et al. Conformationally constrained [p-(ω-aminoalkyl)phenacetyl]-l-seryl-l-lysyl dipeptide amides as potent peptidomimetic inhibitors of Candida albicans and human myristoyl-CoA: protein N-myristoyl transferase. J. Med. Chem. 2623, 1422–1438 (1997).
Wu, W. et al. Super short membrane-active lipopeptides inhibiting the entry of influenza A virus. Biochim. Biophys. Acta Biomembr. 1848, 2344–2350 (2015).
Lin, D. et al. A ‘building block’ approach to the new influenza A virus entry inhibitors with reduced cellular toxicities. Sci. Rep. 6, 1–14 (2016).
Copolovici, D. M., Langel, K., Eriste, E. & Langel, Ü. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano 8, 1972–1994 (2014).
Bode, S. A. et al. Self-assembling mini cell-penetrating peptides enter by both direct translocation and glycosaminoglycan-dependent endocytosis. Chem. Commun. 48, 7179–7181 (2012).
Pham, W., Kircher, M. F., Weissleder, R. & Tung, C. H. Enhancing membrane permeability by fatty acylation of oligoarginine peptides. ChemBioChem 5, 1148–1151 (2004).
Amoura, M. et al. Head to tail cyclisation of cell-penetrating peptides: impact on GAG-dependent internalisation and direct translocation. Chem. Commun. 55, 4566–4569 (2019).
Bhunia, D. et al. Spatial position regulates power of tryptophan: discovery of a major-groove-specific nuclear-localizing, cell-penetrating tetrapeptide. J. Am. Chem. Soc. 140, 1697–1714 (2018). An interesting study in which cell-penetrating short lipopeptides result in nuclear localization, binding to DNA major groove.
Teixeira, R. S. et al. Lysine-based surfactants as chemical permeation enhancers for dermal delivery of local anesthetics. Int. J. Pharm. 474, 212–222 (2014).
Bastiat, G. et al. Tyrosine-based rivastigmine-loaded organogels in the treatment of Alzheimer’s disease. Biomaterials 31, 6031–6038 (2010).
Karra, N. et al. Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: efficacy and biofate in a lung cancer mouse model. Small 9, 4221–4236 (2013). A beautiful example using a very simple lipoamino acid to link an antibody and cetuximab-loaded nanoparticles, to develop a selective antitumour drug delivery system.
Zhang, L., Gao, Z., Zhao, X. & Qi, G. A natural lipopeptide of surfactin for oral delivery of insulin. Drug Deliv. 23, 2084–2093 (2016).
Wang, D. et al. Preparation and characterization of β-casein stabilized lipopeptide lyotropic liquid crystal nanoparticles for delivery of doxorubicin. Soft Matter 15, 9011–9017 (2019).
Javali, N. M., Raj, A., Saraf, P., Li, X. & Jasti, B. Fatty acid-RGD peptide amphiphile micelles as potential paclitaxel delivery carriers to α(v)β(3) integrin overexpressing tumors. Pharm. Res. 29, 3347–3361 (2012).
Du, H., Cui, C., Wang, L., Liu, H. & Cui, G. Novel tetrapeptide, RGDF, mediated tumor specific liposomal doxorubicin (DOX) preparations. Mol. Pharm. 8, 1224–1232 (2011).
Wang, D. et al. Preparation and properties of semi-self-assembled lipopeptide vesicles. Langmuir 35, 13174–13181 (2019). This work describes a new application of lipopeptides in the production of magnetic vesicles.
Silva, E. R. et al. Structural behaviour and gene delivery in complexes formed between DNA and arginine-containing peptide amphiphiles. Soft Matter 12, 9158–9169 (2016).
Ho, J. K., White, P. J. & Pouton, C. W. Self-crosslinking lipopeptide/DNA/PEGylated particles: a new platform for DNA vaccination designed for assembly in aqueous solution. Mol. Ther. Nucleic Acids 12, 504–517 (2018).
Tonellato, U. Catalysis of ester hydrolysis by cationic micelles of surfactants containing the imidazole ring. J. Chem. Soc. Perkin Trans. 2, 771–776 (1976).
Heitmann, P. Reactivity of sulf hydryl groups in micelles: a model for protein. Eur. J. Biochem. 5, 305–315 (1968).
Heitmann, P. A model for sulfhydryl groups in proteins. Hydrophobic interactions of the cysteine side chain in micelles. Eur. J. Biochem. 3, 346–350 (1968).
Moss, R. A., Nahas, R. C. & Lukas, T. J. Preparation and kinetic properties of cysteine surfactants. J. Am. Chem. Soc. 100, 5920–5927 (1978).
Ihara, Y., Hosako, R., Nango, M. & Kuroki, N. Stereoselective micellar catalysis. Part 3. Co-operative effects of N-decanoyl-l-histidine for the hydrolysis of enantiomeric substrates. J. Chem. Soc. Perkin Trans. 2, 5–9 (1983).
Poznik, M. & König, B. Enantioselective ester hydrolysis by an achiral catalyst co-embedded with chiral amphiphiles into a vesicle membrane. RSC Adv. 6, 44456–44458 (2016).
Ihara, Y., Kimura, Y., Nango, M. & Kuroki, N. Catalysis of enantiomeric ester hydrolysis by N-decanoyl-l-histidine in polymer domains. J. Polym. Sci. A 21, 1535–1542 (1983).
You, J. S. et al. Hydrolytic metalloenzyme models enantioselective hydrolysis of long chain α-amino acid esters by chiral metallomicelles composed of lipophilic l-histidinol. J. Mol. Catal. A Chem. 202, 17–22 (2003).
Gayen, K. et al. Amino-acid-based metallo-hydrogel that acts like an esterase. ACS Appl. Bio Mater. 1, 1717–1724 (2018).
Kwak, J., Kim, M. C. & Lee, S. Y. Biomimetic catalytic and sensing cascades built with two designer bolaamphiphilic self-assemblies. ACS Appl. Mater. Interfaces 7, 14150–14156 (2015).
Nothling, M. D. et al. A multifunctional surfactant catalyst inspired by hydrolases. Sci. Adv. 6, 1–13 (2020). A recent representative example of a bioinspired lipoamino acid that catalyses ester hydrolysis.
Gulseren, G., Khalily, M. A., Tekinay, A. B. & Guler, M. O. Catalytic supramolecular self-assembled peptide nanostructures for ester hydrolysis. J. Mater. Chem. B 4, 4605–4611 (2016).
Gulseren, G. et al. Alkaline phosphatase-mimicking peptide nanofibers for osteogenic differentiation. Biomacromolecules 16, 2198–2208 (2015).
Bal, S., Das, K., Ahmed, S. & Das, D. Chemically fueled dissipative self-assembly that exploits cooperative catalysis. Angew. Chem. Int. Ed. 58, 244–247 (2019). A recent use of a very simple histidine lipoamino acid that is able to generate a complex out-of-equilibrium system.
Afrose, S. P., Bal, S., Chatterjee, A., Das, K. & Das, D. Designed negative feedback from transiently formed catalytic nanostructures. Angew. Chem. Int. Ed. 58, 15783–15787 (2019).
Pal, S., Reja, A., Bal, S., Tikader, B. & Das, D. Emergence of a promiscuous peroxidase under non-equilibrium conditions. Angew. Chem. Int. Ed. 61, e202111857 (2022).
Zozulia, O., Dolan, M. A. & Korendovych, I. V. Catalytic peptide assemblies. Chem. Soc. Rev. 47, 3621–3639 (2018).
Li, S., Wu, C., Fu, X. & Miao, Q. Cysteine-based organocatalysts for the highly efficient direct stoichiometric anti- and syn-aldol reactions. Ind. Eng. Chem. Res. 50, 13711–13716 (2011).
Díaz-Oltra, S., Berdugo, C., Miravet, J. F. & Escuder, B. Study of the effect of polymorphism on the self-assembly and catalytic performance of an l-proline based molecular hydrogelator. N. J. Chem. 39, 3785–3791 (2015).
Qin, L. et al. Supramolecular assemblies of amphiphilic l-proline regulated by compressed CO2 as a recyclable organocatalyst for the asymmetric aldol reaction. Angew. Chem. Int. Ed. 52, 7761–7765 (2013).
Singh, N. & Escuder, B. Competition versus cooperation in catalytic hydrogelators for anti-selective Mannich reaction. Chem. A Eur. J. 23, 9946–9951 (2017).
Singh, N. et al. Tandem reactions in self-sorted catalytic molecular hydrogels. Chem. Sci. 7, 5568–5572 (2016).
Soares, B. M. et al. Chiral organocatalysts based on lipopeptide micelles for aldol reactions in water. Phys. Chem. Chem. Phys. 19, 1181–1189 (2017).
Castelletto, V. et al. Self-assembly of a catalytically active lipopeptide and its incorporation into cubosomes. ACS Appl. Bio Mater. 2, 3639–3647 (2019).
Pelin, J. N. B. D. et al. Self-assembly, nematic phase formation, and organocatalytic behavior of a proline-functionalized lipopeptide. ACS Appl. Mater. Interfaces 12, 13671–13679 (2020).
Soares, B. M. et al. Structure optimization of lipopeptide assemblies for aldol reactions in an aqueous medium. Phys. Chem. Chem. Phys. 23, 10953–10963 (2021).
Bhowmick, S., Zhang, L., Ouyang, G. & Liu, M. Self-assembly of amphiphilic dipeptide with homo- and heterochiral centers and their application in asymmetric aldol reaction. ACS Omega 3, 8329–8336 (2018).
Reja, A., Afrose, S. P. & Das, D. Aldolase cascade facilitated by self-assembled nanotubes from short peptide amphiphiles. Angew. Chem. Int. Ed. 59, 4329–4334 (2020).
Hawkins, K., Patterson, A. K., Clarke, P. A. & Smith, D. K. Catalytic gels for a prebiotically relevant asymmetric aldol reaction in water: from organocatalyst design to hydrogel discovery and back again. J. Am. Chem. Soc. 142, 4379–4389 (2020). A beautiful approach in which glutamine lipoamino acid catalyses a model aldol reaction relevant in a prebiotic context.
Rodríguez-Llansola, F., Miravet, J. F. & Escuder, B. Supramolecular catalysis with extended aggregates and gels: inversion of stereoselectivity caused by self-assembly. Chem. A Eur. J. 16, 8480–8486 (2010).
Duschmalé, J., Kohrt, S. & Wennemers, H. Peptide catalysis in aqueous emulsions. Chem. Commun. 50, 8109–8112 (2014).
Delgado, J. A. C. et al. Synthesis of N-alkylated lipopeptides and their application as organocatalysts in asymmetric Michael addition in aqueous environments. N. J. Chem. 45, 14050–14057 (2021).
Khalily, M. A., Ustahuseyin, O., Garifullin, R., Genc, R. & Guler, M. O. A supramolecular peptide nanofiber templated Pd nanocatalyst for efficient Suzuki coupling reactions under aqueous conditions. Chem. Commun. 48, 11358–11360 (2012).
Khalily, M. A., Gulseren, G., Tekinay, A. B. & Guler, M. O. Biocompatible supramolecular catalytic one-dimensional nanofibers for efficient labeling of live cells. Bioconjug. Chem. 26, 2371–2375 (2015).
Kim, M. C. & Lee, S. Y. Carbonic anhydrase-mimetic bolaamphiphile self-assembly for CO2 hydration and sequestration. Chem. A Eur. J. 20, 17019–17024 (2014). An interesting study in which a bolaamphiphile lipoamino acid works as a carbonic anhydrase analogue, reducing CO2 in the presence of zinc.
Khalily, M. A. et al. Facile synthesis of three-dimensional Pt-TiO2 nano-networks: a highly active catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angew. Chem. Int. Ed. 55, 12257–12261 (2016).
Dolan, M. A. et al. Catalytic nanoassemblies formed by short peptides promote highly enantioselective transfer hydrogenation. ACS Nano 13, 9292–9297 (2019). A fascinating example of a short lipopeptide forming an iridium complex that catalysed the enantioselective hydrogenation of ketones in water.
Jasim, B., Sreelakshmi, K. S., Mathew, J. & Radhakrishnan, E. K. Surfactin, iturin, and fengycin biosynthesis by endophytic Bacillus sp. from Bacopa monnieri. Microb. Ecol. 72, 106–119 (2016).
Wu, S., Liu, G., Zhou, S., Sha, Z. & Sun, C. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar. Drugs 17, 199 (2019).
Fewer, D. P. et al. Chemical diversity and cellular effects of antifungal cyclic lipopeptides from cyanobacteria. Physiol. Plant 173, 639–650 (2021).
Rabanal, F. & Cajal, Y. Recent advances and perspectives in the design and development of polymyxins. Nat. Prod. Rep. 34, 886–908 (2017).
Cui, A.-L. et al. Synthesis and bioactivity investigation of the individual components of cyclic lipopeptide antibiotics. J. Med. Chem. 61, 1845–1857 (2018).
Cui, A.-L. et al. Design, synthesis, and bioactivity of cyclic lipopeptide antibiotics with varied polarity, hydrophobicity, and positive charge distribution. ACS Infect. Dis. 6, 1796–1806 (2020).
Nang, S. C., Azad, M. A. K., Velkov, T., Zhou, Q. T. & Li, J. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol. Rev. 73, 679–728 (2021).
Roberts, K. D. et al. A synthetic lipopeptide targeting top-priority multidrug-resistant Gram-negative pathogens. Nat. Commun. 13, 1625 (2022).
Lampel, A., Ulijn, R. V. & Tuttle, T. Guiding principles for peptide nanotechnology through directed discovery. Chem. Soc. Rev. 47, 3737–3758 (2018).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Acknowledgements
The authors acknowledge funding from La Caixa Foundation under the Junior Leader Program (Nos. LCF/BQ/PI19/11690020, ID 100010434), CAM-Atracción de Talento (2019-T1/IND-12683), PID2019-106327GA-I00 (MCIN/AEI/10.13039/501100011033), RyC2019-026674-I (MCIN/AEI/10.13039/501100011033 and FSE) and PIE 20218AT015 (CSIC).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aerogel
-
Solid ordered structure obtained by displacing the liquid component of a gel using a gas, initially developed under supercritical conditions, with slight or no shrinkage and retaining its original shape.
- Critical aggregation concentration
-
(CAC). Minimal concentration of an amphiphile at which self-assembled ordered structures are formed and detected.
- Haemolysis
-
Disruption of erythrocyte membranes, causing the release of haemoglobin, either by an external agent or natural necrosis at the end of erythrocyte life.
- Half-maximal inhibitory concentration (IC50)
-
The concentration of a substance required for 50% inhibition of a specific biological function, used as a measure of drug potency.
- Hydrogel
-
Hydrophilic self-assembled (macro)molecule that is not soluble in water, but absorbs large amounts of water, maintaining well-defined supramolecular structure.
- Hydrophilic–lipophilic balance
-
Measure of the hydrophilic and lipophilic degree of a surfactant molecule that results from its partition between oil and water.
- Hydrophobic collapse
-
Entropy-driven process, commonly observed by polypeptides and amphiphilic species in polar solvents, that produces a more stable 3D conformation as a result of the inability to interact or stabilize the hydrophobic domain by polar solvents.
- Langmuir–Blodgett film
-
Nanostructure formed by organized (multi)layers of amphiphilic molecules, or nanoparticles, as a result of transferring an organized monolayer film from a liquid–air interface into a solid support.
- Low-molecular-weight gelators
-
Small molecules that self-assemble via noncovalent bonding, entrapping molecules of solvent, commonly forming fibres, giving rise to gels.
- Matrix metalloprotein-7
-
(MMP-7). Also known as matrilysin or pump-1 protease (PUMP-1) is a zinc-dependent endonuclease that breaks down macromolecules from the extracellular matrix.
- Melanocortin 1 receptor
-
G-coupled receptor binding to pituitary peptide hormones, normally expressed by melanocytes and overexpressed by melanoma cancerous cells.
- Membrane depolarization
-
A shift in electric charge distribution (potential) within the cell membrane, related to ion transport, that can be physiological, that is, action potential, or lethal, that is antibiotics mechanism.
- Minimal inhibitory concentration
-
(MIC). Lowest concentration of an antimicrobial agent that inhibits the growth of a microorganism.
- Multiresistant bacteria
-
Bacterial strains showing resistance to at least one antimicrobial drug in three or more antimicrobial categories.
- Nonribosomal peptide synthetases
-
Multimodular enzymes that synthesize natural peptides, frequently incorporating non-proteinogenic amino acids and structural modifications, which is an alternative mechanism independent of mRNA, as opposed to ribosomes.
- Organogel
-
Gel formed by an organic liquid and a 3D cross-linked structure, formed by polymerization of reactive monomers or by self-assembling of low-molecular-weight species via noncovalent bonding, retaining the organic solvent.
- Stern layer
-
The first (internal) layer of the electric double layer, which forms at a charged surface in an ionic solution.
- Xerogels
-
Solid ordered structure obtained by drying a gel, for example, evaporating water from a hydrogel, with a notable shrinkage and usually not retaining its original shape.
- Zeta potential
-
Electric potential in the interfacial double layer of a colloidal dispersion, nanoparticle or self-assembled structure with respect to bulk medium, providing a measure of its stability.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vicente-Garcia, C., Colomer, I. Lipopeptides as tools in catalysis, supramolecular, materials and medicinal chemistry. Nat Rev Chem 7, 710–731 (2023). https://doi.org/10.1038/s41570-023-00532-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00532-8