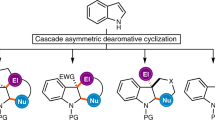
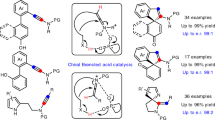
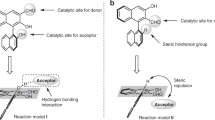
Abstract
Although arynes are usually considered fleeting intermediates, they are highly valuable synthons because they enable the introduction of aromatic rings and the simultaneous formation of new bonds at two sites. Although catalytic reactions using transition metals are excellent method for constructing complex polycyclic aromatic molecules in a single step, the use of asymmetric catalysis for the capture of arynes remains a crucial goal for the progress of aryne chemistry. Catalytic asymmetric reactions of arenes are challenging, requiring sufficient interactions between the neutral and highly reactive short-lived aryne intermediates in a stereo-controlled fashion. In addition, spontaneous decomposition, as well as side reactions, has hindered their development and, until recently, highly enantioselective reactions using arynes had remained elusive. This Review highlights asymmetric reactions using arynes, featuring diastereoselective, enantioselective and catalytic enantioselective reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hoffmann, R. W. Dehydrobenzene and Cycloalkynes (Academic Press, 1967).
Yoshida, H. in Multicomponent Reactions in Organic Synthesis (eds. Zhu, J., Wang, Q., & Wang, M.-X.) 39–72 (Wiley-VCH, 2015).
Kumamoto, T. & Katakawa, K. in Cycloaddition Reactions: Advances in Research and Applications (ed. Margetić, D.) Ch. 1 (Nova, 2019).
Stoermer, R. & Kahlert, B. Ueber das 1- und 2-Brom-type 1cumaron. Ber. Dtsch. Chem. Ges. 35, 1633–1640 (1902).
Bachmann, W. E. & Clarke, H. T. The mechanism of the Wurtz–Fittig reaction. J. Am. Chem. Soc. 49, 2089–2098 (1927).
Wittig, G. Phenyl-Lithium, der Schlüssel zu einer neuen Chemie Metallorganischer Verbindungen. Naturwissenschaften 30, 696–703 (1942).
Roberts, J. D., Simmons, H. E. Jr, Carlsmith, L. A. & Vaughan, C. W. Rearrangement in the reaction of chlorobenzene-1-C14 with potassium amide. J. Am. Chem. Soc. 75, 3290–3291 (1953).
Himeshima, Y., Sonoda, T. & Kobayashi, H. Fluoride-induced 1,2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions. Chem. Lett. 12, 1211–1214 (1983).
Sanz, R. Recent applications of aryne chemistry to organic synthesis. A review. Org. Prep. Proced. Int. 40, 215–291 (2008).
García-López, J.-A. & Greaney, M. F. Synthesis of biaryls using aryne intermediates. Chem. Soc. Rev. 45, 6766–6798 (2016).
Buchwald, S. L. & Nielsen, R. B. Group 4 metal complexes of benzynes, cycloalkynes, acyclic alkynes, and alkenes. Chem. Rev. 88, 1047–1058 (1988).
Bennett, M. A. & Schwemlein, H. P. Metal complexes of small cycloalkynes and arynes. Angew. Chem. Int. Ed. Engl. 28, 1296–1320 (1989).
Bennett, M. A. & Wenger, E. The reactivity of complexes of nickel(0) and platinum(0) containing benzyne and related small-ring alkynes. Chem. Ber. 130, 1029–1042 (1997).
Barluenga, J., Rodríguez, F., Álvarez-Rodrigo, L. & Fañanás, F. J. Coupling reactions of zirconocene complexes and heterosubstituted alkenes. Chem. Soc. Rev. 34, 762–768 (2005).
Dyke, A. M., Hester, A. J. & Lloyd-Jones, G. C. Organometallic generation and capture of ortho-arynes. Synthesis 2006, 4093–4112 (2006).
Bennett, M. A. Aryne complexes of zerovalent metals of the nickel triad. Aust. J. Chem. 63, 1066–1075 (2010).
Shi, J., Li, L. & Li, Y. o-Silylaryl triflates: a journey of kobayashi aryne precursors. Chem. Rev. 121, 3892–4044 (2021).
Peña, D., Escudero, S., Pérez, D., Guitián, E. & Castedo, L. Efficient palladium-catalyzed cyclotrimerization of arynes: synthesis of triphenylenes. Angew. Chem. Int. Ed. 37, 2659–2661 (1998).
Caeiro, J., Peña, D., Cobas, A., Pérez, D. & Guitián, E. Asymmetric catalysis in the [2+2+2] cycloaddition of arynes and alkynes: enantioselective synthesis of a pentahelicene. Adv. Synth. Catal. 348, 2466–2474 (2006). The first paper to show the potential of catalytic asymmetric synthesis using arynes.
Yubuta, A. et al. Enantioselective synthesis of triple helicenes by cross-cyclotrimerization of a helicenyl aryne and alkynes via dynamic kinetic resolution. J. Am. Chem. Soc. 142, 10025–10033 (2020). The first paper to achieve high enantioselectivities in excess of 90% ee in catalytic asymmetric synthesis using arynes.
Pierrot, D. & Marek, I. Synthesis of enantioenriched vicinal tertiary and quaternary carbon stereogenic centers within an acyclic chain. Angew. Chem. Int. Ed. 59, 36–49 (2020).
Zhou, F. et al. Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem. Sci. 11, 9341–9365 (2020).
Ping, Y., Li, Y., Zhu, J. & Kong, W. Construction of quaternary stereocenters by palladium-catalyzed carbopalladation-initiated cascade reactions. Angew. Chem. Int. Ed. 58, 1562–1573 (2019).
Li, Y. & Xu, S. Transition-metal-catalyzed C−H functionalization for construction of quaternary carbon center. Chemistry 24, 16218–16245 (2018).
Feng, J., Holmes, M. & Krische, M. J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 117, 12564–12580 (2017).
Eitzinger, A., Winter, M., Schörgenhumer, J. & Waser, M. Quaternary β2,2-amino acid derivatives by asymmetric addition of isoxazolidin-5-ones to para-quinone methides. Chem. Commun. 56, 579–582 (2020).
Harada, K. et al. Asymmetric construction of vicinal stereocenters containing quaternary and tertiary carbons: application to the formal synthesis of (–)-chenopodene. Eur. J. Org. Chem. 2020, 420–423 (2020).
Qiu, J. et al. Construction of all-carbon chiral quaternary centers through CuI-catalyzed enantioselective reductive hydroxymethylation of 1,1-disubstituted allenes with CO2. Chem. Eur. J. 25, 13874–13878 (2019).
Bratt, E., Suárez-Pantiga, S., Johansson, M. J. & Mendoza, A. Mechanism and regioselectivity of the anionic oxidative rearrangement of 1,3-diketones towards all-carbon quaternary carboxylates. Chem. Commun. 55, 8844–8847 (2019).
Zhang, Q.-Q. et al. Regio- and stereoselective alkenylation of allenoates with gem-difluoroalkenes: facile access to fluorinated 1,4-enynes bearing an all-carbon quaternary center. Org. Lett. 21, 3123–3126 (2019).
Fujita, T. et al. Chemo- and enantioselective Pd/B hybrid catalysis for the construction of acyclic quaternary carbons: migratory allylation of O-allyl esters to α-C-allyl carboxylic acids. J. Am. Chem. Soc. 140, 5899–5903 (2018).
Yu, K. et al. Lithium enolates in the enantioselective construction of tetrasubstituted carbon centers with chiral lithium amides as noncovalent stereodirecting auxiliaries. J. Am. Chem. Soc. 139, 527–533 (2017).
Anthony, S. M., Wonilowicz, L. G., McVeigh, M. S. & Garg, N. K. Leveraging fleeting strained intermediates to access complex scaffolds. JACS Au 1, 897–912 (2021).
Fluegel, L. L. & Hoye, T. R. Hexadehydro-Diels–Alder reaction: benzyne generation via cycloisomerization of tethered triynes. Chem. Rev. 121, 2413–2444 (2021).
Matsuzawa, T., Yoshida, S. & Hosoya, T. Recent advances in reactions between arynes and organosulfur compounds. Tetrahedron Lett. 59, 4197–4208 (2018).
Takikawa, H., Nishii, A., Sakai, T. & Suzuki, K. Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds. Chem. Soc. Rev. 47, 8030–8056 (2018).
Roy, T. & Biju, A. T. Recent advances in molecular rearrangements involving aryne intermediates. Chem. Commun. 54, 2580–2594 (2018).
Shi, J., Li, Y. & Li, Y. Aryne multifunctionalization with benzdiyne and benztriyne equivalents. Chem. Soc. Rev. 46, 1707–1719 (2017).
Idiris, F. I. M. & Jones, C. R. Recent advances in fluoride-free aryne generation from arene precursors. Org. Biomol. Chem. 15, 9044–9056 (2017).
Bhojgude, S. S., Bhunia, A. & Biju, A. T. Employing arynes in Diels–Alder reactions and transition-metal-free multicomponent coupling and arylation reactions. Acc. Chem. Res. 49, 1658–1670 (2016).
Goetz, A. E., Shah, T. K. & Garg, N. K. Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products. Chem. Commun. 51, 34–45 (2015).
Dubrovskiy, A. V., Markina, N. A. & Larock, R. C. Use of benzynes for the synthesis of heterocycles. Org. Biomol. Chem. 11, 191–218 (2013).
Tadross, P. M. & Stoltz, B. M. A comprehensive history of arynes in natural product total synthesis. Chem. Rev. 112, 3550–3577 (2012).
Gampe, C. M. & Carreira, E. M. Arynes and cyclohexyne in natural product synthesis. Angew. Chem. Int. Ed. 51, 3766–3778 (2012).
Wittig, G. & Dürr, H. Dehydrobenzol und acyclische Diene. Justus Liebigs Ann. Chem. 672, 55–62 (1964).
Dockendorff, C., Sahli, S., Olsen, M., Milhau, L. & Lautens, M. Synthesis of dihydronaphthalenes via aryne Diels–Alder reactions: scope and diastereoselectivity. J. Am. Chem. Soc. 127, 15028–15029 (2005).
Webster, R. & Lautens, M. Conformational effects in diastereoselective aryne Diels–Alder reactions: synthesis of benzo-fused [2.2.1] heterobicycles. Org. Lett. 11, 4688–4691 (2009).
Perecim, G. P. et al. Stereoselective total synthesis of (S)- and (R)-nuciferine using benzyne chemistry. Tetrahedron 76, 131461 (2020).
Bilodeau, D. A., Margison, K. D., Serhan, M. & Pezacki, J. P. Bioorthogonal reactions utilizing nitrones as versatile dipoles in cycloaddition reactions. Chem. Rev. 121, 6699–6717 (2021).
Berthet, M., Cheviet, T., Dujardin, G., Parrot, I. & Martinez, J. Isoxazolidine: a privileged scaffold for organic and medicinal chemistry. Chem. Rev. 116, 15235–15283 (2016).
Khangarot, R. K. & Kaliappan, K. P. A stereoselective route to Aza-C-aryl glycosides from arynes and chiral nitrones. Eur. J. Org. Chem. 2012, 5844–5854 (2012).
Ye, W., Zhang, L., Ni, C., Rong, J. & Hu, J. Stereoselective [3+2] cycloaddition of N-tert-butanesulfinyl imines to arynes facilitated by a removable PhSO2CF2 group: synthesis and transformation of cyclic sulfoximines. Chem. Commun. 50, 10596–10599 (2014).
Corsello, M. A., Kim, J. & Garg, N. K. Total synthesis of (–)-tubingensin B enabled by the strategic use of an aryne cyclization. Nat. Chem. 9, 944–949 (2017).
TePaske, M. R., Gloer, J. B., Wicklow, D. T. & Dowd, P. F. Tubingensin A: an antiviral carbazole alkaloid from the sclerotia of Aspergillus tubingensis. J. Org. Chem. 54, 4743–4746 (1989).
TePaske, M. R., Gloer, J. B., Wicklow, D. T. & Dowd, P. F. The structure of tubingensin B: a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis. Tetrahedron Lett. 30, 5965–5968 (1989).
Caubere, P. Applications of sodamide-containing complex bases in organic synthesis. Acc. Chem. Res. 7, 301–308 (1974).
Gregoire, B., Carre, M. C. & Caubere, P. Arynic condensation of ketone enolates. 17. New general access to benzocyclobutene derivatives. J. Org. Chem. 51, 1419–1427 (1986).
Ishida, N., Sawano, S., Masuda, Y. & Murakami, M. Rhodium-catalyzed ring opening of benzycyclobutenols with site-selectivity complementary to thermal ring opening. J. Am. Chem. Soc. 134, 17502–17504 (2012).
Bhojgude, S. S., Thangaraj, M., Suresh, E. & Biju, A. T. Tandem [4 + 2]/[2 + 2] cycloaddition reactions involving indene or benzofurans and arynes. Org. Lett. 16, 3576–3579 (2014).
Alsherimi, A. S. et al. Synthesis of spirocyclic 1-pyrrolines from nitrones and arynes through a dearomative [3,3ʹ]-sigmatropic rearrangement. Angew. Chem. Int. Ed. 59, 15244–15248 (2020).
He, J. et al. Arene trifunctionalization with highly fused ring systems through a domino aryne nucleophilic and Diels–Alder cascade. Angew. Chem. Int. Ed. 58, 18513–18518 (2019).
Swain, S. P. et al. Aryne-induced novel tandem 1,2-addition/(3+2) cycloaddition to generate imidazolidines and pyrrolidines. Angew. Chem. Int. Ed. 54, 9926–9930 (2015).
Jia, H. et al. Tandem nucleophilic addition–cycloaddition of arynes with α-iminoesters: two concurrent pathways to imidazolidines. Chem. Commun. 54, 7050–7053 (2018).
Chen, Z. et al. Aryne 1,4-disubstitution and remote diastereoselective 1,2,4-trisubstitution via a nucleophilic annulation-[5,5]-sigmatropic rearrangement process. Angew. Chem. Int. Ed. 61, e202212160 (2022).
Zhang, J. et al. Aryne-mediated [2,3]-sigmatropic rearrangement of tertiary allylic amines. Org. Lett. 18, 4872–4875 (2016).
Roy, T. et al. The aryne [2,3] Stevens rearrangement. Org. Lett. 18, 5428–5431 (2016).
Huters, A. D., Quasdorf, K. W., Styduhar, E. D. & Garg, N. K. Total synthesis of (−)-N-methylwelwitindolinone C isothiocyanate. J. Am. Chem. Soc. 133, 15797–15799 (2011).
Goetz, A. E., Silberstein, A. L., Corsello, M. A. & Garg, N. K. Concise enantiospecific total synthesis of tubingensin A. J. Am. Chem. Soc. 136, 3036–3039 (2014).
Watsona, I. D. G. & Toste, F. D. Catalytic enantioselective carbon–carbon bond formation using cycloisomerization reactions. Chem. Sci. 3, 2899–2919 (2012).
Trost, B. M., Toste, F. D. & Pinkerton, A. B. Non-metathesis ruthenium-catalyzed C−C bond formation. Chem. Rev. 101, 2067–2096 (2001).
Aubert, C., Buisine, O. & Malacria, M. The behavior of 1,n-enynes in the presence of transition metals. Chem. Rev. 102, 813–834 (2002).
Yang, Y. & Jones, C. R. The arene ene reaction. Synthesis 54, 5042–5054 (2022).
Xu, H. et al. Domino aryne annulation via a nucleophilic–ene process. J. Am. Chem. Soc. 140, 3555–3559 (2018).
Chen, Z., Liang, J., Yin, J., Yu, G.-A. & Liu, S. H. Alder-ene reaction of aryne with olefins. Tetrahedron Lett. 54, 5785–5787 (2013).
Karmakar, R., Mamidipalli, P., Yun, S. Y. & Lee, D. Alder-ene reactions of arynes. Org. Lett. 15, 1938–1941 (2013).
Jayanth, T. T., Jeganmohan, M., Cheng, M.-J., Chu, S.-Y. & Cheng, C.-H. Ene reaction of arynes with alkynes. J. Am. Chem. Soc. 128, 2232–2233 (2006).
Aly, A. A. & Shaker, R. M. 5-Benzyl-1H-tetrazols from the reaction of 1-aryl-5-methyl-1H-tetrazoles with 1,2-dehydrobenzene. Tetrahedron Lett. 46, 2679–2682 (2005).
Aly, A. A., Mohamed, N. K., Hassan, A. A. & Mourad, A. F. E. E. Reaction of diimines and benzyne. Tetrahedron 55, 1111–1118 (1999).
Garsky, V., Koster, D. F. & Arnold, R. T. Studies of the stereochemistry and mechanism of the ene reaction using specifically deuterated pinenes. J. Am. Chem. Soc. 96, 4207–4210 (1974).
Crews, P. & Beard, J. Cycloadditions of benzyne with cyclic olefins. Competition between 2 + 4, ene, and 2 + 2 reaction pathways. J. Org. Chem. 38, 522–528 (1973).
Candito, D. A., Panteleev, J. & Lautens, M. Intramolecular aryne-ene reaction: synthetic and mechanistic studies. J. Am. Chem. Soc. 133, 14200–14203 (2011).
Candito, D. A., Dobrovolsky, D. & Lautens, M. Development of an intramolecular aryne ene reaction and application to the formal synthesis of (±)-crinine. J. Am. Chem. Soc. 134, 15572–15580 (2012).
Cheong, P. H.-Y. et al. Indolyne and aryne distortions and nucleophilic regioselectivites. J. Am. Chem. Soc. 132, 1267–1269 (2010).
Im, G.-Y. J. et al. Indolyne experimental and computational studies: synthetic applications and origins of selectivities of nucleophilic additions. J. Am. Chem. Soc. 132, 17933–17944 (2010).
Goetz, A. E. et al. An efficient computational model to predict the synthetic utility of heterocyclic arynes. Angew. Chem. Int. Ed. 51, 2758–2762 (2012).
Gupta, S., Xie, P., Xia, Y. & Lee, D. Reactivity and selectivity in the intermolecular Alder-ene reactions of arynes with functionalized alkenes. Org. Lett. 19, 5162–5165 (2017).
Hao, Y.-J., Hu, X.-S., Zhou, Y., Zhou, J. & Yu, J.-S. Catalytic enantioselective α-arylation of carbonyl enolates and related compounds. ACS Catal. 10, 955–993 (2020).
Johansson, C. C. C. & Colacot, T. J. Metal-catalyzed α-arylation of carbonyl and related molecules: novel trends in C–C bond formation by C–H bond functionalization. Angew. Chem. Int. Ed. 49, 676–707 (2010).
Jones, E. P., Jones, P. & Barrett, A. G. M. Asymmetric synthesis of α-aryl amino acids; aryne-mediated diastereoselective arylation. Org. Lett. 13, 1012–1015 (2011).
Jones, E. P., Jones, P., White, A. J. P. & Barrett, A. G. M. Asymmetric synthesis of quaternary aryl amino acid derivatives via a three-component aryne coupling reaction. Beilstein J. Org. Chem. 7, 1570–1576 (2011).
Schöllkopf, U. Enantioselective synthesis of non-proteinogenic amino acids via metallated bis-lactim ethers of 2,5-diketopiperazines. Tetrahedron 39, 2085–2091 (1983).
Picazo, E. et al. Arynes and cyclic alkynes as synthetic building blocks for stereodefined quaternary centers. J. Am. Chem. Soc. 140, 7605–7610 (2018). A paper on diastereoselective α-arylation using enamines leading to catalytic asymmetric synthesis in α-arylation.
Kasamatsu, K. et al. α-Arylation of α-amino acid derivatives with arynes via memory of chirality: asymmetric synthesis of benzocyclobutenones with tetrasubstituted carbon. Org. Lett. 19, 352–355 (2017).
Alezra, V. & Kawabata, T. Recent progress in memory of chirality (MOC): an advanced chiral pool. Synthesis 48, 2997–3016 (2016).
Tomohara, K., Yoshimura, T., Hyakutake, R., Yang, P. & Kawabata, T. Asymmetric α-arylation of amino acid derivatives by Clayden rearrangement of ester enolates via memory of chirality. J. Am. Chem. Soc. 135, 13294–13297 (2013).
Kawabata, T., Moriyama, K., Kawakami, S. & Tsubaki, K. Powdered KOH in DMSO: an efficient base for asymmetric cyclization via memory of chirality at ambient temperature. J. Am. Chem. Soc. 130, 4153–4157 (2008).
Kawabata, T., Yahiro, K. & Fuji, K. Memory of chirality: enantioselective alkylation reactions at an asymmetric carbon adjacent to a carbonyl group. J. Am. Chem. Soc. 113, 9694–9696 (1991).
Zhao, H., Hsu, D. C. & Carlier, P. R. Memory of chirality: an emerging strategy for asymmetric synthesis. Synthesis 2005, 1–16 (2005).
Bringmann, G. et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5384–5427 (2005).
Tanaka, K. Transition-metal-catalyzed enantio-selective [2+2+2] cycloadditions for the synthesis of axially chiral biaryls. Chem. Asian J. 4, 508–518 (2009).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Zilate, B., Castrogiovanni, A. & Sparr, C. Catalyst-controlled stereoselective synthesis of atropisomers. ACS Catal. 8, 2981–2988 (2018).
Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via transition metal-catalysed asymmetric C–H functionalization. Chem. Commun. 55, 8514–8523 (2019).
Bao, X., Rodriguez, J. & Bonne, D. Enantioselective synthesis of atropisomers with multiple stereogenic axes. Angew. Chem. Int. Ed. 59, 12623–12634 (2020).
Zhao, Q., Peng, C., Wang, Y.-T., Zhan, G. & Han, B. Recent progress on the construction of axial chirality through transition-metal-catalyzed benzannulation. Org. Chem. Front. 8, 2772–2785 (2021).
Wittig, G., Pieper, G. & Fuhrmann, G. Über die bildung von diphenyl aus fluorbenzol und phenyl-lithium (IV. Mitteil. über Austauschreaktionen mit phenyl-lithium). Ber. Dtsch. Chem. Ges. A B 73, 1193–1197 (1940).
Gilman, H. & Gaj, B. Coupling reactions with some organolithium compounds in tetrahydrofuran. J. Org. Chem. 22, 447–449 (1957).
Leroux, F. & Schlosser, M. The ‘aryne’ route to biaryls featuring uncommon substituent patterns. Angew. Chem. Int. Ed. 41, 4272–4274 (2002).
Berthelot-Bréhier, A., Panossian, A., Colobert, F. & Leroux, F. R. Atroposelective synthesis of axially chiral P,S-ligands based on arynes. Org. Chem. Front. 2, 634–644 (2015).
Leroux, F. R., Berthelot, A., Bonnafoux, L., Panossian, A. & Colobert, F. Transition-metal-free atropo-selective synthesis of biaryl compounds based on arynes. Chemistry 18, 14232–14236 (2012).
Augros, D. et al. Atropo-diastereoselective coupling of aryllithiums and arynes — variations around the chiral auxiliary. Tetrahedron 72, 5208–5220 (2016).
Yalcouye, B. et al. Access to atropisomerically enriched biaryls by the coupling of aryllithiums with arynes under control by homochiral oxazolines. Eur. J. Org. Chem. 2016, 725–732 (2016).
Augros, D. et al. Transition-metal-free synthesis of a known intermediate in the formal synthesis of (–)-steganacin. Eur. J. Org. Chem. 2017, 497–503 (2017).
Augros, D. et al. The winding road towards an atropo-enantioselective ‘ARYNE coupling’. Eur. J. Org. Chem. 2021, 1971–1978 (2021).
Wei, Y.-L., Dauvergne, G., Rodriguez, J. & Coquerel, Y. Enantiospecific generation and trapping reactions of aryne atropisomers. J. Am. Chem. Soc. 142, 16921–16925 (2020). The first example showing that an aryne with an axially chiral biaryl skeleton can be used in a reaction while retaining its axial chirality.
Dauvergne, G. et al. Determination of the rate constant of the [4 + 2] cycloaddition between an aryne atropisomer and furan in solution. J. Org. Chem. 87, 11141–11147 (2022).
Mohanan, K., Coquerel, Y. & Rodriguez, J. Transition-metal-free α-arylation of β-keto amides via an interrupted insertion reaction of arynes. Org. Lett. 14, 4686–4689 (2012). The first paper showing the possibility of catalytic asymmetric synthesis in α-arylation of β-keto amide.
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α-arylation of cyclic β-ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020). The second paper on catalytic asymmetric synthesis using arynes to achieve high enantioselectivities in excess of 90% ee and the first example in central asymmetry control.
Acknowledgements
The author gratefully acknowledges a Grant-in-Aid for Scientific Research (B) (22H02081), a Grant-in-Aid for Challenging Exploratory Research (22K19035) and a Toshiaki Ogasawara Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Christopher Jones and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamikawa, K. Asymmetric reactions involving aryne intermediates. Nat Rev Chem 7, 496–510 (2023). https://doi.org/10.1038/s41570-023-00485-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00485-y