Abstract
The construction of complex polymer architectures with well-defined topology, composition and functionality has been extensively explored as the molecular basis for the development of modern polymer materials. The unique reaction kinetics of free-radical polymerization leads to the concurrent formation of crosslinks between polymer chains and rings within an individual chain and, thus, free-radical (co)polymerization of multivinyl monomers provides a facile method to manipulate chain topology and functionality. Regulating the relative contribution of these intermolecular and intramolecular chain-propagation reactions is the key to the construction of architecturally complex polymers. This can be achieved through the design of new monomers or by spatially or kinetically controlling crosslinking reactions. These mechanisms enable the synthesis of various polymer architectures, including linear, cyclized, branched and star polymer chains, as well as crosslinked networks. In this Review, we highlight some of the contemporary experimental strategies to prepare complex polymer architectures using radical polymerization of multivinyl monomers. We also examine the recent development of characterization techniques for sub-chain connections in such complex macromolecules. Finally, we discuss how these crosslinking reactions have been engineered to generate advanced polymer materials for use in a variety of biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
PlasticsEurope. Plastics — the Facts 2018 https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (2018).
Nesvadba, P. in Encyclopedia of Radicals in Chemistry, Biology and Materials https://doi.org/10.1002/9781119953678.rad080 (Wiley, 2012).
Matyjaszewski, K. & Tsarevsky, N. V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 1, 276–288 (2009).
Matyjaszewski, K. Architecturally complex polymers with controlled heterogeneity. Science 333, 1104–1105 (2011).
Georges, M. K., Veregin, R. P. N., Kazmaier, P. M. & Hamer, G. K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules 26, 2987–2988 (1993).
Wang, J.-S. & Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 117, 5614–5615 (1995).
Chiefari, J. et al. Living free-radical polymerization by reversible addition–fragmentation chain transfer: the RAFT process. Macromolecules 31, 5559–5562 (1998).
Stals, P. J. M. et al. How far can we push polymer architectures? J. Am. Chem. Soc. 135, 11421–11424 (2013).
Newland, B. et al. Single cyclized molecule versus single branched molecule: a simple and efficient 3D “knot” polymer structure for nonviral gene delivery. J. Am. Chem. Soc. 134, 4782–4789 (2012).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Terashima, T., Kamigaito, M., Baek, K.-Y., Ando, T. & Sawamoto, M. Polymer catalysts from polymerization catalysts: direct encapsulation of metal catalyst into star polymer core during metal-catalyzed living radical polymerization. J. Am. Chem. Soc. 125, 5288–5289 (2003).
Staudinger, H. & Husemann, E. Über hochpolymere Verbindungen, 116. Mitteil.: Über das begrenzt quellbare Poly-styrol. Ber. Dtsch. Chem. Ges. 68, 1618–1634 (1935).
Flory, P. J. Molecular size distribution in three dimensional polymers. I. Gelation. J. Am. Chem. Soc. 63, 3083–3090 (1941).
Stockmayer, W. H. Theory of molecular size distribution and gel formation in branched polymers II. General cross linking. J. Chem. Phys. 12, 125–131 (1944).
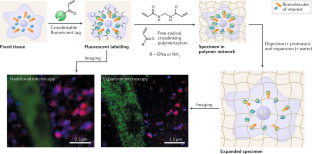
Chen, F. F., Tillberg, P. W. & Boyden, E. S. Expansion microscopy. Science 347, 543–548 (2015). This work shows the application of free-radical copolymerization of monovinyl/divinyl monomers for biological imaging.
Distefano, G. et al. Highly ordered alignment of a vinyl polymer by host–guest cross-polymerization. Nat. Chem. 5, 335–341 (2013). This work describes the free-radical copolymerization of monovinyl/divinyl monomers in porous coordination polymers, yielding a pseudo-crystalline polymeric network.
Gu, Y., Zhao, J. & Johnson, J. A. A (macro)molecular-level understanding of polymer network topology. Trends Chem. 1, 318–334 (2019).
Seiffert, S. Origin of nanostructural inhomogeneity in polymer-network gels. Polym. Chem. 8, 4472–4487 (2017).
Matsumoto, A., Miwa, Y., Inoue, S., Enomoto, T. & Aota, H. Discussion of “greatly delayed gelation from theory in free-radical cross-linking multivinyl polymerization accompanied by microgel formation” based on multiallyl polymerization. Macromolecules 43, 6834–6842 (2010).
Okay, O., Kurz, M., Lutz, K. & Funke, W. Cyclization and reduced pendant vinyl group reactivity during the free-radical crosslinking polymerization of 1,4-divinylbenzene. Macromolecules 28, 2728–2737 (1995).
Norisuye, T. et al. Small angle neutron scattering studies on structural inhomogeneities in polymer gels: irradiation cross-linked gels vs chemically cross-linked gels. Polymer 43, 5289–5297 (2002).
Adibnia, V. & Hill, R. J. Universal aspects of hydrogel gelation kinetics, percolation and viscoelasticity from PA-hydrogel rheology. J. Rheol. 60, 541–548 (2016).
Polanowski, P., Jeszka, J. K., Li, W. & Matyjaszewski, K. Effect of dilution on branching and gelation in living copolymerization of monomer and divinyl cross-linker: modeling using dynamic lattice liquid model (DLL) and Flory–Stockmayer (FS) model. Polymer 52, 5092–5101 (2011).
Lyu, J. et al. Monte Carlo simulations of atom transfer radical (homo)polymerization of divinyl monomers: applicability of Flory–Stockmayer theory. Macromolecules 51, 6673–6681 (2018).
Elliott, J. E., Anseth, J. W. & Bowman, C. N. Kinetic modeling of the effect of solvent concentration on primary cyclization during polymerization of multifunctional monomers. Chem. Eng. Sci. 56, 3173–3184 (2001).
Cerid, H. & Okay, O. Minimization of spatial inhomogeneity in polystyrene gels formed by free-radical mechanism. Eur. Polym. J. 40, 579–587 (2004).
Elliott, J. E. & Bowman, C. N. Effects of solvent quality during polymerization on network structure of cross-linked methacrylate copolymers. J. Phys. Chem. B 106, 2843–2847 (2002).
Doura, M., Naka, Y., Aota, H. & Matsumoto, A. Control of intermolecular cross-linking reaction in free-radical cross-linking monovinyl/divinyl copolymerizations by the aid of amphiphilic nature of primary polymer chains and cross-link units with opposite polarities. Macromolecules 38, 5955–5963 (2005).
Mori, H. & Tsukamoto, M. RAFT polymerization of diacrylate derivatives having different spacers in dilute conditions. Polymer 52, 635–645 (2011).
Yu, Q., Zhu, Y., Ding, Y. & Zhu, S. Reaction behavior and network development in RAFT radical polymerization of dimethacrylates. Macromol. Chem. Phys. 209, 551–556 (2008).
Van Camp, W., Gao, H., Du Prez, F. E. & Matyjaszewski, K. Effect of crosslinker multiplicity on the gel point in ATRP. J. Polym. Sci. A Polym. Chem. 48, 2016–2023 (2010).
Elliott, J. E. & Bowman, C. N. Monomer functionality and polymer network formation. Macromolecules 34, 4642–4649 (2001).
Patras, G., Qiao, G. G. & Solomon, D. H. Novel cross-linked homogeneous polyacrylamide gels with improved separation properties: Investigation of the cross-linker functionality. Electrophoresis 22, 4303–4310 (2001).
Denisin, A. K. & Pruitt, B. L. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl. Mater. Interfaces 8, 21893–21902 (2016).
Shi, Y., Graff, R. W., Cao, X., Wang, X. & Gao, H. Chain-growth click polymerization of AB2 monomers for the formation of hyperbranched polymers with low polydispersities in a one-pot process. Angew. Chem. Int. Ed. 54, 7562–7562 (2015).
O’Brien, N., McKee, A., Sherrington, D. C. C., Slark, A. T. T. & Titterton, A. Facile, versatile and cost effective route to branched vinyl polymers. Polymer 41, 6027–6031 (2000). This work proposes the ‘Strathclyde route’ for synthesizing branched polymers from radical polymerization of multivinyl monomers with the addition of chain-transfer agents.
Besenius, P., Slavin, S., Vilela, F. & Sherrington, D. C. Synthesis and characterization of water-soluble densely branched glycopolymers. React. Funct. Polym. 68, 1524–1533 (2008).
Baudry, R. & Sherrington, D. C. Synthesis of highly branched poly(methyl methacrylate)s using the “Strathclyde methodology” in aqueous emulsion. Macromolecules 39, 1455–1460 (2006).
Chisholm, M., Hudson, N., Kirtley, N., Vilela, F. & Sherrington, D. C. Application of the “Strathclyde route” to branched vinyl polymers in suspension polymerization: architectural, thermal, and rheological characterization of the derived branched products. Macromolecules 42, 7745–7752 (2009).
Xiang, L., Song, Y., Qiu, M. & Su, Y. Synthesis of branched poly(butyl acrylate) using the Strathclyde method in continuous-flow microreactors. Ind. Eng. Chem. Res. 58, 21312–21322 (2019).
Guan, Z. Control of polymer topology through transition-metal catalysis: synthesis of hyperbranched polymers by cobalt-mediated free radical polymerization. J. Am. Chem. Soc. 124, 5616–5617 (2002).
McEwan, K. A. & Haddleton, D. M. Combining catalytic chain transfer polymerisation (CCTP) and thio-Michael addition: enabling the synthesis of peripherally functionalised branched polymers. Polym. Chem. 2, 1992–1999 (2011).
Smeets, N. M. B. Amphiphilic hyperbranched polymers from the copolymerization of a vinyl and divinyl monomer: the potential of catalytic chain transfer polymerization. Eur. Polym. J. 49, 2528–2544 (2013).
Kurochkin, S. A., Silant’ev, M. A., Perepelitsyna, E. O. & Grachev, V. P. Synthesis of branched polymers via radical copolymerization under oxygen inflow. Eur. Polym. J. 57, 202–212 (2014).
Hirano, T., Kamiike, R., Hsu, Y., Momose, H. & Ute, K. Multivariate analysis of 13C NMR spectra of branched copolymers prepared by initiator-fragment incorporation radical copolymerization of ethylene glycol dimethacrylate and tert-butyl methacrylate. Polym. J. 48, 793–800 (2016).
Liang, S., Li, X., Wang, W.-J. J., Li, B.-G. & Zhu, S. Toward understanding of branching in RAFT copolymerization of methyl methacrylate through a cleavable dimethacrylate. Macromolecules 49, 752–759 (2016).
Bannister, I., Billingham, N. C., Armes, S. P., Rannard, S. P. & Findlay, P. Development of branching in living radical copolymerization of vinyl and divinyl monomers. Macromolecules 39, 7483–7492 (2006).
Rosselgong, J., Armes, S. P., Barton, W. & Price, D. Synthesis of highly branched methacrylic copolymers: observation of near-ideal behavior using RAFT polymerization. Macromolecules 42, 5919–5924 (2009).
Bannister, I., Billingham, N. C. & Armes, S. P. Monte Carlo modelling of living branching copolymerisation of monovinyl and divinyl monomers: comparison of simulated and experimental data for ATRP copolymerisation of methacrylic monomers. Soft Matter 5, 3495–3504 (2009).
Bouhier, M.-H., Cormack, P. A. G., Graham, S. & Sherrington, D. C. Synthesis of densely branched poly(methyl methacrylate)s via ATR copolymerization of methyl methacrylate and ethylene glycol dimethacrylate. J. Polym. Sci. A Polym. Chem. 45, 2375–2386 (2007).
Liu, B., Kazlauciunas, A., Guthrie, J. T. & Perrier, S. One-pot hyperbranched polymer synthesis mediated by reversible addition fragmentation chain transfer (RAFT) polymerization. Macromolecules 38, 2131–2136 (2005).
Gao, H., Min, K. & Matyjaszewski, K. Determination of gel point during atom transfer radical copolymerization with cross-linker. Macromolecules 40, 7763–7770 (2007).
Yang, H. et al. Synthesis of highly branched polymers by reversible complexation-mediated copolymerization of vinyl and divinyl monomers. Polym. Chem. 8, 2137–2144 (2017).
Flynn, S., Dwyer, A. B., Chambon, P. & Rannard, S. Expanding the monomer scope of linear and branched vinyl polymerisations via copper-catalysed reversible-deactivation radical polymerisation of hydrophobic methacrylates using anhydrous alcohol solvents. Polym. Chem. 10, 5103–5115 (2019).
Rosselgong, J., Armes, S. P., Barton, W. R. S. & Price, D. Synthesis of branched methacrylic copolymers: comparison between RAFT and ATRP and effect of varying the monomer concentration. Macromolecules 43, 2145–2156 (2010).
Gao, Y., Newland, B., Zhou, D., Matyjaszewski, K. & Wang, W. Controlled polymerization of multivinyl monomers: formation of cyclized/knotted single-chain polymer architectures. Angew. Chem. Int. Ed. 56, 450–460 (2017).
Wang, W. et al. Controlling chain growth: a new strategy to hyperbranched materials. Macromolecules 40, 7184–7194 (2007).
Zhao, T. et al. Water soluble hyperbranched polymers from controlled radical homopolymerization of PEG diacrylate. RSC Adv. 5, 33823–33830 (2015).
Zhao, T., Zheng, Y., Poly, J. & Wang, W. Controlled multi-vinyl monomer homopolymerization through vinyl oligomer combination as a universal approach to hyperbranched architectures. Nat. Commun. 4, 1873 (2013). This work describes a universal approach to synthesize hyperbranched polymers from kinetically controlled multivinyl monomers.
Odian, G. in Principles of Polymerization (Wiley, 2004).
Gao, H., Miasnikova, A. & Matyjaszewski, K. Effect of cross-linker reactivity on experimental gel points during ATRcP of monomer and cross-linker. Macromolecules 41, 7843–7849 (2008).
Nagelsdiek, R., Mennicken, M., Maier, B., Keul, H. & Höcker, H. Synthesis of polymers containing cross-linkable groups by atom transfer radical polymerization: poly(allyl methacrylate) and copolymers of allyl methacrylate and styrene. Macromolecules 37, 8923–8932 (2004).
Yhaya, F., Sutinah, A., Gregory, A. M., Liang, M. & Stenzel, M. H. RAFT polymerization of vinyl methacrylate and subsequent conjugation via enzymatic thiol-ene chemistry. J. Polym. Sci. A Polym. Chem. 50, 4085–4093 (2012).
Akiyama, M., Yoshida, K. & Mori, H. Controlled synthesis of vinyl-functionalized homopolymers and block copolymers by RAFT polymerization of vinyl methacrylate. Polymer 55, 813–823 (2014).
Ma, J., Cheng, C., Sun, G. & Wooley, K. L. Well-defined polymers bearing pendent alkene functionalities via selective RAFT polymerization. Macromolecules 41, 9080–9089 (2008).
Ma, J., Cheng, C. & Wooley, K. L. Cycloalkenyl-functionalized polymers and block copolymers: syntheses via selective RAFT polymerizations and demonstration of their versatile reactivity. Macromolecules 42, 1565–1573 (2009).
Qu, Q., Liu, G., Lv, X., Zhang, B. & An, Z. In situ cross-linking of vesicles in polymerization-induced self-assembly. ACS Macro Lett. 5, 316–320 (2016).
Chen, L. et al. Chemoselective RAFT polymerization of a trivinyl monomer derived from carbon dioxide and 1,3-butadiene: from linear to hyperbranched. Macromolecules 50, 9598–9606 (2017).
Dong, Z. M., Liu, X. H., Tang, X. L. & Li, Y. S. Synthesis of hyperbranched polymers with pendent norbornene functionalities via raft polymerization of a novel asymmetrical divinyl monomer. Macromolecules 42, 4596–4603 (2009).
Ren, J. M. et al. Star polymers. Chem. Rev. 116, 6743–6836 (2016).
Gao, H. & Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: from stars to gels. Prog. Polym. Sci. 34, 317–350 (2009).
Wang, D. et al. Kinetics and modeling of semi-batch RAFT copolymerization with hyperbranching. Macromolecules 45, 28–38 (2012). This work describes the synthesis of branched polymers from semi-batch copolymerization of multivinyl monomers.
Wang, D., Wang, W.-J., Li, B.-G. & Zhu, S. Semibatch RAFT polymerization for branched polyacrylamide production: effect of divinyl monomer feeding policies. AIChE J. 59, 1322–1333 (2013).
Xia, J., Zhang, X. & Matyjaszewski, K. Synthesis of star-shaped polystyrene by atom transfer radical polymerization using an ‘arm first’ approach. Macromolecules 32, 4482–4484 (1999).
Gao, H., Ohno, S., & Matyjaszewski, K. Low Polydispersity Star Polymers via Cross-Linking Macromonomers by ATRP. J. Am. Chem. Soc. 128, 15111–15113 (2006).
Connal, L. A., Vestberg, R., Hawker, C. J. & Qiao, G. G. Synthesis of dendron functionalized core cross-linked star polymers. Macromolecules 40, 7855–7863 (2007).
Hatton, F. L., Chambon, P., McDonald, T. O., Owen, A. & Rannard, S. P. Hyperbranched polydendrons: a new controlled macromolecular architecture with self-assembly in water and organic solvents. Chem. Sci. 5, 1844–1853 (2014).
Hern, F. Y., Hill, A., Owen, A. & Rannard, S. P. Co-initiated hyperbranched-polydendron building blocks for the direct nanoprecipitation of dendron-directed patchy particles with heterogeneous surface functionality. Polym. Chem. 9, 1767–1771 (2018).
Kanaoka, S., Sawamoto, M. & Higashimura, T. Star-shaped polymers by living cationic polymerization. 1. Synthesis of star-shaped polymers of alkyl and vinyl ethers. Macromolecules 24, 2309–2313 (1991).
Gao, H. & Matyjaszewski, K. Structural control in ATRP synthesis of star polymers using the arm-first method. Macromolecules 39, 3154–3160 (2006).
Baek, K.-Y. Y., Kamigaito, M. & Sawamoto, M. Star-shaped polymers by metal-catalyzed living radical polymerization. 1. Design of Ru(II)-based systems and divinyl linking agents. Macromolecules 34, 215–221 (2001).
Baek, K.-Y., Kamigaito, M. & Sawamoto, M. Core-functionalized star polymers by transition metal-catalyzed living radical polymerization. 1. Synthesis and characterization of star polymers with PMMA arms and amide cores. Macromolecules 34, 7629–7635 (2001).
Li, W. & Matyjaszewski, K. Star polymers via cross-linking amphiphilic macroinitiators by AGET ATRP in aqueous media. J. Am. Chem. Soc. 131, 10378–10379 (2009).
Zhang, X., Xia, J. & Matyjaszewski, K. End-functional poly(tert-butyl acrylate) star polymers by controlled radical polymerization. Macromolecules 33, 2340–2345 (2000).
Pasquale, A. J. & Long, T. E. Synthesis of star-shaped polystyrenes via nitroxide-mediated stable free-radical polymerization. J. Polym. Sci. A Polym. Chem. 39, 216–223 (2001).
Gao, H. & Matyjaszewski, K. Synthesis of star polymers by a new “core-first” method: sequential polymerization of cross-linker and monomer. Macromolecules 41, 1118–1125 (2008).
Butler, G. B. & Bunch, R. L. Preparation and polymerization of unsaturated quaternary ammonium compounds. J. Am. Chem. Soc. 71, 3120–3122 (1949).
Butler, G. B. & Ingley, F. L. Preparation and polymerization of unsaturated quaternary ammonium compounds. II. Halogenated allyl derivatives. J. Am. Chem. Soc. 73, 895–896 (1951).
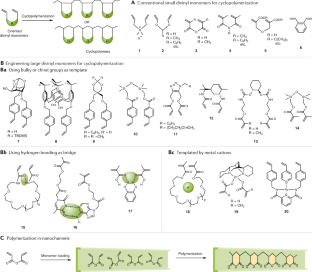
Pasini, D. & Takeuchi, D. Cyclopolymerizations: synthetic tools for the precision synthesis of macromolecular architectures. Chem. Rev. 118, 8983–9057 (2018).
Saito, Y. & Saito, R. The effect of the distance between neighboring vinyl groups on template polymerization. Polymer 52, 3565–3569 (2011).
Matsumoto, A. et al. Crystal engineering for topochemical polymerization of muconic esters using halogen–halogen and CH/π interactions as weak intermolecular interactions. J. Am. Chem. Soc. 124, 8891–8902 (2002).
Crawshaw, A. & Butler, G. B. The formation of linear polymers from diene monomers by a cyclic polymerization mechanism. II. Polyacrylic anhydride and the derived polyacrylic acid. J. Am. Chem. Soc. 80, 5464–5466 (1958).
Tsukino, M. & Kunitake, T. Radical cyclopolymerization of divinyl acetals — structure variation with polymerization conditions. Polym. J. 17, 943–951 (1985).
Costa, L., Chiantore, O. & Guaita, M. Free radical polymerization of unconjugated dienes: 19. Temperature dependence of the cyclopolymerization of o-divinylbenzene. Polymer 19, 202–204 (1978).
Jones, R. G., Harry Cragg, R. & Swain, A. C. Structure and mechanism in the cyclopolymerization of diallylsilanes. Eur. Polym. J. 28, 651–655 (1992).
Erkoc, S., Mathias, L. J. & Acar, A. E. Cyclopolymerization of tert-butyl α-(hydroxymethyl) acrylate (TBHMA) ether dimer via atom transfer radical polymerization (ATRP). Macromolecules 39, 8936–8942 (2006).
Erkoc, S. & Acar, A. E. Controlled/living cyclopolymerization of tert-butyl α-(hydroxymethyl) acrylate ether dimer via reversible addition fragmentation chain transfer polymerization. Macromolecules 41, 9019–9024 (2008).
Yokota, K., Matsumura, M., Yamaguchi, K. & Takada, Y. Synthesis of polymers with benzo-19-crown-6 units via cyclopolymerization of divinyl ethers. Macromol. Rapid Commun. 4, 721–724 (1983).
Wulff, G., Schmidt, H., Witt, H. & Zentel, R. Cooperativity and transfer of chirality in liquid-crystalline polymers. Angew. Chem. Int. Ed. 33, 188–191 (1994).
Kakuchi, T. et al. Chirality induction in cyclocopolymerization. 14. Template effect of 1,2-cycloalkanediol in the cyclocopolymerization of bis(4-vinylbenzoate)s with styrene. Macromolecules 34, 38–43 (2001).
Zhao, X. & Liu, H. Synthesis and characterization of PEG polymer brushes via cyclopolymerization of 1,2,3-triazole tethered diacrylates. Chin. J. Chem. Phys. 28, 739–745 (2015).
Ochiai, B., Shiomi, T. & Yoshita, H. Cyclopolymerization of a bisacrylate through selective formation of a 19-membered ring. Polym. J. 48, 859–862 (2016).
Ochiai, B., Ootani, Y. & Endo, T. Controlled cyclopolymerization through quantitative 19-membered ring formation. J. Am. Chem. Soc. 130, 10832–10833 (2008).
Kim, T.-H. et al. Cyclopolymerisation of an oriented 4,6-bis(4-vinylbenzyl)-myo-inositol orthoformate. Chem. Commun. 24, 2419–2420 (2000).
Kim, T.-H., Holmes, A. B. & Giles, M. Ring closing metathesis of a 4,6-diallyl-myo-inositol orthoformate as a model for an inositol cyclopolymer. Chem. Commun. 24, 2419–2420 (2000).
Li, J., Du, M., Zhao, Z. & Liu, H. Cyclopolymerization of disiloxane-tethered divinyl monomers to synthesize chirality-responsive helical polymers. Macromolecules 49, 445–454 (2016).
Saito, Y. & Saito, R. Synthesis of syndiotactic poly(methacrylic acid) by free-radical polymerization of the pseudo-divinyl monomer formed with methacrylic acid and catechol. J. Appl. Polym. Sci. 128, 3528–3533 (2013).
Kang, Y., Lu, A., Ellington, A., Jewett, M. C. & O’Reilly, R. K. Effect of complementary nucleobase interactions on the copolymer composition of RAFT copolymerizations. ACS Macro Lett. 2, 581–586 (2013).
Kimura, Y., Miyabara, Y., Terashima, T. & Sawamoto, M. Polyacrylamide pseudo crown ethers via hydrogen bond-assisted cyclopolymerization. J. Polym. Sci. A Polym. Chem. 54, 3294–3302 (2016).
Mathur, A. M. & Scranton, A. B. Synthesis and ion-binding properties of polymeric pseudocrown ethers II: template ion induced cyclization of oligomeric ethylene glycol diacrylates. Sep. Sci. Technol. 32, 285–301 (1997).
Terashima, T., Kawabe, M., Miyabara, Y., Yoda, H. & Sawamoto, M. Polymeric pseudo-crown ether for cation recognition via cation template-assisted cyclopolymerization. Nat. Commun. 4, 2321 (2013). This work presents the cyclopolymerization of divinyl monomers in the presence of cation template.
Jana, S. & Sherrington, D. C. Transfer of chirality from (−)-sparteine zinc(II) (meth)acrylate complexes to the main chains of their (meth)acrylate polymer derivatives. Angew. Chem. Int. Ed. 44, 4804–4808 (2005).
Hibi, Y., Ouchi, M. & Sawamoto, M. Sequence-regulated radical polymerization with a metal-templated monomer: repetitive ABA sequence by double cyclopolymerization. Angew. Chem. Int. Ed. 50, 7434–7437 (2011). This work describes the synthesis of sequence-controlled polymers via cyclopolymerization of multivinyl monomers.
Mochizuki, S., Kitao, T. & Uemura, T. Controlled polymerizations using metal–organic frameworks. Chem. Commun. 54, 11843–11856 (2018).
Uemura, T., Hiramatsu, D., Kubota, Y., Takata, M. & Kitagawa, S. Topotactic linear radical polymerization of divinylbenzenes in porous coordination polymers. Angew. Chem. Int. Ed. 46, 4987–4990 (2007).
Uemura, T. et al. Controlled cyclopolymerization of difunctional vinyl monomers in coordination nanochannels. Macromolecules 47, 7321–7326 (2014).
Gao, Y. et al. Intramolecular cyclization dominating homopolymerization of multivinyl monomers toward single-chain cyclized/knotted polymeric nanoparticles. Macromolecules 48, 6882–6889 (2015).
Elliott, J. E. & Bowman, C. N. Kinetics of primary cyclization reactions in cross-linked polymers: an analytical and numerical approach to heterogeneity in network formation. Macromolecules 32, 8621–8628 (1999).
Taylor, W. R. & Lin, K. Protein knots: a tangled problem. Nature 421, 25 (2003).
Zheng, Y. et al. 3D single cyclized polymer chain structure from controlled polymerization of multi-vinyl monomers: beyond Flory–Stockmayer theory. J. Am. Chem. Soc. 133, 13130–13137 (2011). This work describes the synthesis of single-cyclized polymers from intramolecular cyclization-dominated polymerization of multivinyl monomers.
Li, Y. & Armes, S. P. Synthesis and chemical degradation of branched vinyl polymers prepared via ATRP: use of a cleavable disulfide-based branching agent. Macromolecules 38, 8155–8162 (2005).
Rosselgong, J. & Armes, S. P. Quantification of intramolecular cyclization in branched copolymers by 1H NMR spectroscopy. Macromolecules 45, 2731–2737 (2012).
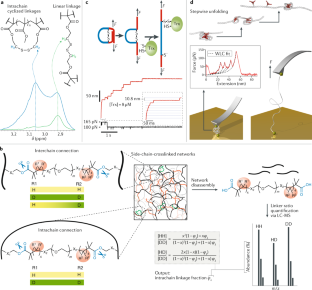
Zhou, H. et al. Counting primary loops in polymer gels. Proc. Natl Acad. Sci. USA 109, 19119–19124 (2012).
Wang, J. et al. Counting secondary loops is required for accurate prediction of end-linked polymer network elasticity. ACS Macro Lett. 7, 244–249 (2018).
Zhong, M., Wang, R., Kawamoto, K., Olsen, B. D. & Johnson, J. A. Quantifying the impact of molecular defects on polymer network elasticity. Science 353, 1264–1268 (2016). This work proposes a technique, called symmetric isotopic labelling disassembly spectrometry (SILDaS), to quantify the intrachain links (loops) in polymer networks.
Wang, J. et al. Counting loops in sidechain-crosslinked polymers from elastic solids to single-chain nanoparticles. Chem. Sci. 10, 5332–5337 (2019).
Wang, D. & Russell, T. P. Advances in atomic force microscopy for probing polymer structure and properties. Macromolecules 51, 3–24 (2018).
Pavliček, N. & Gross, L. Generation, manipulation and characterization of molecules by atomic force microscopy. Nat. Rev. Chem. 1, 0005 (2017).
Krieg, M. et al. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 1, 41–57 (2019).
Burdyńska, J. et al. Synthesis and arm dissociation in molecular stars with a spoked wheel core and bottlebrush arms. J. Am. Chem. Soc. 136, 12762–12770 (2014).
Lafferentz, L. et al. Conductance of a single conjugated polymer as a continuous function of its length. Science 323, 1193–1197 (2009).
Chung, J., Kushner, A. M., Weisman, A. C. & Guan, Z. Direct correlation of single-molecule properties with bulk mechanical performance for the biomimetic design of polymers. Nat. Mater. 13, 1055–1062 (2014).
Beedle, A. E. M. et al. Forcing the reversibility of a mechanochemical reaction. Nat. Commun. 9, 3155 (2018).
Wiita, A. P. et al. Probing the chemistry of thioredoxin catalysis with force. Nature 450, 124–127 (2007).
Garcia-Manyes, S., Liang, J., Szoszkiewicz, R., Kuo, T.-L. & Fernández, J. M. Force-activated reactivity switch in a bimolecular chemical reaction. Nat. Chem. 1, 236–242 (2009).
Hosono, N. et al. Forced unfolding of single-chain polymeric nanoparticles. J. Am. Chem. Soc. 137, 6880–6888 (2015).
Levy, A., Feinstein, R. & Diesendruck, C. E. Mechanical unfolding and thermal refolding of single-chain nanoparticles using ligand–metal bonds. J. Am. Chem. Soc. 141, 7256–7260 (2019).
Liu, N. & Zhang, W. Feeling inter- or intramolecular interactions with the polymer chain as probe: recent progress in SMFS studies on macromolecular interactions. ChemPhysChem 13, 2238–2256 (2012).
Huang, Z. et al. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 6, 2487–2495 (2018).
Kakkar, A., Traverso, G., Farokhzad, O. C., Weissleder, R. & Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 1, 0063 (2017).
Breslow, D. S., Edwards, E. I. & Newburg, N. R. Divinyl ether-maleic anhydride (pyran) copolymer used to demonstrate the effect of molecular weight on biological activity. Nature 246, 160–162 (1973).
Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug. Discov. 2, 347–360 (2003).
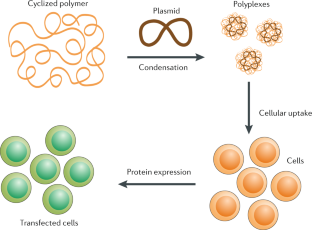
Zhou, D. et al. The transition from linear to highly branched poly(β-amino ester)s: branching matters for gene delivery. Sci. Adv. 2, e1600102 (2016).
Wei, H., Chu, D. S. H., Zhao, J., Pahang, J. A. & Pun, S. H. Synthesis and evaluation of cyclic cationic polymers for nucleic acid delivery. ACS Macro Lett. 2, 1047–1050 (2013).
Cortez, M. A. et al. The synthesis of cyclic poly(ethylene imine) and exact linear analogues: an evaluation of gene delivery comparing polymer architectures. J. Am. Chem. Soc. 137, 6541–6549 (2015).
Newland, B. et al. GDNF gene delivery via a 2-(dimethylamino)ethyl methacrylate based cyclized knot polymer for neuronal cell applications. ACS Chem. Neurosci. 4, 540–546 (2013).
Cook, A. B. et al. Branched poly (trimethylphosphonium ethylacrylate-co-PEGA) by RAFT: alternative to cationic polyammoniums for nucleic acid complexation. J. Interdiscip. Nanomed. 3, 164–174 (2018).
Cho, H. Y. et al. Synthesis of biocompatible PEG-based star polymers with cationic and degradable core for siRNA delivery. Biomacromolecules 12, 3478–3486 (2011).
Dai, F., Sun, P., Liu, Y. & Liu, W. Redox-cleavable star cationic PDMAEMA by arm-first approach of ATRP as a nonviral vector for gene delivery. Biomaterials 31, 559–569 (2010).
Zhao, T. et al. Significance of branching for transfection: synthesis of highly branched degradable functional poly(dimethylaminoethyl methacrylate) by vinyl oligomer combination. Angew. Chem. Int. Ed. 53, 6095–6100 (2014).
Jackson, A. W. et al. Synthesis and in vivo magnetic resonance imaging evaluation of biocompatible branched copolymer nanocontrast agents. Int. J. Nanomed. 10, 5895–5907 (2015).
Hatton, F. L. et al. Hyperbranched polydendrons: a new nanomaterials platform with tuneable permeation through model gut epithelium. Chem. Sci. 6, 326–334 (2015).
Xu, Q. et al. Bacteria-resistant single chain cyclized/knotted polymer coatings. Angew. Chem. Int. Ed. 58, 10616–10620 (2019).
Namivandi-Zangeneh, R. et al. The effects of polymer topology and chain length on the antimicrobial activity and hemocompatibility of amphiphilic ternary copolymers. Polym. Chem. 9, 1735–1744 (2018).
Rolfe, B. E. et al. Multimodal polymer nanoparticles with combined 19F magnetic resonance and optical detection for tunable, targeted, multimodal imaging in vivo. J. Am. Chem. Soc. 136, 2413–2419 (2014).
Tai, H. et al. Thermoresponsive and photocrosslinkable PEGMEMA-PPGMA-EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules 10, 822–828 (2009).
Dong, Y. et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Funct. Mater. 27, 1606619 (2017).
Sigen, A. et al. Hyperbranched PEG-based multi-NHS polymer and bioconjugation with BSA. Polym. Chem. 8, 1283–1287 (2017).
Chen, X. et al. Conformational manipulation of scale-up prepared single-chain polymeric nanogels for multiscale regulation of cells. Nat. Commun. 10, 2705 (2019).
Zhao, T., Sellers, D. L., Cheng, Y., Horner, P. J. & Pun, S. H. Tunable, injectable hydrogels based on peptide-cross-linked, cyclized polymer nanoparticles for neural progenitor cell delivery. Biomacromolecules 18, 2723–2731 (2017).
Yoshimatsu, K. et al. Temperature-responsive “catch and release” of proteins by using multifunctional polymer-based nanoparticles. Angew. Chem. Int. Ed. 51, 2405–2408 (2012).
Lee, S. H. et al. Engineered synthetic polymer nanoparticles as IgG affinity ligands. J. Am. Chem. Soc. 134, 15765–15772 (2012).
Hoshino, Y. et al. Recognition, neutralization, and clearance of target peptides in the bloodstream of living mice by molecularly imprinted polymer nanoparticles: a plastic antibody. J. Am. Chem. Soc. 132, 6644–6645 (2010).
Pan, G., Guo, Q., Ma, Y., Yang, H. & Li, B. Thermo-responsive hydrogel layers imprinted with RGDS peptide: a system for harvesting cell sheets. Angew. Chem. Int. Ed. 52, 6907–6911 (2013).
Ma, Y., Pan, G., Zhang, Y., Guo, X. & Zhang, H. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed. 52, 1511–1514 (2013).
Kloxin, A. M., Kasko, A. M., Salinas, C. N. & Anseth, K. S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009).
Hook, A. L. et al. Combinatorial discovery of polymers resistant to bacterial attachment. Nat. Biotechnol. 30, 868–875 (2012).
Vining, K. H. et al. Synthetic light-curable polymeric materials provide a supportive niche for dental pulp stem cells. Adv. Mater. 30, 1704486 (2018).
Mei, Y. et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 9, 768–778 (2010).
Yan, M. et al. Single siRNA nanocapsules for enhanced RNAi delivery. J. Am. Chem. Soc. 134, 13542–13545 (2012).
Yan, M. et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat. Nanotechnol. 5, 48–53 (2010).
Koide, H. et al. A polymer nanoparticle with engineered affinity for a vascular endothelial growth factor (VEGF165). Nat. Chem. 9, 715–722 (2017).
Tillberg, P. W. et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat. Biotechnol. 34, 987–992 (2016).
Chen, F. et al. Nanoscale imaging of RNA with expansion microscopy. Nat. Methods 13, 679–684 (2016).
Zhao, Y. et al. Nanoscale imaging of clinical specimens using pathology-optimized expansion microscopy. Nat. Biotechnol. 35, 757–764 (2017).
Tang, W. & Matyjaszewski, K. Kinetic modeling of normal ATRP, normal ATRP with [CuII]0, reverse ATRP and SR&NI ATRP. Macromol. Theory Simul. 17, 359–375 (2008).
Soeriyadi, A. H. et al. Synthesis and modification of thermoresponsive poly(oligo(ethylene glycol) methacrylate) via catalytic chain transfer polymerization and thiol–ene Michael addition. Polym. Chem. 2, 815–822 (2011).
Baudry, R. & Sherrington, D. C. Facile synthesis of branched poly(vinyl alcohol)s. Macromolecules 39, 5230–5237 (2006).
Bao, Y., Shen, G., Liu, X. & Li, Y. RAFT polymerization of a novel allene-derived asymmetrical divinyl monomer: a facile strategy to alkene-functionalized hyperbranched vinyl polymers with high degrees of branching. J. Polym. Sci. A Polym. Chem. 51, 2959–2969 (2013).
Jia, Y.-B. et al. Controlled divinyl monomer polymerization mediated by Lewis pairs: a powerful synthetic strategy for functional polymers. ACS Macro Lett. 3, 896–899 (2014).
Butler, G. B. & Myers, G. R. The fundamental basis for cyclopolymerization. IV. Radiation initiated solid-state polymerization of certain dimethacrylamides. J. Macromol. Sci. A. Chem. 5, 135–166 (1971).
Costa, A. I., Barata, P. D. & Prata, J. V. Radical cyclopolymerization of a divinylbenzyl-p-tert-butylcalix[4]arene derivative. React. Funct. Polym. 66, 465–470 (2006).
Edizer, S. et al. Efficient free-radical cyclopolymerization of oriented styrenic difunctional monomers. Macromolecules 42, 1860–1866 (2009).
Ochiai, B., Ito, S. & Endo, T. Chiral interaction between aromatic aldehydes and a polymer bearing large chiral rings obtained by cyclopolymerization of bisacrylamide. Polym. J. 42, 138–141 (2010).
Sharma, A. K., Cornaggia, C. & Pasini, D. Controlled RAFT cyclopolymerization of oriented styrenic difunctional monomers. Macromol. Chem. Phys. 211, 2254–2259 (2010).
Acknowledgements
The authors acknowledge the Science Foundation Ireland (SFI) Principal Investigator (PI) Programme (13/IA/1962) (to W.W.), National Science Foundation (NSF) Division of Materials Research (DMR) (1501324) (to K.M.), National Natural Science Foundation of China (NSFC) (51873179) (to W.W.), Senior Visiting Scholarship of State Key Laboratory of Molecular Engineering of Polymers, Fudan University (19FGJ07) (to W.W.), Irish Research Council (IRC) Employment-Based Postgraduate Programme (EBPPG/2018/159) (to J.L.) and University College Dublin (to Y.G.) for financial support. The authors apologize to those whose work is relevant but could not be cited owing to space limitations.
Author information
Authors and Affiliations
Contributions
Y.G., D.Z. and J.L. contributed equally to the research, writing and review of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks A. Zhukhovitskiy, H. Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Intramolecular cyclization
-
In the context of free-radical polymerization, a special type of crosslinking reaction that produces a link similar to a standard crosslink but between two monomer units in the same primary chain, resulting in a loop structure.
- Instantaneous kinetic chain length
-
The number of double bonds added during one activation/deactivation cycle in ATRP (Equation 2 of Box 3).
- Degree of branching
-
In radical polymerization of MVMs, degree of branching is defined as the number of branch points (that is, fully reacted MVMs) per repeat unit.
- ‘Grafting-from’ polymerization
-
A type of polymerization process in which initiating moieties are covalently bonded to the main polymer backbone and monomers are polymerized as side chains.
- ‘5-Å rule’
-
Effective intramolecular cyclization occurs only when two vinyl units are within 5 Å of one another.
- Mark–Houwink exponent (α)
-
A constant from the Mark–Houwink equation, which correlates the intrinsic viscosity ([𝜂]) of polymer with its molecular weight (M): [𝜂] = kMα. The α values depend on the configuration of polymer chains in the solvent environment.
Rights and permissions
About this article
Cite this article
Gao, Y., Zhou, D., Lyu, J. et al. Complex polymer architectures through free-radical polymerization of multivinyl monomers. Nat Rev Chem 4, 194–212 (2020). https://doi.org/10.1038/s41570-020-0170-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-020-0170-7
This article is cited by
-
Recent progress of non-linear topological structure polymers: synthesis, and gene delivery
Journal of Nanobiotechnology (2024)
-
Exploring the Interplay between Local Chain Structure and Stress Distribution in Polymer Networks
Chinese Journal of Polymer Science (2024)
-
Constructing stable transparent hydrophobic POSS@epoxy-group coatings for waterproofing protection of decorative-painting surfaces
Polymer Bulletin (2024)
-
One-pot synthesis of hyperbranched polymers via visible light regulated switchable catalysis
Nature Communications (2023)
-
Methods of controlled radical polymerization in the synthesis of functional polymers and macromolecular structures
Russian Chemical Bulletin (2023)



