Abstract
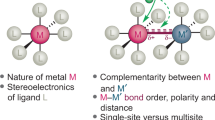
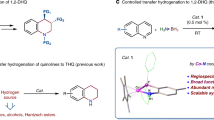
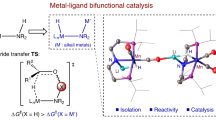
In conventional homogeneous catalysis, supporting ligands act as spectators that do not interact directly with substrates. However, in metal–ligand cooperative catalysis, ligands are involved in facilitating reaction pathways that would be less favourable were they to occur solely at the metal centre. This catalysis paradigm has been known for some time, in part because it is at play in enzyme catalysis. For example, studies of hydrogenative and dehydrogenative enzymes have revealed striking details of metal–ligand cooperative catalysis that involve functional groups proximal to metal active sites. In addition to the more well-known [FeFe]-hydrogenase and [NiFe]-hydrogenase enzymes, [Fe]-hydrogenase, lactate racemase and alcohol dehydrogenase each makes use of cooperative catalysis. This Perspective highlights these enzymatic examples of metal–ligand cooperative catalysis and describes functional bioinspired molecular catalysts that also make use of these motifs. Although progress has been made in developing molecular catalysts, considerable challenges will need to be addressed before we have synthetic catalysts of practical value.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Khusnutdinova, J. R. & Milstein, D. Metal–ligand cooperation. Angew. Chem. Int. Ed. 54, 12236–12273 (2015).
van der Vlugt, J. I. Cooperative catalysis with first-row late transition metals. Eur. J. Inorg. Chem. 2012, 363–375 (2012).
Collman, J. P. Synthetic models for the oxygen-binding hemoproteins. Acc. Chem. Res. 10, 265–272 (1977).
Collman, J. P. & Fu, L. Synthetic models for hemoglobin and myoglobin. Acc. Chem. Res. 32, 455–463 (1999).
Vignais, P. M., Billoud, B. & Meyer, J. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 25, 455–501 (2001).
Lubitz, W., Ogata, H., Rüdiger, O. & Reijerse, E. Hydrogenases. Chem. Rev. 114, 4081–4148 (2014).
Zampella, G., Greco, C., Fantucci, P. & De Gioia, L. Proton reduction and dihydrogen oxidation on models of the [2Fe]H cluster of [Fe] hydrogenases. A density functional theory investigation. Inorg. Chem. 45, 4109–4118 (2006).
Rauchfuss, T. B. Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere. Acc. Chem. Res. 48, 2107–2116 (2015).
Schilter, D., Camara, J. M., Huynh, M. T., Hammes-Schiffer, S. & Rauchfuss, T. B. Hydrogenase enzymes and their synthetic models: the role of metal hydrides. Chem. Rev. 116, 8693–8749 (2016).
Evans, R. M. et al. Mechanism of hydrogen activation by [NiFe] hydrogenases. Nat. Chem. Biol. 12, 46–50 (2016).
Berggren, G. et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66–69 (2013).
Esselborn, J. et al. Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nat. Chem. Biol. 9, 607–609 (2013).
Schilter, D. & Rauchfuss, T. B. And the winner is ... azadithiolate: an amine proton relay in the [FeFe] hydrogenases. Angew. Chem. Int. Ed. 52, 13518–13520 (2013).
Esselborn, J. et al. A structural view of synthetic cofactor integration into [FeFe]-hydrogenases. Chem. Sci. 7, 959–968 (2016).
Siebel, J. F. et al. Hybrid [FeFe]-hydrogenases with modified active sites show remarkable residual enzymatic activity. Biochemistry 54, 1474–1483 (2015).
Shima, S. & Thauer, R. K. A third type of hydrogenase catalyzing H2 activation. Chem. Rec. 7, 37–46 (2007).
Shima, S. & Ermler, U. Structure and function of [Fe]-hydrogenase and its iron–guanylylpyridinol (FeGP) cofactor. Eur. J. Inorg. Chem. 2011, 963–972 (2011).
Shima, S. et al. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science 321, 572–575 (2008).
Tamura, H. et al. Crystal structures of [Fe]-hydrogenase in complex with inhibitory isocyanides: implications for the H2-activation site. Angew. Chem. Int. Ed. 52, 9656–9659 (2013).
Yang, X. & Hall, M. B. Monoiron hydrogenase catalysis: hydrogen activation with the formation of a dihydrogen, Fe–Hδ−···Hδ+–O, bond and methenyl-H4MPT+ triggered hydride transfer. J. Am. Chem. Soc. 131, 10901–10908 (2009).
Finkelmann, A. R., Stiebritz, M. T. & Reiher, M. Kinetic modeling of hydrogen conversion at [Fe] hydrogenase active-site models. J. Phys. Chem. B 117, 4806–4817 (2013).
Finkelmann, A. R., Senn, H. M. & Reiher, M. Hydrogen-activation mechanism of [Fe] hydrogenase revealed by multi-scale modeling. Chem. Sci. 5, 4474–4482 (2014).
Shima, S. et al. Reconstitution of [Fe]-hydrogenase using model complexes. Nat. Chem. 7, 995–1002 (2015).
Schultz, K. M., Chen, D. & Hu, X. [Fe]-hydrogenase and models that contain iron–acyl ligation. Chem. Asian J. 8, 1068–1075 (2013).
Murray, K. A., Wodrich, M. D., Hu, X. & Corminboeuf, C. Toward functional type III [Fe]-hydrogenase biomimics for H2 activation: insights from computation. Chemistry 21, 3987–3996 (2015).
Xu, T. et al. A functional model of [Fe]-hydrogenase. J. Am. Chem. Soc. 138, 3270–3273 (2016).
Moore, C. M., Dahl, E. W. & Szymczak, N. K. Beyond H2: exploiting 2-hydroxypyridine as a design element from [Fe]-hydrogenase for energy-relevant catalysis. Curr. Opin. Chem. Biol. 25, 9–17 (2015).
Desguin, B. et al. A tethered niacin-derived pincer complex with a nickel–carbon bond in lactate racemase. Science 349, 66–69 (2015).
Xu, T., Bauer, G. & Hu, X. A novel nickel pincer complex in the active site of lactate racemase. Chembiochem 17, 31–32 (2016).
Desguin, B., Soumillion, P., Hols, P., Hu, J. & Hausinger, R. P. in The Biological Chemistry of Nickel (eds Zamble, D., Rowinska-Zyrek, M. & Kozlowski, H. ) 220–236 (Royal Society of Chemistry, 2017).
Cantwell, A. & Dennis, D. Lactate racemase. Direct evidence for an α-carbonyl intermediate. Biochemistry 13, 287–291 (1974).
Zhang, X. & Chung, L. W. Alternative mechanistic strategy for enzyme catalysis in a Ni-dependent lactate racemase (LarA): intermediate destabilization by the cofactor. Chemistry 23, 3623–3630 (2017).
Yu, M.-J. & Chen, S.-L. From NAD+ to nickel pincer complex: a significant cofactorevolution presented by lactate racemase. Chemistry 23, 7545–7557 (2017).
Eklund, H., Plapp, B. V., Samama, J.-P. & Brädén, C.-I. Binding of substrate in a ternary complex of horse liver alcohol dehydrogenase. J. Biol. Chem. 257, 14349–14358 (1982).
Cook, P. F. & Cleland, W. W. pH Variation of isotope effects in enzyme-catalyzed reactions. 2. Isotope-dependent step not pH dependent. Kinetic mechanism of alcohol dehydrogenase. Biochemistry 20, 1805–1816 (1981).
Eklund, H. et al. Three-dimensional structure of horse liver alcohol dehydrogenase at 2.4 Å resolution. J. Mol. Biol. 102, 27–59 (1976).
Ramaswamy, S., Eklund, H. & Plapp, B. V. Structures of horse liver alcohol dehydrogenase complexed with NAD+ and substituted benzyl alcohols. Biochemistry 33, 5230–5237 (1994).
Inoue, J., Tomioka, N., Itai, A. & Harayama, S. Proton transfer in benzyl alcohol dehydrogenase during catalysis: alternate proton-relay routes. Biochemistry 37, 3305–3311 (1998).
Xu, T., Wodrich, M. D., Scopelliti, R., Corminboeuf, C. & Hu, X. Nickel pincer model of the active site of lactate racemase involves ligand participation in hydride transfer. Proc. Natl Acad. Sci. USA 114, 1242–1245 (2017).
Dauth, A., Gellrich, U., Diskin-Posner, Y., Ben-David, Y. & Milstein, D. The ferraquinone–ferrahydroquinone couple: combining quinonic and metal-based reactivity. J. Am. Chem. Soc. 139, 2799–2807 (2017).
McSkimming, A., Chan, B., Bhadbhade, M. M., Ball, G. E. & Colbran, S. B. Bio-inspired transition metal–organic hydride conjugates for catalysis of transfer hydrogenation: experiment and theory. Chemistry 21, 2821–2834 (2015).
Can, M., Armstrong, F. A. & Ragsdale, S. W. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl-CoA synthase. Chem. Rev. 114, 4149–4174 (2014).
Dobbek, H., Svetlitchnyi, V., Gremer, L., Huber, R. & Meyer, O. Crystal structure of a carbon monoxide dehydrogenase reveals a [Ni-4Fe-5S] cluster. Science 293, 1281–1285 (2001).
Jeoung, J.-H. & Dobbek, H. Carbon dioxide activation at the Ni, Fe-cluster of anaerobic carbon monoxide dehydrogenase. Science 318, 1461–1464 (2007).
Lindahl, P. A. The Ni-containing carbon monoxide dehydrogenase family: light at the end of the tunnel? Biochemistry 41, 2097–2105 (2002).
Feng, J. & Lindahl, P. A. Carbon monoxide dehydrogenase from Rhodospirillum rubrum: effect of redox potential on catalysis. Biochemistry 43, 1552–1559 (2004).
Fesseler, J., Jeoung, J.-H. & Dobbek, H. How the [NiFe4S4] cluster of CO dehydrogenase activates CO2 and NCO−. Angew. Chem. Int. Ed. 54, 8560–8564 (2015).
Kim, E. J., Feng, J., Bramlett, M. R. & Lindahl, P. A. Evidence for a proton transfer network and a required persulfide-bond-forming cysteine residue in Ni-containing carbon monoxide dehydrogenases. Biochemistry 43, 5728–5734 (2004).
Rao, P. V. & Holm, R. H. Synthetic analogues of the active sites of iron–sulfur proteins. Chem. Rev. 104, 527–560 (2004).
Beley, M., Collin, J.-P., Ruppert, R. & Sauvage, J.-P. Electrocatalytic reduction of CO2 by Ni cyclam2+ in water: study of the factors affecting the efficiency and the selectivity of the process. J. Am. Chem. Soc. 108, 7461–7467 (1986).
Costentin, C., Drouet, S., Robert, M. & Savéant, J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 338, 90–94 (2012).
Rakowski DuBois, M. & DuBois, D. L. The roles of the first and second coordination spheres in the design of molecular catalysts for H2 production and oxidation. Chem. Soc. Rev. 38, 62–72 (2009).
Wilson, A. D. et al. Nature of hydrogen interactions with Ni(II) complexes containing cyclic phosphine ligands with pendant nitrogen bases. Proc. Natl Acad. Sci. USA 104, 6951–6956 (2007).
Brazzolotto, D. et al. Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase. Nat. Chem. 8, 1054–1060 (2016).
Crabtree, R. H. Dihydrogen complexation. Chem. Rev. 116, 8750–8769 (2016).
DuBois, D. L. & Bullock, R. M. Molecular electrocatalysts for the oxidation of hydrogen and the production of hydrogen — the role of pendant amines as proton relays. Eur. J. Inorg. Chem. 2011, 1017–1027 (2011).
Xu, T., Chen, D. & Hu, X. Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 303, 32–41 (2015).
Ginovska-Pangovska, B., Dutta, A., Reback, M. L., Linehan, J. C. & Shaw, W. J. Beyond the active site: the impact of the outer coordination sphere on electrocatalysts for hydrogen production and oxidation. Acc. Chem. Res. 47, 2621–2630 (2014).
Acknowledgements
The authors thank the Swiss National Science Foundation for financial support (200020_172486/1). M.D.W. acknowledges C. Corminboeuf (École Polytechnique Fédérale de Lausanne, Switzerland) for financial support. G. Gryn’ova is acknowledged for artistic contributions.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Wodrich, M., Hu, X. Natural inspirations for metal–ligand cooperative catalysis. Nat Rev Chem 2, 0099 (2018). https://doi.org/10.1038/s41570-017-0099
Published:
DOI: https://doi.org/10.1038/s41570-017-0099
This article is cited by
-
Catalytic hydrogenation of olefins by a multifunctional molybdenum-sulfur complex
Nature Communications (2024)
-
Carbon neutral hydrogen storage and release cycles based on dual-functional roles of formamides
Nature Communications (2023)
-
Cell-inspired design of cascade catalysis system by 3D spatially separated active sites
Nature Communications (2023)
-
Programming conformational cooperativity to regulate allosteric protein-oligonucleotide signal transduction
Nature Communications (2023)
-
Synthesis of atomic platinum with high loading on metal-organic sulfide
Science China Materials (2022)