Abstract
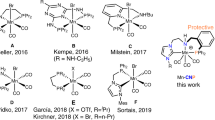
The high efficiency of widely applied Noyori-type hydrogenation catalysts arises from the N–H moiety coordinated to a metal centre, which stabilizes rate-determining transition states through hydrogen-bonding interactions. It was proposed that a higher efficiency could be achieved by substituting an N–M′ group (M′ = alkali metals) for the N–H moiety using a large excess of metal alkoxides (M′OR); however, such a metal-hydride amidate intermediate has not yet been isolated. Here we present the synthesis, isolation and reactivity of a metal-hydride amidate complex (HMn–NLi). Kinetic studies show that the rate of hydride transfer from HMn–NLi to a ketone is 24-fold higher than that of the corresponding amino metal-hydride complex (HMn–NH). Moreover, the hydrogenation of N-alkyl-substituted aldimines was realized using HMn–NLi as the active catalyst, whereas HMn–NH is much less effective. These results highlight the superiority of M/NM′ bifunctional catalysis over the classic M/NH bifunctional catalysis for hydrogenation reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The crystallographic data for the structures of [Mn]-2*, [Mn]-5, [Mn]-7 and [Mn]-9 reported in this work have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2089177, 2128243, 2128247 and 2128248, respectively. The data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Schrock, R. R. & Osborn, J. A. Rhodium catalysts for the homogeneous hydrogenation of ketones. J. Chem. Soc. D, 567–568 (1970).
Clapham, S. E., Hadzovic, A. & Morris, R. H. Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coord. Chem. Rev. 248, 2201–2237 (2004).
Dub, P. A., Scott, B. L. & Gordon, J. C. Why does alkylation of the N–H functionality within M/NH bifunctional Noyori-type catalysts lead to turnover? J. Am. Chem. Soc. 139, 1245–1260 (2017).
Noyori, R. & Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 40, 40–73 (2001).
Ohkuma, T., Ooka, H., Hashiguchi, S., Ikariya, T. & Noyori, R. Practical enantioselective hydrogenation of aromatic ketones. J. Am. Chem. Soc. 117, 2675–2676 (1995).
Doucet, H. et al. Trans-[RuCl2(phosphane)2(1,2-diamine)] and chiral trans-[RuCl2(diphosphane)(1,2-diamine)]: shelf-stable precatalysts for the rapid, productive, and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 37, 1703–1707 (1998).
Ohkuma, T. et al. Asymmetric hydrogenation of alkenyl, cyclopropyl, and aryl ketones. RuCl2(xylbinap)(1,2-diamine) as a precatalyst exhibiting a wide scope. J. Am. Chem. Soc. 120, 13529–13530 (1998).
Ohkuma, T., Ishii, D., Takeno, H. & Noyori, R. Asymmetric hydrogenation of amino ketones using chiral RuCl2(diphophine)(1,2-diamine) complexes. J. Am. Chem. Soc. 122, 6510–6511 (2000).
Ohkuma, T. et al. Trans-RuH(η-BH4)(binap)(1,2-diamine): a catalyst for asymmetric hydrogenation of simple ketones under base-free conditions. J. Am. Chem. Soc. 124, 6508–6509 (2002).
Balaraman, E., Gunanathan, C., Zhang, J., Shimon, L. J. W. & Milstein, D. Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO2 and CO. Nat. Chem. 3, 609–614 (2011).
Xie, J.-H., Liu, X.-Y., Xie, J.-B., Wang, L.-X. & Zhou, Q.-L. An additional coordination group leads to extremely efficient chiral iridium catalysts for asymmetric hydrogenation of ketones. Angew. Chem. Int. Ed. 50, 7329–7332 (2011).
Zuo, W., Lough, A. J., Li, Y. F. & Morris, R. H. Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 342, 1080–1083 (2013).
Pan, H.-J. et al. A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor. Nat. Chem. 11, 669–675 (2019).
Ikariya, T. & Gridnev, I. D. Bifunctional transition metal-based molecular catalysts for asymmetric C–C and C–N bond formation. Chem. Rec. 9, 106–123 (2009).
Zhao, B., Han, Z. & Ding, K. The N–H Functional group in organometallic catalysis. Angew. Chem. Int. Ed. 52, 4744–4788 (2013).
Khusnutdinova, J. R. & Milstein, D. Metal–ligand cooperation. Angew. Chem. Int. Ed. 54, 12236–12273 (2015).
Haack, K.-J., Hashiguchi, S., Fujii, A., Ikariya, T. & Noyori, R. The catalyst precursor, catalyst, and intermediate in the RuII-promoted asymmetric hydrogen transfer between alcohols and ketones. Angew. Chem. Int. Ed. Engl. 36, 285–288 (1997).
Yamakawa, M., Ito, H. & Noyori, R. The metal–ligand bifunctional catalysis: a theoretical study on the ruthenium(II)-catalyzed hydrogen transfer between alcohols and carbonyl compounds. J. Am. Chem. Soc. 122, 1466–1478 (2000).
Abdur-Rashid, K., Faatz, M., Lough, A. J. & Morris, R. H. Catalytic cycle for the asymmetric hydrogenation of prochiral ketones to chiral alcohols: direct hydride and proton transfer from chiral catalysts trans-Ru(H)2(diphosphine)(diamine) to ketones and direct addition of dihydrogen to the resulting hydridoamido complexes. J. Am. Chem. Soc. 123, 7473–7474 (2001).
Abdur-Rashid, K. et al. Mechanism of the hydrogenation of ketones catalyzed by trans-dihydrido(diamine)ruthenium(II) complexes. J. Am. Chem. Soc. 124, 15104–15118 (2002).
Sandoval, C. A., Ohkuma, T., Muñiz, K. & Noyori, R. Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1,2-Diamine–ruthenium(II) complexes. J. Am. Chem. Soc. 125, 13490–13503 (2003).
Abbel, R. et al. A succession of isomers of ruthenium dihydride complexes. Which one is the ketone hydrogenation catalyst? J. Am. Chem. Soc. 127, 1870–1882 (2005).
Hamilton, R. J., Leong, C. G., Bigam, G., Miskolzie, M. & Bergens, S. H. A ruthenium–dihydrogen putative intermediate in ketone hydrogenation. J. Am. Chem. Soc. 127, 4152–4153 (2005).
Hamilton, R. J. & Bergens, S. H. An unexpected possible role of base in asymmetric catalytic hydrogenations of ketones. Synthesis and characterization of several key catalytic intermediates. J. Am. Chem. Soc. 128, 13700–13701 (2006).
Hamilton, R. J. & Bergens, S. H. Direct observations of the metal–ligand bifunctional addition step in an enantioselective ketone hydrogenation. J. Am. Chem. Soc. 130, 11979–11987 (2008).
Takebayashi, S., Dabral, N., Miskolzie, M. & Bergens, S. H. Experimental investigations of a partial Ru–O Bond during the metal–ligand bifunctional addition in Noyori-type enantioselective ketone hydrogenation. J. Am. Chem. Soc. 133, 9666–9669 (2011).
Dub, P. A., Henson, N. J., Martin, R. L. & Gordon, J. C. Unravelling the mechanism of the asymmetric hydrogenation of acetophenone by [RuX2(diphosphine)(1,2-diamine)] catalysts. J. Am. Chem. Soc. 136, 3505–3521 (2014).
Hasanayn, F. & Morris, R. H. Symmetry aspects of H2 splitting by five-coordinate d6 ruthenium amides, and calculations on acetophenone hydrogenation, ruthenium alkoxide formation, and subsequent hydrogenolysis in a model trans-Ru(H)2(diamine)(diphosphine) system. Inorg. Chem. 51, 10808–10818 (2012).
Dub, P. A. & Ikariya, T. Quantum chemical calculations with the inclusion of nonspecific and specific solvation: asymmetric transfer hydrogenation with bifunctional ruthenium catalysts. J. Am. Chem. Soc. 135, 2604–2619 (2013).
Dub, P. A. & Gordon, J. C. The mechanism of enantioselective ketone reduction with Noyori and Noyori–Ikariya bifunctional catalysts. Dalton Trans. 45, 6756–6781 (2016).
Dub, P. A. & Gordon, J. C. Metal–ligand bifunctional catalysis: the ‘accepted’ mechanism, the issue of concertedness, and the function of the ligand in catalytic cycles involving hydrogen atoms. ACS Catal. 7, 6635–6655 (2017).
Dub, P. A. & Gordon, J. C. The role of the metal-bound N–H functionality in Noyori-type molecular catalysts. Nat. Rev. Chem. 2, 396–408 (2018).
Hartmann, R. & Chen, P. Noyori’s hydrogenation catalyst needs a Lewis acid cocatalyst for high activity. Angew. Chem. Int. Ed. 40, 3581–3585 (2001).
Hartmann, R. & Chen, P. Numerical modeling of differential kinetics in the asymmetric hydrogenation of acetophenone by Noyori’s catalyst. Adv. Synth. Catal. 345, 1353–1359 (2003).
John, J. M., Takebayashi, S., Dabral, N., Miskolzie, M. & Bergens, S. H. Base-catalyzed bifunctional addition to amides and imides at low temperature. A new pathway for carbonyl hydrogenation. J. Am. Chem. Soc. 135, 8578–8584 (2013).
Nakane, S., Yamamura, T., Manna, S. K., Tanaka, S. & Kitamura, M. Mechanistic study of the Ru-catalyzed asymmetric hydrogenation of nonchelatable and chelatable tert-alkyl ketones using the linear tridentate sp3P/sp3NH/sp2N-combined ligand PN(H)N: RuNH- and RuNK-involved dual catalytic cycle. ACS Catal. 8, 11059–11075 (2018).
Dub, P. A. Alkali metal alkoxides in Noyori-type hydrogenations. Eur. J. Inorg. Chem. 2021, 4884–4889 (2021).
Liu, C., van Putten, R., Kulyaev, P. O., Filonenko, G. A. & Pidko, E. A. Computational insights into the catalytic role of the base promoters in ester hydrogenation with homogeneous non-pincer-based Mn–P,N catalyst. J. Catal. 363, 136–143 (2018).
Cui, C.-X. et al. Mechanism of Ir-catalyzed hydrogenation: a theoretical view. Coord. Chem. Rev. 412, 213251 (2020).
Wang, Y., Wang, M., Li, Y. & Liu, Q. Homogeneous manganese-catalyzed hydrogenation and dehydrogenation reactions. Chem 7, 1180–1223 (2021).
Fu, S., Shao, Z., Wang, Y. & Liu, Q. Manganese-catalyzed upgrading of ethanol into 1-butanol. J. Am. Chem. Soc. 139, 11941–11948 (2017).
Wang, Y., Shao, Z., Zhang, K. & Liu, Q. Manganese-catalyzed dual-deoxygenative coupling of primary alcohols with 2-arylethanols. Angew. Chem. Int. Ed. 57, 15143–15147 (2018).
Wang, Y. et al. Unmasking the ligand effect in manganese-catalyzed hydrogenation: mechanistic insight and catalytic application. J. Am. Chem. Soc. 141, 17337–17349 (2019).
Shao, Z. et al. Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation. Nat. Commun. 11, 591 (2020).
Liu, C. et al. Manganese-catalyzed asymmetric hydrogenation of quinolines enabled by π–π interaction. Angew. Chem. Int. Ed. 60, 5108–5113 (2021).
Elangovan, S. et al. Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes. Nat. Commun. 7, 12641 (2016).
Nguyen, D. H. et al. Manganese pincer complexes for the base-free, acceptorless dehydrogenative coupling of alcohols to esters: development, scope, and understanding. ACS Catal. 7, 2022–2032 (2017).
Choualeb, A., Lough, A. J. & Gusev, D. G. Hemilabile pincer-type hydride complexes of iridium. Organometallics 26, 5224–5229 (2007).
Hadzovic, A., Song, D., MacLaughlin, C. M. & Morris, R. H. A mechanism displaying autocatalysis: the hydrogenation of acetophenone catalyzed by RuH(S-binap)(app) where app is the amido ligand derived from 2-amino-2-(2-pyridyl)propane. Organometallics 26, 5987–5999 (2007).
Chakraborty, S., Brennessel, W. W. & Jones, W. D. A molecular iron catalyst for the acceptorless dehydrogenation and hydrogenation of N-heterocycles. J. Am. Chem. Soc. 136, 8564–8567 (2014).
Seo, C. S. G., Tsui, B. T. H., Gradiski, M. V., Smith, S. A. M. & Morris, R. H. Enantioselective direct, base-free hydrogenation of ketones by a manganese amido complex of a homochiral, unsymmetrical P–N–P′ ligand. Catal. Sci. Technol. 11, 3153–3163 (2021).
Freitag, F., Irrgang, T. & Kempe, R. Mechanistic studies of hydride transfer to imines from a highly active and chemoselective manganate catalyst. J. Am. Chem. Soc. 141, 11677–11685 (2019).
Seo, C. S. G., Tannoux, T., Smith, S. A. M., Lough, A. J. & Morris, R. H. Enantioselective hydrogenation of activated aryl imines catalyzed by an iron(II) P–NH–P′ Complex. J. Org. Chem. 84, 12040–12049 (2019).
Lagaditis, P. O. et al. Iron(II) complexes containing unsymmetrical P–N–P′ pincer ligands for the catalytic asymmetric hydrogenation of ketones and imines. J. Am. Chem. Soc. 136, 1367–1380 (2014).
Acknowledgements
Financial support from the National Key R&D Program of China 2021YFF0701600 (Q.L.), the National Natural Science Foundation of China 22171159 (Q.L.), 21822106 (Q.L.), the Foundation of the Department of Education of Guangdong Province 2021KTSCX140 (Q.L.) and the China Postdoctoral Science Foundation 2020M680021 (Y.W.), 2021T140366 (Y.W.) is greatly appreciated. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank the National Supercomputing Center, Zhengzhou. We are very grateful to the referees for their valuable comments and suggestions that improved the quality of this paper. We thank Y. Xia and R. Jian from Tsinghua University for high-resolution mass spectroscopy analysis of [Mn]-5. We also acknowledge G. Sun from Chongqing University for helpful discussions about theoretical calculations. This paper is dedicated to Prof. Matthias Beller on occasion of his 60th birthday.
Author information
Authors and Affiliations
Contributions
Y.W. and Q.L. conceived and designed this research. Y.W. synthesized and characterized all the HMn–NM′ complexes, performed the kinetic studies, designed the catalytic reactions and optimized reaction conditions. H.Y. and H.L. helped to explore the substrate scope. S.L. and Y.L. carried out DFT calculations and discussed the manuscript. Y.W. wrote the original draft with the contribution of S.L., which was reviewed and edited by Y.L. and Q.L. Q.L. directed the project and all the authors have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Pavel Dub and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30, Tables 1–4, experimental procedures and characterization data, DFT calculation results, discussion and references.
Supplementary Data 1
x,y,z coordinates for DFT calculations.
Supplementary Data 2
Crystallographic data for compound [Mn]-2*; CCDC reference 2089177.
Supplementary Data 3
Crystallographic data for compound [Mn]-5; CCDC reference 2128243.
Supplementary Data 4
Crystallographic data for compound [Mn]-7; CCDC reference 2128247.
Supplementary Data 5
Crystallographic data for compound [Mn]-9; CCDC reference 2128248.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Liu, S., Yang, H. et al. Structure, reactivity and catalytic properties of manganese-hydride amidate complexes. Nat. Chem. 14, 1233–1241 (2022). https://doi.org/10.1038/s41557-022-01036-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01036-6
This article is cited by
-
A 13-million turnover-number anionic Ir-catalyst for a selective industrial route to chiral nicotine
Nature Communications (2023)
-
Isolating intermediates
Nature Chemistry (2022)