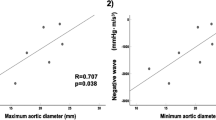
Abstract
Aortic blood flow patterns are closely linked to the morphology and function of the left ventricle, aortic valve and aorta. These flow patterns demonstrate the exceptional adaptability of the cardiovascular system to maintain blood circulation under a broad range of haemodynamic workloads and can be altered in various pathophysiological states. For instance, normal ascending aortic systolic flow is predominantly laminar, whereas abnormal aortic systolic flow is associated with increased eccentricity, vorticity and flow reversal. These flow abnormalities result in reduced aortic conduit function and increased energy loss in the cardiovascular system. Emerging evidence details the association of these flow patterns with loss of aortic compliance, which leads to adverse left ventricular remodelling, poor tissue perfusion, and an increased risk of morbidity and death. In this Perspective article, we review the evidence for the link between aortic flow-related abnormalities and cardiovascular disease and how these changes in aortic flow patterns are emerging as a therapeutic target for aortic valve intervention in first-in-human studies.
Key points
-
Aortic flow is a complex and dynamic process that is influenced by several factors, including the aortic valve, the left ventricle and the aorta itself.
-
Aortic flow after aortic valve intervention is characterized by eccentric jets and abnormal vorticity.
-
Doppler echocardiography allows basic aortic flow visualization and quantification; complex flow assessment is restricted by the limitations of ultrasonography.
-
2D and 4D flow cardiac MRI are non-invasive imaging modalities that can accurately quantify aortic flow and provide insights into aortic flow patterns.
-
Systolic flow displacement on cardiac MRI is a marker of flow eccentricity that is associated with further ascending aortic dilatation, whereas the systolic flow reversal ratio can be used to quantify aortic conduit function; increases in these biomarkers are associated with ageing and reduced exercise capacity.
-
Novel prosthetic aortic valves are being developed to restore normal aortic flow and increase prosthesis longevity, preserve the aortic root, and improve patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Vahanian, A. et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 43, 561–632 (2022).
von Knobelsdorff-Brenkenhoff, F. et al. Evaluation of aortic blood flow and wall shear stress in aortic stenosis and its association with left ventricular remodeling. Circ. Cardiovasc. Imaging 9, e004038 (2016).
Ajmone Marsan, N. et al. Valvular heart disease: shifting the focus to the myocardium. Eur. Heart J. 44, 28–40 (2022).
Takehara, Y. Clinical application of 4D flow MR imaging for the abdominal aorta. Magn. Reson. Med. Sci. 21, 354–364 (2022).
Hansen, K. L. et al. Analysis of systolic backflow and secondary helical blood flow in the ascending aorta using vector flow imaging. Ultrasound Med. Biol. 42, 899–908 (2016).
Gimbrone, M. A., Topper, J. N., Nagel, T., Anderson, K. R. & Garcia-Cardeña, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 902, 230–239 (2000).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282, 2035–2042 (1999).
Bogren, H. G. et al. The function of the aorta in ischemic heart disease: a magnetic resonance and angiographic study of aortic compliance and blood flow patterns. Am. Heart J. 118, 234–247 (1989).
Ozdemi R, M., Asoglu, R., Aladag, N. & Asoglu, E. Aortic flow propagation velocity and neutrophil-to-lymphocyte ratio in coronary slow flow. Bratisl. Lek. Listy 122, 513–518 (2021).
Guala, A. et al. Wall shear stress predicts aortic dilation in patients with bicuspid aortic valve. JACC Cardiovasc. Imaging 15, 46–56 (2022).
Burris, N. S. et al. Systolic flow displacement correlates with future ascending aortic growth in patients with bicuspid aortic valves undergoing magnetic resonance surveillance. Invest. Radiol. 49, 635–639 (2014).
Minderhoud, S. C. S. et al. Wall shear stress angle is associated with aortic growth in bicuspid aortic valve patients. Eur. Heart J. Cardiovasc. Imaging 23, 1680–1689 (2022).
Soulat, G. et al. Association of regional wall shear stress and progressive ascending aorta dilation in bicuspid aortic valve. JACC Cardiovasc. Imaging 15, 33–42 (2022).
Zhang, X., Wang, W., Yan, J. & Wang, Q. Surgical treatment results of secondary tunnel-like subaortic stenosis after congenital heart disease operations: a 7-year, single-center experience in 25 patients. J. Card. Surg. 35, 335–340 (2020).
Kiema, M. et al. Wall shear stress predicts media degeneration and biomechanical changes in thoracic aorta. Front. Physiol. 13, 934941 (2022).
Tse, K. M. et al. A computational fluid dynamics study on geometrical influence of the aorta on haemodynamics. Eur. J. Cardiothorac. Surg. 43, 829–838 (2013).
Johnson, N. P. et al. Pressure gradient vs. flow relationships to characterize the physiology of a severely stenotic aortic valve before and after transcatheter valve implantation. Eur. Heart J. 39, 2646–2655 (2018).
Burris, N. S. & Hope, M. D. 4D flow MRI applications for aortic disease. Magn. Reson. Imaging Clin. N. Am. 23, 15–23 (2015).
Kilner, P. J., Yang, G. Z., Mohiaddin, R. H., Firmin, D. N. & Longmore, D. B. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation 88, 2235–2247 (1993).
Gautier, M. et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am. J. Cardiol. 105, 888–894 (2010).
Vasan, R. S., Larson, M. G. & Levy, D. Determinants of echocardiographic aortic root size. The Framingham Heart Study. Circulation 91, 734–740 (1995).
Son, J.-W. et al. Differences in aortic vortex flow pattern between normal and patients with stroke: qualitative and quantitative assessment using transesophageal contrast echocardiography. Int. J. Cardiovasc. Imaging 32, 45–52 (2016).
Taylor, R. Evolution of the continuity equation in the Doppler echocardiographic assessment of the severity of valvular aortic stenosis. J. Am. Soc. Echocardiogr. 3, 326–330 (1990).
Meaume, S., Benetos, A., Henry, O. F., Rudnichi, A. & Safar, M. E. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler. Thromb. Vasc. Biol. 21, 2046–2050 (2001).
Calabia, J. et al. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc. Ultrasound 9, 13 (2011).
Rabben, S. I. et al. An ultrasound-based method for determining pulse wave velocity in superficial arteries. J. Biomech. 37, 1615–1622 (2004).
Szmigielski, C. et al. Pulse wave velocity correlates with aortic atherosclerosis assessed with transesophageal echocardiography. J. Hum. Hypertens. 30, 90–94 (2016).
Galal, H., Ammar, M., Ismail, M. & Elshazly, A. Echocardiographic assessment of aortic pulse wave velocity as a diagnostic and prognostic parameter for left ventricular diastolic dysfunction. Minerva Cardioangiol. 68, 313–318 (2020).
Daae, A. S., Wigen, M. S., Fadnes, S., Løvstakken, L. & Støylen, A. Intraventricular vector flow imaging with blood speckle tracking in adults: feasibility, normal physiology and mechanisms in healthy volunteers. Ultrasound Med. Biol. 47, 3501–3513 (2021).
Li, C. et al. Quantification of chronic aortic regurgitation by vector flow mapping: a novel echocardiographic method. Eur. J. Echocardiogr. 11, 119–124 (2010).
Cantinotti, M. et al. Characterization of aortic flow patterns by high-frame-rate blood speckle tracking echocardiography in children. J. Am. Heart Assoc. 12, e026335 (2023).
Juffermans, J. F. et al. Multicenter consistency assessment of valvular flow quantification with automated valve tracking in 4D flow CMR. JACC Cardiovasc. Imaging 14, 1354–1366 (2021).
Garg, P. et al. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. Cardiol. 17, 298–312 (2020).
Vejpongsa, P., Xu, J., Quinones, M. A., Shah, D. J. & Zoghbi, W. A. Differences in cardiac remodeling in left-sided valvular regurgitation: implications for optimal definition of significant aortic regurgitation. JACC Cardiovasc. Imaging 15, 1730–1741 (2022).
Hashimoto, G. et al. Association of left ventricular remodeling assessment by cardiac magnetic resonance with outcomes in patients with chronic aortic regurgitation. JAMA Cardiol. 7, 924–933 (2022).
Nayak, K. S. et al. Cardiovascular magnetic resonance phase contrast imaging. J. Cardiovasc. Magn. Reson. 17, 71 (2015).
Morbiducci, U. et al. Mechanistic insight into the physiological relevance of helical blood flow in the human aorta: an in vivo study. Biomech. Model. Mechanobiol. 10, 339–355 (2011).
Frydrychowicz, A. et al. Interdependencies of aortic arch secondary flow patterns, geometry, and age analysed by 4-dimensional phase contrast magnetic resonance imaging at 3 Tesla. Eur. Radiol. 22, 1122–1130 (2012).
Stonebridge, P. A. et al. Non spiral and spiral (helical) flow patterns in stenoses. In vitro observations using spin and gradient echo magnetic resonance imaging (MRI) and computational fluid dynamic modeling. Int. Angiol. J. 23, 276–283 (2004).
Frazin, L. J. et al. Functional chiral asymmetry in descending thoracic aorta. Circulation 82, 1985–1994 (1990).
Ding, Z., Fan, Y., Deng, X., Zhan, F. & Kang, H. Effect of swirling flow on the uptakes of native and oxidized LDLs in a straight segment of the rabbit thoracic aorta. Exp. Biol. Med. 235, 506–513 (2010).
Liu, X. et al. A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. Am. J. Physiol. Heart Circ. Physiol. 297, H163–H170 (2009).
Liu, X., Fan, Y. & Deng, X. Effect of spiral flow on the transport of oxygen in the aorta: a numerical study. Ann. Biomed. Eng. 38, 917–926 (2010).
Shipkowitz, T., Rodgers, V. G., Frazin, L. J. & Chandran, K. B. Numerical study on the effect of secondary flow in the human aorta on local shear stresses in abdominal aortic branches. J. Biomech. 33, 717–728 (2000).
Linge, F., Hye, M. A. & Paul, M. C. Pulsatile spiral blood flow through arterial stenosis. Comput. Methods Biomech. Biomed. Engin. 17, 1727–1737 (2014).
Oechtering, T. H. et al. Time-resolved 3-dimensional magnetic resonance phase contrast imaging (4D Flow MRI) reveals altered blood flow patterns in the ascending aorta of patients with valve-sparing aortic root replacement. J. Thorac. Cardiovasc. Surg. 159, 798–810.e1 (2020).
Bürk, J. et al. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J. Cardiovasc. Magn. Reson. 14, 84 (2012).
Lorenz, R. et al. 4D flow magnetic resonance imaging in bicuspid aortic valve disease demonstrates altered distribution of aortic blood flow helicity. Magn. Reson. Med. 71, 1542–1553 (2014).
Ebel, S. et al. Automated quantitative extraction and analysis of 4d flow patterns in the ascending aorta: an intraindividual comparison at 1.5 T and 3 T. Sci. Rep. 10, 2949 (2020).
Hatoum, H., Moore, B. L. & Dasi, L. P. On the significance of systolic flow waveform on aortic valve energy loss. Ann. Biomed. Eng. 46, 2102–2111 (2018).
Yap, C.-H., Dasi, L. P. & Yoganathan, A. P. Dynamic hemodynamic energy loss in normal and stenosed aortic valves. J. Biomech. Eng. 132, 021005 (2010).
Martin, C., Sun, W., Primiano, C., McKay, R. & Elefteriades, J. Age-dependent ascending aortic mechanics assessed through multiphase CT. Ann. Biomed. Eng. 41, 2565–2574 (2013).
Bellhouse, B. J. & Bellhouse, F. H. Mechanism of closure of the aortic valve. Nature 217, 86–87 (1968).
Meuschke, M., Köhler, B., Preim, U., Preim, B. & Lawonn, K. Semi-automatic vortex flow classification in 4D PC-MRI data of the aorta. Comput. Graph. Forum 35, 351–360 (2016).
Tyszka, J. M., Laidlaw, D. H., Asa, J. W. & Silverman, J. M. Three-dimensional, time-resolved (4D) relative pressure mapping using magnetic resonance imaging. J. Magn. Reson. Imaging 12, 321–329 (2000).
von Spiczak, J. et al. Quantitative analysis of vortical blood flow in the thoracic aorta using 4D phase contrast MRI. PLoS One 10, e0139025 (2015).
Sigovan, M., Hope, M. D., Dyverfeldt, P. & Saloner, D. Comparison of four-dimensional flow parameters for quantification of flow eccentricity in the ascending aorta. J. Magn. Reson. Imaging 34, 1226–1230 (2011).
Ayaon-Albarran, A. et al. Systolic flow displacement using 3D magnetic resonance imaging in an experimental model of ascending aorta aneurysm: impact of rheological factors. Eur. J. Cardiothorac. 50, 685–692 (2016).
Rodríguez-Palomares, J. F. et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 20, 28 (2018).
Lenz, A. et al. 4D flow cardiovascular magnetic resonance for monitoring of aortic valve repair in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 22, 29 (2020).
Barker, A. J., Lanning, C. & Shandas, R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann. Biomed. Eng. 38, 788–800 (2010).
Ha, H. et al. Association between flow skewness and aortic dilatation in patients with aortic stenosis. Int. J. Cardiovasc. Imaging 33, 1969–1978 (2017).
Zhao, X. et al. Aortic flow is associated with aging and exercise capacity. Eur. Heart J. Open 3, oead079 (2023).
Wentland, A. L., Grist, T. M. & Wieben, O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc. Diagn. Ther. 4, 193–206 (2014).
Pereira, T., Correia, C. & Cardoso, J. Novel methods for pulse wave velocity measurement. J. Med. Biol. Eng. 35, 555–565 (2015).
Westenberg, J. J. M. et al. Improved aortic pulse wave velocity assessment from multislice two-directional in-plane velocity-encoded magnetic resonance imaging. J. Magn. Reson. Imaging 32, 1086–1094 (2010).
Jarvis, K. et al. Aortic pulse wave velocity evaluated by 4D flow MRI across the adult lifespan. J. Magn. Reson. Imaging 56, 464–473 (2022).
Kaolawanich, Y. & Boonyasirinant, T. Prognostic value of aortic stiffness using cardiovascular magnetic resonance in the elderly with known or suspected coronary artery disease. Arq. Bras. Cardiol. 118, 961–971 (2022).
Kaolawanich, Y. & Boonyasirinant, T. Incremental prognostic value of aortic stiffness in addition to myocardial ischemia by cardiac magnetic resonance imaging. BMC Cardiovasc. Disord. 20, 287 (2020).
Meiszterics, Z., Simor, T., van der Geest, R. J., Farkas, N. & Gaszner, B. Evaluation of pulse wave velocity for predicting major adverse cardiovascular events in post-infarcted patients; comparison of oscillometric and MRI methods. Rev. Cardiovasc. Med. 22, 1701–1710 (2021).
Kaolawanich, Y. & Boonyasirinant, T. Impact of aortic stiffness by velocity-encoded magnetic resonance imaging on late gadolinium enhancement to predict cardiovascular events. Int. J. Cardiol. Heart Vasc. 30, 100635 (2020).
Cave, D. G. W., Panayiotou, H. & Bissell, M. M. Hemodynamic profiles before and after surgery in bicuspid aortic valve disease — a systematic review of the literature. Front. Cardiovasc. Med. 8, 629227 (2021).
Keller, E. J. et al. Reduction of aberrant aortic haemodynamics following aortic root replacement with a mechanical valved conduit. Interact. Cardiovasc. Thorac. Surg. 23, 416–423 (2016).
David, T. E. et al. Results of aortic valve-sparing operations. J. Thorac. Cardiovasc. Surg. 122, 39–46 (2001).
Sarikaya, S. & Kirali, K. Long-term experience of the modified David V re-implantation technique for valve-sparing aortic root replacement. Cardiovasc. J. Afr. 34, 1–8 (2023).
von Knobelsdorff-Brenkenhoff, F. et al. Blood flow characteristics in the ascending aorta after aortic valve replacement–a pilot study using 4D-flow MRI. Int. J. Cardiol. 170, 426–433 (2014).
Akutsu, T. & Matsumoto, A. Influence of three mechanical bileaflet prosthetic valve designs on the three-dimensional flow field inside a simulated aorta. J. Artif. Organs 13, 207–217 (2010).
Midha, P. A. et al. The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 136, 1598–1609 (2017).
Meduri, C. et al. TCT-471 first-in-human study results with the novel anteris DurAVRTM three-dimensional single-piece TAVR device. J. Am. Coll. Cardiol. 80, B190 (2022).
Kodali, S. K. et al. Early safety and feasibility of a first-in-class biomimetic transcatheter aortic valve - DurAVR. EuroIntervention 19, e352–e362 (2023).
Suzuki, E. et al. Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 24, 2107–2114 (2001).
Author information
Authors and Affiliations
Contributions
P.G., S.L.S. and J.C. researched data for the article. All the authors were involved in writing, reviewing, editing and discussing the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
P.G. has an advisory role with Medis Medical Imaging and Pie Medical, and is a consultant for Anteris Technologies and Edward Lifesciences. M.M. has received research support from Circle Cardiovascular Imaging and Siemens. J.S. is a consultant for Anteris, Boston Scientific, Edwards Lifesciences and Medtronic, has received research funding from Edwards Lifesciences and Medtronic, and has received speaking fees from New Valve Technology. S.L.S. is a consultant for Anteris, Edwards Lifesciences and Medtronic, has received research support from Edwards Lifesciences, Heartflow, Medtronic and Vivitro Labs, and is supported by a Scholar award from Michael Smith Health Research BC. C.M. is the Chief Medical Officer of Anteris Technologies, has received research support from Boston Scientific, and has received honoraria, consultation or proctoring fees from Abbott, Alleviant, Boston Scientific, Cardiovalve, V2V, VDyne and XDot. J.C. has received consulting fees from 4C Medical, Abbott Structural, Anteris, AriaCV, Boston Scientific, Edwards Lifesciences, Medtronic, VDyne, WL Gore and Xylocor, and has received research grant support from Abbott Northwestern Hospital Foundation.
Peer review
Peer review information
Nature Reviews Cardiology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, P., Markl, M., Sathananthan, J. et al. Restoration of flow in the aorta: a novel therapeutic target in aortic valve intervention. Nat Rev Cardiol 21, 264–273 (2024). https://doi.org/10.1038/s41569-023-00943-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00943-6