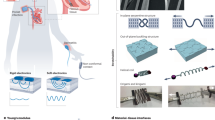
Abstract
Cardiovascular electronic devices have enormous benefits for health and quality of life but the long-term operation of these implantable and wearable devices remains a huge challenge owing to the limited life of batteries, which increases the risk of device failure and causes uncertainty among patients. A possible approach to overcoming the challenge of limited battery life is to harvest energy from the body and its ambient environment, including biomechanical, solar, thermal and biochemical energy, so that the devices can be self-powered. This strategy could allow the development of advanced features for cardiovascular electronic devices, such as extended life, miniaturization to improve comfort and conformability, and functions that integrate with real-time data transmission, mobile data processing and smart power utilization. In this Review, we present an update on self-powered cardiovascular implantable electronic devices and wearable active sensors. We summarize the existing self-powered technologies and their fundamental features. We then review the current applications of self-powered electronic devices in the cardiovascular field, which have two main goals. The first is to harvest energy from the body as a sustainable power source for cardiovascular electronic devices, such as cardiac pacemakers. The second is to use self-powered devices with low power consumption and high performance as active sensors to monitor physiological signals (for example, for active endocardial monitoring). Finally, we present the current challenges and future perspectives for the field.
Key points
-
The introduction of implantable or wearable electronic devices has revolutionized diagnosis and therapy in cardiovascular medicine, reducing morbidity and mortality of millions for patients with cardiovascular disease.
-
Current battery-powered cardiovascular electronic devices have a limited life and do not allow long-term, uninterrupted monitoring or treatment of cardiovascular disease, which is crucial for preventing death and/or improving quality of life.
-
Abundant sources of energy exist in the human body and the surrounding environment, such as biomechanical, solar, thermal and biochemical energy.
-
Self-powered technology, which converts energy from the human body or surrounding environment into electricity, can provide a sustainable source of power to replace or supplement battery technology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beaglehole, R., Saracci, R. & Panico, S. Cardiovascular diseases: causes, surveillance and prevention. Int. J. Epidemiol. 30, S1–S4 (2001).
World Health Organization. Cardiovascular diseases (CVDs) https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (WHO, 2017).
Auricchio, A. & Moccetti, T. Electronic cardiac medicine: present and future opportunities. Swiss Med. Wkly 140, w13052 (2010).
Wang, Z. L. Self-powered nanotech. Sci. Am. 298, 82–87 (2008).
Larsson, B., Elmqvist, H., Ryden, L. & Schuller, H. Lessons from the first patient with an implanted pacemaker: 1958-2001. Pacing Clin. Electrophysiol. 26, 114–124 (2003).
European Society of Cardiology. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 15, 1070–1118 (2013).
Madhavan, M., Mulpuru, S. K., McLeod, C. J., Cha, Y. M. & Friedman, P. A. Advances and future directions in cardiac pacemakers: part 2 of a 2-part series. J. Am. Coll. Cardiol. 69, 211–235 (2017).
Dotsenko, O., Barsheshet, A. & Huang, D. T. Cardiac resynchronization therapy for prevention of heart failure events in elderly patients with left ventricular dysfunction. Expert Rev. Cardiovasc. Ther. 10, 1319–1327 (2012).
Moss, A. J. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361, 1329–1338 (2009).
Leclercq, C. et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J. Am. Coll. Cardiol. 51, 1455–1462 (2008).
Ellenbogen, K. A. & Vijayaraman, P. His bundle pacing: a new promise in heart failure therapy? JACC Clin. Electrophysiol. 1, 592–595 (2015).
Keene, D. et al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: insights from a large international observational study. J. Cardiovasc. Electrophysiol. 30, 1984–1993 (2019).
Boriani, G. et al. Role of ventricular Autocapture function in increasing longevity of DDDR pacemakers: a prospective study. Europace 8, 216–220 (2006).
Biffi, M. et al. Actual pacemaker longevity: the benefit of stimulation by automatic capture verification. Pacing Clin. Electrophysiol. 33, 873–881 (2010).
Milasinovic, G. et al. Percent ventricular pacing with managed ventricular pacing mode in standard pacemaker population. Europace 10, 151–155 (2008).
Gillis, A. M. et al. Reducing unnecessary right ventricular pacing with the managed ventricular pacing mode in patients with sinus node disease and AV block. Pacing Clin. Electrophysiol. 29, 697–705 (2006).
Moss, A. J. et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 346, 877–883 (2002).
Bardy, G. H. et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 352, 225–237 (2005).
Glatter, K. A., Young, J. N. & McElvany, M. D. Implantable cardioverter-defibrillators: a new preventive medical option. Prev. Cardiol. 9, 49–53 (2006).
Brignole, M. et al. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 11, 671–687 (2009).
Farwell, D. J. & Sulke, A. N. Does the use of a syncope diagnostic protocol improve the investigation and management of syncope? Heart 90, 52–58 (2004).
Roy, D. et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian trial of atrial fibrillation investigators. N. Engl. J. Med. 342, 913–920 (2000).
Wyse, D. G. et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 347, 1825–1833 (2002).
Dunn, J., Runge, R. & Snyder, M. Wearables and the medical revolution. Per. Med. 15, 429–448 (2018).
Pevnick, J. M., Birkeland, K., Zimmer, R., Elad, Y. & Kedan, I. Wearable technology for cardiology: an update and framework for the future. Trends Cardiovasc. Med. 28, 144–150 (2018).
National Heart Foundation and High Blood Pressure Research Council or Australia Ambulatory Blood Pressure Monitoring Consensus Committee. Ambulatory blood pressure monitoring. Aust. Fam. Phys. 40, 877–880 (2011).
Mukkamala, R. et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans. Biomed. Eng. 62, 1879–1901 (2015).
Teplitzky, B. A. & McRoberts, M. in 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN) 58–61 (IEEE, 2018).
Vandenberk, T. et al. Clinical validation of heart rate apps: mixed-methods evaluation study. JMIR Mhealth Uhealth 5, e129 (2017).
Orchard, J. et al. Screening for atrial fibrillation during influenza vaccinations by primary care nurses using a smartphone electrocardiograph (iECG): a feasibility study. Eur. J. Prev. Cardiol. 23, 13–20 (2016).
Lau, J. K. et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int. J. Cardiol. 165, 193–194 (2013).
de Zambotti, M., Rosas, L., Colrain, I. M. & Baker, F. C. The sleep of the ring: comparison of the OURA sleep tracker against polysomnography. Behav. Sleep Med. 17, 124–136 (2019).
Chung, M. K. et al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J. Am. Coll. Cardiol. 56, 194–203 (2010).
Selvaraj, N. in 2014 IEEE Healthcare Innovations Conference (HIC) 83–86 (IEEE, 2014).
Turakhia, M. P. et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am. J. Cardiol. 112, 520–524 (2013).
Solomon, M. D. et al. Incidence and timing of potentially high-risk arrhythmias detected through long term continuous ambulatory electrocardiographic monitoring. BMC Cardiovasc. Disord. 16, 35 (2016).
Glassninja. HeartIn ECG-monitoring T-shirt. Cool Wearable https://www.coolwearable.com/heartin-ecg-monitoring-t-shirt/ (2020).
Corday, E. et al. Detection of phantom arrhythmias and evanescent electrocardiographic abnormalities: use of prolonged direct electrocardiocording. JAMA 193, 417–421 (1965).
Kamal, A. A., Harness, J. B., Irving, G. & Mearns, A. J. Skin photoplethysmography — a review. Comput. Methods Prog. Biomed. 28, 257–269 (1989).
Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 28, R1–R39 (2007).
Cadmus-Bertram, L., Gangnon, R., Wirkus, E. J., Thraen-Borowski, K. M. & Gorzelitz-Liebhauser, J. The accuracy of heart rate monitoring by some wrist-worn activity trackers. Ann. Intern. Med. 166, 610–612 (2017).
Wang, R. et al. Accuracy of wrist-worn heart rate monitors. JAMA Cardiol. 2, 104–106 (2017).
Krumholz, H. M. et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ. Cardiovasc. Qual. Outcomes 2, 407–413 (2009).
Adamson, P. B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr. Heart Fail. Rep. 6, 287–292 (2009).
Amir, O., Rappaport, D., Zafrir, B. & Abraham, W. T. A novel approach to monitoring pulmonary congestion in heart failure: initial animal and clinical experiences using remote dielectric sensing technology. Congest. Heart Fail. 19, 149–155 (2013).
Malfatto, G. et al. Correlation between trans and intra-thoracic impedance and conductance in patients with chronic heart failure. J. Cardiovasc. Med. 17, 276–282 (2016).
Evenson, K. R., Goto, M. M. & Furberg, R. D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 12, 159 (2015).
Chiauzzi, E., Rodarte, C. & DasMahapatra, P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 13, 77 (2015).
Lau, E. W. Technologies for prolonging cardiac implantable electronic device longevity. Pacing Clin. Electrophysiol. 40, 75–96 (2017).
Lekkerkerker, J. C. et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 95, 715–720 (2009).
Munawar, D. A. et al. Predicted longevity of contemporary cardiac implantable electronic devices: a call for industry-wide “standardized” reporting. Heart Rhythm 15, 1756–1763 (2018).
Wang, Z. L. & Song, J. H. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312, 242–246 (2006).
Wang, X., Song, J., Liu, J. & Wang, Z. L. Direct-current nanogenerator driven by ultrasonic waves. Science 316, 102–105 (2007).
Xu, S. et al. Self-powered nanowire devices. Nat. Nanotechnol. 5, 366–373 (2010).
Hu, Y., Zhang, Y., Xu, C., Zhu, G. & Wang, Z. L. High-output nanogenerator by rational unipolar assembly of conical nanowires and its application for driving a small liquid crystal display. Nano Lett. 10, 5025–5031 (2010).
Wang, Z. L. From nanogenerators to piezotronics: a decade-long study of ZnO nanostructures. MRS Bull. 37, 814–827 (2012).
Fan, F.-R., Tian, Z.-Q. & Lin Wang, Z. Flexible triboelectric generator. Nano Energy 1, 328–334 (2012).
Wang, Z. L. Triboelectric nanogenerators as new energy technology and self-powered sensors – principles, problems and perspectives. Faraday Discuss. 176, 447–458 (2014).
Zhu, G. et al. Triboelectric-generator-driven pulse electrodeposition for micropatterning. Nano Lett. 12, 4960–4965 (2012).
Niu, S. et al. Theoretical study of contact-mode triboelectric nanogenerators as an effective power source. Energy Environ. Sci. 6, 3576–3583 (2013).
Wang, S. et al. Sliding-triboelectric nanogenerators based on in-plane charge-separation mechanism. Nano Lett. 13, 2226–2233 (2013).
Niu, S. et al. Theoretical investigation and structural optimization of single-electrode triboelectric nanogenerators. Adv. Funct. Mater. 24, 3332–3340 (2014).
Wang, S., Xie, Y., Niu, S., Lin, L. & Wang, Z. L. Freestanding triboelectric-layer-based nanogenerators for harvesting energy from a moving object or human motion in contact and non-contact modes. Adv. Mater. 26, 2818–2824 (2014).
Niu, S. et al. Theory of freestanding triboelectric-layer-based nanogenerators. Nano Energy 12, 760–774 (2015).
Bowen, C. R. et al. Pyroelectric materials and devices for energy harvesting applications. Energy Environ. Sci. 7, 3836–3856 (2014).
Cuadras, A., Gasulla, M. & Ferrari, V. Thermal energy harvesting through pyroelectricity. Sens. Actuators A Phys. 158, 132–139 (2010).
Uchida, K. et al. Observation of the spin Seebeck effect. Nature 455, 778–781 (2008).
Mano, N., Mao, F. & Heller, A. Characteristics of a miniature compartment-less glucose-O2 biofuel cell and its operation in a living plant. J. Am. Chem. Soc. 125, 6588–6594 (2003).
Rapoport, B. I., Kedzierski, J. T. & Sarpeshkar, R. A glucose fuel cell for implantable brain–machine interfaces. PLoS ONE 7, e38436 (2012).
Zebda, A. et al. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 3, 1516 (2013).
Dagdeviren, C., Li, Z. & Wang, Z. L. Energy harvesting from the animal/human body for self-powered electronics. Annu. Rev. Biomed. Eng. 19, 85–108 (2017).
Tian, B. et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 449, 885–889 (2007).
Vazquez-Mena, O. et al. Performance enhancement of a graphene-zinc phosphide solar cell using the electric field-effect. Nano Lett. 14, 4280–4285 (2014).
Chang, T. H. et al. Planar heterojunction perovskite solar cells incorporating metal-organic framework nanocrystals. Adv. Mater. 27, 7229–7235 (2015).
Goto, H., Sugiura, T., Harada, Y. & Kazui, T. Feasibility of using the automatic generating system for quartz watches as a leadless pacemaker power source. Med. Biol. Eng. Comput. 37, 377–380 (1999).
Mercier, P. P., Lysaght, A. C., Bandyopadhyay, S., Chandrakasan, A. P. & Stankovic, K. M. Energy extraction from the biologic battery in the inner ear. Nat. Biotechnol. 30, 1240–1243 (2012).
Li, Z., Zhu, G., Yang, R., Wang, A. C. & Wang, Z. L. Muscle-driven in vivo nanogenerator. Adv. Mater. 22, 2534–2537 (2010).
Ben Amar, A., Kouki, A. B. & Cao, H. Power approaches for implantable medical devices. Sensors 15, 28889–28914 (2015).
Burleson, W., Clark, S. S., Ransford, B. & Fu, K. in DAC Design Automation Conference 2012 12–17 (IEEE, 2012).
Joung, Y. H. Development of implantable medical devices: from an engineering perspective. Int. Neurourol. J. 17, 98–106 (2013).
Shi, B., Li, Z. & Fan, Y. Implantable energy-harvesting devices. Adv. Mater. 30, e1801511 (2018).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111, 1927–1932 (2014).
Lu, B. et al. Ultra-flexible piezoelectric devices integrated with heart to harvest the biomechanical energy. Sci. Rep. 5, 16065 (2015).
Kim, D. H. et al. In vivo self-powered wireless transmission using biocompatible flexible energy harvesters. Adv. Funct. Mater. 27, 1700341 (2017).
Zhang, H. et al. A flexible and implantable piezoelectric generator harvesting energy from the pulsation of ascending aorta: in vitro and in vivo studies. Nano Energy 12, 296–304 (2015).
Lin, H.-I., Wuu, D.-S., Shen, K.-C. & Horng, R.-H. Fabrication of an ultra-flexible ZnO nanogenerator for harvesting energy from respiration. ECS J. Solid State Sci. Technol. 2, P400–P404 (2013).
Dagdeviren, C. et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 1, 807–817 (2017).
Zheng, Q. et al. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2, e1501478 (2016).
Song, P. et al. A self-powered implantable drug-delivery system using biokinetic energy. Adv. Mater. 29, 1605668 (2017).
Zheng, Q. et al. In vivo powering of pacemaker by breathing-driven implanted triboelectric nanogenerator. Adv. Mater. 26, 5851–5856 (2014).
Zheng, Q. et al. In vivo self-powered wireless cardiac monitoring via implantable triboelectric nanogenerator. ACS Nano 10, 6510–6518 (2016).
Ouyang, H. et al. Symbiotic cardiac pacemaker. Nat. Commun. 10, 1821 (2019).
Zurbuchen, A. et al. Energy harvesting from the beating heart by a mass imbalance oscillation generator. Ann. Biomed. Eng. 41, 131–141 (2013).
Zurbuchen, A. et al. Towards batteryless cardiac implantable electronic devices–the Swiss way. IEEE Trans. Biomed. Circuits Syst. 11, 78–86 (2017).
Bereuter, L. et al. Energy harvesting by subcutaneous solar cells: a long-term study on achievable energy output. Ann. Biomed. Eng. 45, 1172–1180 (2017).
Chaar, L. E., lamont, L. A. & Zein, N. E. Review of photovoltaic technologies. Renew. Sust. Energ. Rev. 15, 2165–2175 (2011).
Hovel, H. J. in Semiconductors and Semimetals Vol. 11 (eds Willardson, R. K. & Beer, A. C.) 181–207 (Academic Press, 1975).
Haeberlin, A. et al. Successful pacing using a batteryless sunlight-powered pacemaker. Europace 16, 1534–1539 (2014).
Haeberlin, A. et al. The first batteryless, solar-powered cardiac pacemaker. Heart Rhythm 12, 1317–1323 (2015).
Song, K. et al. Subdermal flexible solar cell arrays for powering medical electronic implants. Adv. Healthc. Mater. 5, 1572–1580 (2016).
Zhao, L. M., Li, H., Meng, J. P. & Li, Z. The recent advances in self-powered medical information sensors. InfoMat 2, 212–234 (2020).
Sun, J. Y., Yang, A. P., Zhao, C., Liu, F. & Li, Z. Recent progress of nanogenerators acting as biomedical sensors in vivo. Sci. Bull. 64, 1336–1347 (2019).
Liu, Z. et al. Wearable and implantable triboelectric nanogenerators. Adv. Funct. Mater. 29, 1808820 (2019).
Feng, H. et al. Nanogenerator for biomedical applications. Adv. Healthc. Mater. 7, e1701298 (2018).
Lin, Z. et al. Triboelectric nanogenerator enabled body sensor network for self-powered human heart-rate monitoring. ACS Nano 11, 8830–8837 (2017).
Li, Z., Zheng, Q., Wang, Z. L. & Li, Z. Nanogenerator-based self-powered sensors for wearable and implantable electronics. Research 2020, 8710686 (2020).
Dagdeviren, C. et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496 (2014).
Park, D. Y. et al. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv. Mater. 29, 1702308 (2017).
Ouyang, H. et al. Self-powered pulse sensor for antidiastole of cardiovascular disease. Adv. Mater. 29, 1703456 (2017).
Meng, K. et al. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv. Funct. Mater. 29, 1806388 (2019).
Mahmud, M. A. P., Huda, N., Farjana, S. H., Asadnia, M. & Lang, C. Recent advances in nanogenerator-driven self-powered implantable biomedical devices. Adv. Energy Mater. 8, 1701210 (2018).
Ma, Y. et al. Self-powered, one-stop, and multifunctional implantable triboelectric active sensor for real-time biomedical monitoring. Nano Lett. 16, 6042–6051 (2016).
Cheng, X. L. et al. Implantable and self-powered blood pressure monitoring based on a piezoelectric thinfilm: simulated, in vitro and in vivo studies. Nano Energy 22, 453–460 (2016).
Liu, Z. et al. Transcatheter self-powered ultrasensitive endocardial pressure sensor. Adv. Funct. Mater. 29, 1807560 (2019).
Wang, M. et al. Air-flow-driven triboelectric nanogenerators for self-powered real-time respiratory monitoring. ACS Nano 12, 6156–6162 (2018).
Wang, A., Hu, M., Zhou, L. & Qiang, X. Self-powered wearable pressure sensors with enhanced piezoelectric properties of aligned P(VDF-TrFE)/MWCNT composites for monitoring human physiological and muscle motion signs. Nanomaterials 8, 1021 (2018).
Wang, S., Wang, Z. L. & Yang, Y. A one-structure-based hybridized nanogenerator for scavenging mechanical and thermal energies by triboelectric-piezoelectric-pyroelectric effects. Adv. Mater. 28, 2881–2887 (2016).
Wang, X. et al. Light-triggered pyroelectric nanogenerator based on a pn-junction for self-powered near-infrared photosensing. ACS Nano 11, 8339–8345 (2017).
Xue, H. et al. A wearable pyroelectric nanogenerator and self-powered breathing sensor. Nano Energy 38, 147–154 (2017).
Ohayon, D. et al. Biofuel powered glucose detection in bodily fluids with an n-type conjugated polymer. Nat. Mater. 19, 456–463 (2020).
Wang, X. et al. Bionic single-electrode electronic skin unit based on piezoelectric nanogenerator. ACS Nano 12, 8588–8596 (2018).
Xia, K., Zhu, Z., Zhang, H. & Xu, Z. W. A triboelectric nanogenerator as self-powered temperature sensor based on PVDF and PTFE. Appl. Phys. A 124, 520 (2018).
Zhang, F., Zang, Y., Huang, D., Di, C. A. & Zhu, D. Flexible and self-powered temperature-pressure dual-parameter sensors using microstructure-frame-supported organic thermoelectric materials. Nat. Commun. 6, 8356 (2015).
Feiner, R. & Dvir, T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 3, 17076 (2017).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Hong, G., Yang, X., Zhou, T. & Lieber, C. M. Mesh electronics: a new paradigm for tissue-like brain probes. Curr. Opin. Neurobiol. 50, 33–41 (2018).
Obidin, N., Tasnim, F. & Dagdeviren, C. The future of neuroimplantable devices: a materials science and regulatory perspective. Adv. Mater. 32, e1901482 (2020).
Pfenniger, A., Jonsson, M., Zurbuchen, A., Koch, V. M. & Vogel, R. Energy harvesting from the cardiovascular system, or how to get a little help from yourself. Ann. Biomed. Eng. 41, 2248–2263 (2013).
Tjong, F. V. & Reddy, V. Y. Permanent leadless cardiac pacemaker therapy: a comprehensive review. Circulation 135, 1458–1470 (2017).
Tan, P. et al. A battery-like self-charge universal module for motional energy harvest. Adv. Energy Mater. 9, 1901857 (2019).
Schwefel, J. et al. Wireless communication by an autonomous self-powered cyborg insect. J. Electrochem. Soc. 161, H3113–H3116 (2014).
Cinquin, P. et al. A glucose biofuel cell implanted in rats. PLoS ONE 5, e10476 (2010).
El Ichi, S. et al. Bioelectrodes modified with chitosan for long-term energy supply from the body. Energy Environ. Sci. 8, 1017–1026 (2015).
Shi, B. et al. A packaged self-powered system with universal connectors based on hybridized nanogenerators. Adv. Mater. 28, 846–852 (2016).
Yu, J. R. et al. Current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv. Healthc. Mater. 8, e1801471 (2019).
Castorena-Gonzalez, J. A. et al. Biofuel cell operating in vivo in rat. Electroanalysis 25, 1579–1584 (2013).
Dong, L. et al. In vivo cardiac power generation enabled by an integrated helical piezoelectric pacemaker lead. Nano Energy 66, 104085 (2019).
Acknowledgements
The authors’ work on self-powered medical devices is funded by the National Natural Science Foundation of China (no. 61875015 and 81971770), the Key Project of the National Natural Science Foundation (no. 81530012), the National Key R&D Project from the Ministry of Science and Technology, China (2016YFA0202703), the Beijing Natural Science Foundation (7204333), the University of Chinese Academy of Sciences and the National Youth Talent Support Program.
Author information
Authors and Affiliations
Contributions
Q.Z. and Z.L. researched the data for the article and wrote the manuscript. All the authors discussed the content of the article and reviewed and/or edited it before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks S.-G. Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Piezoelectric effect
-
The capacity of certain materials to generate an electrical charge in response to applied mechanical force.
- Crystal
-
A solid material whose constituents (such as atoms, molecules and ions) are arranged in a highly ordered microscopic structure to form a crystal lattice that extends in all directions.
- Electric dipole moment
-
The separation of a positive charge and a negative charge by a distance; a measure of the polarity of a system.
- Wurtzite structure
-
A hexagonal crystal structure that occurs in various binary compounds; named after the mineral wurtzite.
- C-axis
-
In crystal drawings, by convention, the c-axis is usually oriented vertically in the plane of the paper; all crystals except those with a cubic (or isometric) crystal structure have a c-axis.
- Charge centre
-
The position in a charge distribution with non-zero total charge where the electric dipole moment vanishes.
- Superposition
-
Superposition is the capacity of a quantum system to be in multiple states at the same time until it is measured.
- Triboelectrification
-
A type of contact electrification whereby certain materials become electrically charged after they are separated from a different material with which they were in contact.
- Electrostatic induction
-
A method to create or generate static electricity in a material by bringing an electrically charged object near to it, which causes the electrical charges to be redistributed in the material, resulting in one side having an excess of either positive or negative charges.
- Electron-capture properties
-
During electron capture, an electron in the inner shell of an atom is drawn into the nucleus where it combines with a proton, forming a neutron and a neutrino; the neutrino is ejected from the atom’s nucleus, and the overall effect is for an unstable atom to become more stable.
- Photovoltaic effect
-
A process in which two dissimilar materials in close contact produce an electrical voltage when struck by light or other form of radiant energy.
- Electromagnetic effect
-
A process in which either a stationary conductor is put in a moving magnetic field or a moving conductor is put in a stationary magnetic field, producing a voltage or electromotive force across the electrical conductor.
- Open-circuit voltage
-
(Voc). The difference in electric potential between two terminals of a device when disconnected from any circuit (no external load is connected and no external electric current is flowing between the terminals).
- Short-circuit current
-
The excess current flowing through an electrical circuit as a result of an unintended path in the circuit with no or very low electrical impedance.
- Rectifier
-
An electrical device that converts alternating current, which periodically reverses direction, into direct current, which flows in only one direction.
- Quartz clock
-
Inside a quartz clock or watch, the battery sends electricity to the quartz crystal through an electronic circuit; the quartz crystal oscillates (vibrates back and forth) at the precise frequency of 32,768 times per second.
- Spin Seebeck effect
-
The generation of spin ‘voltage’ as a result of a temperature gradient; when a metallic magnet is subjected to a temperature gradient, it generates different driving powers of electrons in different spin channels along the temperature gradient.
- n-Type organic semiconductor
-
Organic materials that are generally electrical insulators but which become semiconducting when charges are injected from appropriate electrodes; n-type organic semiconductors are electron acceptors with a low-lying lowest unoccupied molecular orbital.
- Organic electrochemical transistor
-
A transistor in which the drain current is controlled by the injection of ions from an electrolyte into a semiconductor channel; the injection of ions in the channel is controlled through the application of a voltage to the gate electrode.
- Young’s modulus
-
A measure of the capacity of a material to withstand changes in length when under lengthwise tension or compression.
- Impedance mismatch
-
In electrical engineering, an impedance mismatch occurs when the input impedance of an electrical load does not match the output impedance of the signal source, resulting in signal reflection or an inefficient power transfer (depending on the type of matching required).
- DC-to-DC converters
-
Electronic circuits or electromechanical devices that convert a source of direct current (DC) from one voltage level to another.
- Charge pumps
-
DC-to-DC converters that use capacitors for electrical charge storage to raise or lower the voltage.
- Buffer capacitors
-
A capacitor designed to suppress voltage surges that might otherwise damage other components in an electrical circuit.
- Sputtered metals
-
A process by which metals form a thin layer of conducting material on a surface as a result of sputtering, a method of physical vapour deposition.
Rights and permissions
About this article
Cite this article
Zheng, Q., Tang, Q., Wang, Z.L. et al. Self-powered cardiovascular electronic devices and systems. Nat Rev Cardiol 18, 7–21 (2021). https://doi.org/10.1038/s41569-020-0426-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-0426-4
This article is cited by
-
A self-powered intracardiac pacemaker in swine model
Nature Communications (2024)
-
Multifunctional and Reconfigurable Electronic Fabrics Assisted by Artificial Intelligence for Human Augmentation
Advanced Fiber Materials (2024)
-
Artificial intelligence enhanced sensors - enabling technologies to next-generation healthcare and biomedical platform
Bioelectronic Medicine (2023)
-
CNT-PDMS foams as self-powered humidity sensors based on triboelectric nanogenerators driven by finger tapping
Scientific Reports (2023)
-
Arrayed piezoresistive and inertial measurement unit sensor-integrated assistant training tennis racket for multipoint hand pressure monitoring and representative action recognition
Science China Technological Sciences (2023)