Abstract
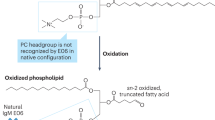
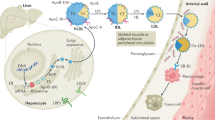
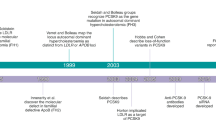
Epidemiological and clinical studies over the past decade have firmly established that elevated plasma concentrations of lipoprotein(a) (Lp(a)) are an important, independent and probably causal risk factor for the development of cardiovascular diseases. Whereas a link between Lp(a) levels and atherosclerotic cardiovascular disease (ASCVD) has been appreciated for decades, the role of Lp(a) in calcific aortic valve disease (CAVD) and aortic stenosis has come into focus only in the past 5 years. ASCVD and CAVD are aetiologically distinct but have several risk factors in common and similar pathological processes at the cellular and molecular levels. Oxidized phospholipids, which modify Lp(a) primarily by covalent binding to its unique apolipoprotein(a) (apo(a)) component, might hold the key to Lp(a) pathogenicity and provide a mechanistic link between ASCVD and CAVD. Oxidized phospholipids colocalize with apo(a)–Lp(a) in arterial and aortic valve lesions and directly participate in the pathogenesis of these disorders by promoting endothelial dysfunction, lipid deposition, inflammation and osteogenic differentiation, leading to calcification. The advent of potent Lp(a)-lowering therapies provides the opportunity to address directly the causality of Lp(a) in ASCVD and CAVD and, more importantly, to provide both a novel approach to reduce the residual risk of ASCVD and a long-sought medical treatment for CAVD.
Key points
-
Elevated plasma concentrations of lipoprotein(a) (Lp(a)) are an independent — and possibly causal — risk factor for atherothrombotic cardiovascular disease and calcific aortic valve disease.
-
The mechanisms by which Lp(a) accelerates these disorders are not fully understood, but the oxidized phospholipids present on apolipoprotein(a) might have an important role.
-
Lp(a) is the major carrier of oxidized phospholipids in human plasma, and interventions that lower plasma Lp(a) levels also reduce the oxidized phospholipid concentration in plasma.
-
The oxidized phospholipids on apolipoprotein(a) have been shown to mediate numerous, and partially overlapping, molecular and cellular events underlying atherothrombotic cardiovascular disease and calcific aortic valve disease.
-
Direct evidence demonstrating that lowering plasma Lp(a) levels reduces the risk of cardiovascular disease is lacking, as are conclusive data on the specific role of Lp(a)-associated oxidized phospholipids.
-
Cardiovascular outcome studies and further basic science investigations will be required to address these important questions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ellis, K. L., Boffa, M. B., Sahebkar, A., Koschinsky, M. L. & Watts, G. F. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog. Lipid Res. 68, 57–82 (2017).
Witztum, J. L. & Ginsberg, H. N. Lipoprotein (a): coming of age at last. J. Lipid Res. 57, 336–339 (2016).
Berg, K. A. New serum type system in man — the Lp system. Acta Pathol. Microbiol. Scand. 59, 369–382 (1963).
McLean, J. W. et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 330, 132–137 (1987).
van der Hoek, Y. Y., Wittekoek, M. E., Beisiegel, U., Kastelein, J. J. & Koschinsky, M. L. The apolipoprotein(a) kringle IV repeats which differ from the major repeat kringle are present in variably-sized isoforms. Hum. Mol. Genet. 2, 361–366 (1993).
Lackner, C., Cohen, J. C. & Hobbs, H. H. Molecular definition of the extreme size polymorphism in apolipoprotein(a). Hum. Mol. Genet. 2, 933–940 (1993).
Koschinsky, M. L., Cote, G. P., Gabel, B. & van der Hoek, Y. Y. Identification of the cysteine residue in apolipoprotein(a) that mediates extracellular coupling with apolipoprotein B-100. J. Biol. Chem. 268, 19819–19825 (1993).
Guevara, J. et al. Comparison of ligand-binding sites of modeled apo[a] kringle-like sequences in human lipoprotein[a]. Arterioscler. Thromb. 13, 758–770 (1993).
Guevara, J. Jr. et al. Proposed mechanisms for binding of apo[a] kringle type 9 to apo B-100 in human lipoprotein[a]. Biophys. J. 64, 686–700 (1993).
Rahman, M., Jia, Z., Gabel, B. R., Marcovina, S. M. & Koschinsky, M. L. Expression of apolipoprotein(a) kringle IV type 9 in Escherichia coli: demonstration of a specific interaction between kringle IV type 9 and apolipoproteinB-100. Protein Eng. 11, 1249–1256 (1998).
McCormick, S. P. et al. Mutagenesis of the human apolipoprotein B gene in a yeast artificial chromosome reveals the site of attachment for apolipoprotein(a). Proc. Natl Acad. Sci. USA 92, 10147–10151 (1995).
Gabel, B. R. & Koschinsky, M. I. Analysis of the proteolytic activity of a recombinant form of apolipoprotein(a). Biochemistry 34, 15777–15784 (1995).
Boffa, M. B. & Koschinsky, M. L. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J. Lipid Res. 57, 745–757 (2016).
Castellino, F. J. & McCance, S. G. The kringle domains of human plasminogen. Ciba Found. Symp. 212, 46–60; discussion 60–65 (1997).
Rahman, M. N. et al. Comparative analyses of the lysine binding site properties of apolipoprotein(a) kringle IV types 7 and 10. Biochemistry 41, 1149–1155 (2002).
Harpel, P. C., Gordon, B. R. & Parker, T. S. Plasmin catalyzes binding of lipoprotein (a) to immobilized fibrinogen and fibrin. Proc. Natl Acad. Sci. USA 86, 3847–3851 (1989).
Hoover-Plow, J. & Huang, M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism 62, 479–491 (2013).
Ernst, A. et al. Identification of two functionally distinct lysine-binding sites in kringle 37 and in kringles 32–36 of human apolipoprotein(a). J. Biol. Chem. 270, 6227–6234 (1995).
Gabel, B. R., May, L. F., Marcovina, S. M. & Koschinsky, M. L. Lipoprotein(a) assembly. Quantitative assessment of the role of apo(a) kringle IV types 2–10 in particle formation. Arterioscler. Thromb. Vasc. Biol. 16, 1559–1567 (1996).
Reyes-Soffer, G., Ginsberg, H. N. & Ramakrishnan, R. The metabolism of lipoprotein (a): an ever-evolving story. J. Lipid Res. 58, 1756–1764 (2017).
Kostner, K. M. & Kostner, G. M. Lipoprotein (a): a historical appraisal. J. Lipid Res. 58, 1–14 (2017).
Chiesa, G. et al. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a). J. Biol. Chem. 267, 24369–24374 (1992).
White, A. L. & Lanford, R. E. Cell surface assembly of lipoprotein(a) in primary cultures of baboon hepatocytes. J. Biol. Chem. 269, 28716–28723 (1994).
Bonen, D. K., Hausman, A. M., Hadjiagapiou, C., Skarosi, S. F. & Davidson, N. O. Expression of a recombinant apolipoprotein(a) in HepG2 cells. Evidence for intracellular assembly of lipoprotein(a). J. Biol. Chem. 272, 5659–5667 (1997).
Su, W., Campos, H., Judge, H., Walsh, B. W. & Sacks, F. M. Metabolism of Apo(a) and ApoB100 of lipoprotein(a) in women: effect of postmenopausal estrogen replacement. J. Clin. Endocrinol. Metab. 83, 3267–3276 (1998).
Frischmann, M. E. et al. In vivo stable-isotope kinetic study suggests intracellular assembly of lipoprotein(a). Atherosclerosis 225, 322–327 (2012).
Demant, T., Seeberg, K., Bedynek, A. & Seidel, D. The metabolism of lipoprotein(a) and other apolipoprotein B-containing lipoproteins: a kinetic study in humans. Atherosclerosis 157, 325–339 (2001).
Jenner, J. L. et al. The metabolism of apolipoproteins (a) and B-100 within plasma lipoprotein (a) in human beings. Metabolism 54, 361–369 (2005).
Diffenderfer, M. R. et al. Distinct metabolism of apolipoproteins (a) and B-100 within plasma lipoprotein(a). Metabolism 65, 381–390 (2016).
Rader, D. J., Cain, W., Zech, L. A., Usher, D. & Brewer, H. B. Jr. Variation in lipoprotein(a) concentrations among individuals with the same apolipoprotein (a) isoform is determined by the rate of lipoprotein(a) production. J. Clin. Invest. 91, 443–447 (1993).
Krempler, F., Kostner, G. M., Bolzano, K. & Sandhofer, F. Turnover of lipoprotein (a) in man. J. Clin. Invest. 65, 1483–1490 (1980).
Rader, D. J. et al. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J. Clin. Invest. 93, 2758–2763 (1994).
White, A. L., Guerra, B. & Lanford, R. E. Influence of allelic variation on apolipoprotein(a) folding in the endoplasmic reticulum. J. Biol. Chem. 272, 5048–5055 (1997).
White, A. L., Hixson, J. E., Rainwater, D. L. & Lanford, R. E. Molecular basis for “null” lipoprotein(a) phenotypes and the influence of apolipoprotein(a) size on plasma lipoprotein(a) level in the baboon. J. Biol. Chem. 269, 9060–9066 (1994).
Marcovina, S. M. et al. Differences in Lp[a] concentrations and apo[a] polymorphs between black and white Americans. J. Lipid Res. 37, 2569–2585 (1996).
Coassin, S. et al. A novel but frequent variant in LPA KIV-2 is associated with a pronounced Lp(a) and cardiovascular risk reduction. Eur. Heart J. 38, 1823–1831 (2017).
Boffelli, D., Zajchowski, D. A., Yang, Z. & Lawn, R. M. Estrogen modulation of apolipoprotein(a) expression. Identification of a regulatory element. J. Biol. Chem. 274, 15569–15574 (1999).
Zysow, B. R., Kauser, K., Lawn, R. M. & Rubanyi, G. M. Effects of estrus cycle, ovariectomy, and treatment with estrogen, tamoxifen, and progesterone on apolipoprotein(a) gene expression in transgenic mice. Arterioscler. Thromb. Vasc. Biol. 17, 1741–1745 (1997).
Chennamsetty, I. et al. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J. Lipid Res. 53, 2405–2412 (2012).
Chennamsetty, I. et al. Farnesoid X receptor represses hepatic human APOA gene expression. J. Clin. Invest. 121, 3724–3734 (2011).
Muller, N. et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J. Lipid Res. 56, 1034–1042 (2015).
Cain, W. J. et al. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a]. J. Lipid Res. 46, 2681–2691 (2005).
Hofmann, S. L. et al. Overexpression of human low density lipoprotein receptors leads to accelerated catabolism of Lp(a) lipoprotein in transgenic mice. J. Clin. Invest. 85, 1542–1547 (1990).
Romagnuolo, R. et al. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J. Biol. Chem. 290, 11649–11662 (2015).
Raal, F. J. et al. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: an analysis of 10 clinical trials and the LDL receptor’s role. J. Lipid Res. 57, 1086–1096 (2016).
Tam, S. P., Zhang, X. & Koschinsky, M. L. Interaction of a recombinant form of apolipoprotein[a] with human fibroblasts and with the human hepatoma cell line HepG2. J. Lipid Res. 37, 518–533 (1996).
Sharma, M., Redpath, G. M., Williams, M. J. & McCormick, S. P. Recycling of apolipoprotein(a) after PlgRKT-mediated endocytosis of lipoprotein(a). Circ. Res. 120, 1091–1102 (2017).
Yang, X. P. et al. Scavenger receptor-BI is a receptor for lipoprotein(a). J. Lipid Res. 54, 2450–2457 (2013).
Gaudet, D. et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am. J. Cardiol. 114, 711–715 (2014).
Raal, F. J. et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J. Am. Coll. Cardiol. 63, 1278–1288 (2014).
Watts, G. F. et al. Controlled study of the effect of proprotein convertase subtilisin-kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur. Heart J. 39, 2577–2585 (2018).
Villard, E. F. et al. PCSK9 modulates the secretion but not the cellular uptake of lipoprotein(a) ex vivo. JACC Basic Transl Sci. 1, 419–427 (2016).
Boffelli, D., Cheng, J. F. & Rubin, E. M. Convergent evolution in primates and an insectivore. Genomics 83, 19–23 (2004).
Schneider, M. et al. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J. Lipid Res. 46, 769–778 (2005).
Schaefer, E. J. et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA 271, 999–1003 (1994).
Bostom, A. G. et al. A prospective investigation of elevated lipoprotein (a) detected by electrophoresis and cardiovascular disease in women. The Framingham Heart Study. Circulation 90, 1688–1695 (1994).
Ridker, P. M., Hennekens, C. H. & Stampfer, M. J. A prospective study of lipoprotein(a) and the risk of myocardial infarction. JAMA 270, 2195–2199 (1993).
Cantin, B. et al. Is lipoprotein(a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J. Am. Coll. Cardiol. 31, 519–525 (1998).
Marcovina, S. M. & Albers, J. J. Lipoprotein (a) measurements for clinical application. J. Lipid Res. 57, 526–537 (2016).
Emerging, Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 302, 412–423 (2009).
Clarke, R. et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361, 2518–2528 (2009).
Kamstrup, P. R., Tybjaerg-Hansen, A., Steffensen, R. & Nordestgaard, B. G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 301, 2331–2339 (2009).
Nordestgaard, B. G. & Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J. Lipid Res. 57, 1953–1975 (2016).
Kamstrup, P. R. & Nordestgaard, B. G. Lipoprotein(a) concentrations, isoform size, and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 1, 220–227 (2013).
Kamstrup, P. R. & Nordestgaard, B. G. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 4, 78–87 (2016).
Thanassoulis, G. et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368, 503–512 (2013).
Kamstrup, P. R., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 63, 470–477 (2014).
Kamstrup, P. R., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 32, 1732–1741 (2012).
Kivimaki, M. et al. Conventional and Mendelian randomization analyses suggest no association between lipoprotein(a) and early atherosclerosis: the Young Finns Study. Int. J. Epidemiol. 40, 470–478 (2011).
Langsted, A., Kamstrup, P. R. & Nordestgaard, B. G. High lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: a Mendelian randomization study. Clin. Chem. 63, 1714–1723 (2017).
Saleheen, D. et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol. 5, 524–533 (2017).
Helgadottir, A. et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J. Am. Coll. Cardiol. 60, 722–729 (2012).
Hopewell, J. C. et al. Lipoprotein(a) genetic variants associated with coronary and peripheral vascular disease but not with stroke risk in the Heart Protection Study. Circ. Cardiovasc. Genet. 4, 68–73 (2011).
Laschkolnig, A. et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc. Res. 103, 28–36 (2014).
Gurdasani, D. et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 32, 3058–3065 (2012).
Emdin, C. A. et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J. Am. Coll. Cardiol. 68, 2761–2772 (2016).
Afshar, M. et al. Estimating the population impact of Lp(a) lowering on the incidence of myocardial infarction and aortic stenosis — brief report. Arterioscler. Thromb. Vasc. Biol. 36, 2421–2423 (2016).
Burgess, S. et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 3, 619–627 (2018).
Albers, J. J. et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 62, 1575–1579 (2013).
Tsimikas, S. et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386, 1472–1483 (2015).
Viney, N. J. et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 388, 2239–2253 (2016).
Berg, K. et al. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin. Genet. 52, 254–261 (1997).
Shlipak, M. G. et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA 283, 1845–1852 (2000).
Nestel, P. J. et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 33, 2902–2908 (2013).
Arsenault, B. J. et al. Prediction of cardiovascular events in statin-treated stable coronary patients of the treating to new targets randomized controlled trial by lipid and non-lipid biomarkers. PLOS ONE 9, e114519 (2014).
O’Donoghue, M. L. et al. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J. Am. Coll. Cardiol. 63, 520–527 (2014).
Schwartz, G. G. et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: Analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol. 3, 164–168 (2017).
Zewinger, S. et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 5, 534–543 (2017).
Dahabreh, I. J. & Kent, D. M. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA 305, 822–823 (2011).
Smits, L. J. et al. Index event bias-a numerical example. J. Clin. Epidemiol. 66, 192–196 (2013).
Varvel, S., McConnell, J. P. & Tsimikas, S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler. Thromb. Vasc. Biol. 36, 2239–2245 (2016).
O’Donoghue, M. L. et al. Lipoprotein(a), PCSK9 inhibition and Cardiovascular Risk: Insights from the FOURIER Trial. Circulation https://doi.org/10.1161/CIRCULATIONAHA.118.037184 (2018).
Bittner, V. et al. Lp(a) and cardiovascular outcomes: an analysis from the ODYSSEY OUTCOMES trial. Atheroscler. Suppl. 32, 24–25 (2018).
Alonso, R. et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J. Am. Coll. Cardiol. 63, 1982–1989 (2014).
Langsted, A., Kamstrup, P. R., Benn, M., Tybjaerg-Hansen, A. & Nordestgaard, B. G. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 4, 577–587 (2016).
Ellis, K. L. et al. Elevated lipoprotein(a) and familial hypercholesterolemia in the coronary care unit: between Scylla and Charybdis. Clin. Cardiol. 41, 378–384 (2018).
van Capelleveen, J. C., van der Valk, F. M. & Stroes, E. S. Current therapies for lowering lipoprotein (a). J. Lipid Res. 57, 1612–1618 (2016).
O’Brien, K. D. et al. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 16, 523–532 (1996).
Stewart, B. F. et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 29, 630–634 (1997).
Gotoh, T. et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol. 76, 928–932 (1995).
Thanassoulis, G. Lipoprotein (a) in calcific aortic valve disease: from genomics to novel drug target for aortic stenosis. J. Lipid Res. 57, 917–924 (2016).
Mathieu, P., Arsenault, B. J., Boulanger, M. C., Bosse, Y. & Koschinsky, M. L. Pathobiology of Lp(a) in calcific aortic valve disease. Expert Rev. Cardiovasc. Ther. 15, 797–807 (2017).
Chan, K. L. et al. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 121, 306–314 (2010).
Kvidal, P., Bergstrom, R., Horte, L. G. & Stahle, E. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 35, 747–756 (2000).
Dweck, M. R., Boon, N. A. & Newby, D. E. Calcific aortic stenosis: a disease of the valve and the myocardium. J. Am. Coll. Cardiol. 60, 1854–1863 (2012).
Schnitzler, J. G., Dallinga-Thie, G. M. & Kroon, J. The role of (modified) lipoproteins in vascular function: a duet between monocytes and the endothelium. Curr. Med. Chem. https://doi.org/10.2174/0929867325666180316121015 (2018).
Berliner, J. A., Leitinger, N. & Tsimikas, S. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 50, S207–S212 (2009).
Yeang, C. et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J. Clin. Lipidol. 10, 594–603 (2016).
Tsimikas, S. et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation 110, 1406–1412 (2004).
Friedman, P., Horkko, S., Steinberg, D., Witztum, J. L. & Dennis, E. A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 277, 7010–7020 (2002).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018).
van Dijk, R. A. et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J. Lipid Res. 53, 2773–2790 (2012).
Torzewski, M. et al. Lipoprotein(a) associated molecules are prominent components in plasma and valve leaflets in calcific aortic valve stenosis. JACC Basic Transl Sci. 2, 229–240 (2017).
Bergmark, C. et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49, 2230–2239 (2008).
Tsimikas, S. et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J. Am. Coll. Cardiol. 41, 360–370 (2003).
Tsimikas, S. et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353, 46–57 (2005).
Kiechl, S. et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 27, 1788–1795 (2007).
Tsimikas, S. et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J. Am. Coll. Cardiol. 56, 946–955 (2010).
Capoulade, R. et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66, 1236–1246 (2015).
Kamstrup, P. R., Hung, M. Y., Witztum, J. L., Tsimikas, S. & Nordestgaard, B. G. Oxidized phospholipids and risk of calcific aortic valve disease: the Copenhagen General Population Study. Arterioscler. Thromb. Vasc. Biol. 37, 1570–1578 (2017).
Tsimikas, S. et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation 109, 3164–3170 (2004).
Arai, K. et al. The I4399M variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein B-100 particles. Atherosclerosis 209, 498–503 (2010).
Tsimikas, S., Tsironis, L. D. & Tselepis, A. D. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 27, 2094–2099 (2007).
Tselepis, A. D. Oxidized phospholipids and lipoprotein-associated phospholipase A2 as important determinants of Lp(a) functionality and pathophysiological role. J. Biomed. Res. 32, 13–22 (2016).
Leibundgut, G. et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a). J. Lipid Res. 54, 2815–2830 (2013).
Scipione, C. A. et al. Mechanistic insights into Lp(a)-induced IL-8 expression: a role for oxidized phospholipid modification of apo(a). J. Lipid Res. 56, 2273–2285 (2015).
Taleb, A., Witztum, J. L. & Tsimikas, S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 5, 673–694 (2011).
Tsironis, L. D., Mitsios, J. V., Milionis, H. J., Elisaf, M. & Tselepis, A. D. Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc. Res. 63, 130–138 (2004).
Blencowe, C., Hermetter, A., Kostner, G. M. & Deigner, H. P. Enhanced association of platelet-activating factor acetylhydrolase with lipoprotein (a) in comparison with low density lipoprotein. J. Biol. Chem. 270, 31151–31157 (1995).
Karabina, S. A. et al. PAF-acetylhydrolase activity of Lp(a) before and during Cu(2+)-induced oxidative modification in vitro. Atherosclerosis 125, 121–134 (1996).
Binder, C. J., Papac-Milicevic, N. & Witztum, J. L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 16, 485–497 (2016).
Tsimikas, S. et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J. Am. Coll. Cardiol. 63, 1724–1734 (2014).
Edelstein, C. et al. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a). J. Biol. Chem. 278, 52841–52847 (2003).
Seimon, T. A. et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12, 467–482 (2010).
van der Valk, F. M. et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 134, 611–624 (2016).
Cho, T., Jung, Y. & Koschinsky, M. L. Apolipoprotein(a), through its strong lysine-binding site in KIV(10′), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. J. Biol. Chem. 283, 30503–30512 (2008).
Cho, T., Romagnuolo, R., Scipione, C., Boffa, M. B. & Koschinsky, M. L. Apolipoprotein(a) stimulates nuclear translocation of beta-catenin: a novel pathogenic mechanism for lipoprotein(a). Mol. Biol. Cell 24, 210–221 (2013).
Liu, L. et al. Apolipoprotein(a) stimulates vascular endothelial cell growth and migration and signals through integrin alphaVbeta3. Biochem. J. 418, 325–336 (2009).
Boonmark, N. W. et al. Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice. J. Clin. Invest. 100, 558–564 (1997).
Hughes, S. D. et al. Lipoprotein(a) vascular accumulation in mice. In vivo analysis of the role of lysine binding sites using recombinant adenovirus. J. Clin. Invest. 100, 1493–1500 (1997).
Bouchareb, R. et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation 132, 677–690 (2015).
Nsaibia, M. J. et al. Autotaxin interacts with lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J. Intern. Med. 280, 509–517 (2016).
Yu, B. et al. Lipoprotein(a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci. 2, 358–371 (2017).
Tsimikas, S. The re-emergence of lipoprotein(a) in a broader clinical arena. Prog. Cardiovasc. Dis. 59, 135–144 (2016).
Boffa, M. B. & Koschinsky, M. L. The journey towards understanding lipoprotein(a) and cardiovascular disease risk: are we there yet? Curr. Opin. Lipidol. 29, 259–267 (2018).
Waldmann, E. & Parhofer, K. G. Lipoprotein apheresis to treat elevated lipoprotein(a). J. Lipid Res. 57, 1751–1757 (2016).
Mochalkin, I., Cheng, B., Klezovitch, O., Scanu, A. M. & Tulinsky, A. Recombinant kringle IV-10 modules of human apolipoprotein(a): structure, ligand binding modes, and biological relevance. Biochemistry 38, 1990–1998 (1999).
Koschinsky, M. L. & Marcovina, S. M. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr. Opin. Lipidol. 15, 167–174 (2004).
Koschinsky, M. L., Boffa, M. B. o Marcovina, S. M. in Clinical Lipidology: A Companion to Braunwald’s Heart Disease (ed. Ballantyne, C. M.) 109–127 (Elsevier, 2015).
Acknowledgements
M.L.K. acknowledges grant support for her lipoprotein(a) research programme from the Heart and Stroke Foundation of Canada, the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada and ASPIRE Cardiovascular Research Awards.
Reviewer information
Nature Reviews Cardiology thanks G. Kostner, J. Witztum and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article and discussed its content. M.B.B. wrote the manuscript, and M.L.K. reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
M.B.B. has a research contract from IONIS. M.L.K. has research contracts from CardioVax, Eli Lilly and IONIS and is on the advisory board and/or speakers’ bureau of Amgen, Eli Lilly, IONIS and Sanofi.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boffa, M.B., Koschinsky, M.L. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol 16, 305–318 (2019). https://doi.org/10.1038/s41569-018-0153-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0153-2
This article is cited by
-
Models for calcific aortic valve disease in vivo and in vitro
Cell Regeneration (2024)
-
Enhancing the evaluation of acute ischemic stroke risk in individuals with non-valvular atrial fibrillation by including laboratory indicators
Scientific Reports (2024)
-
Targeting Lipoprotein(a): Can RNA Therapeutics Provide the Next Step in the Prevention of Cardiovascular Disease?
Cardiology and Therapy (2024)
-
Cholesterol Lowering Biotechnological Strategies: From Monoclonal Antibodies to Antisense Therapies. A Pre-Clinical Perspective Review
Cardiovascular Drugs and Therapy (2023)
-
Lipoprotein(a) predicts recurrent cardiovascular events in patients with prior cardiovascular events post-PCI: five-year findings from a large single center cohort study
Thrombosis Journal (2022)