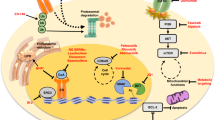
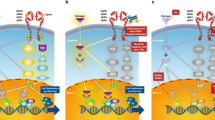
Abstract
The hormone receptor oestrogen receptor-α (ER) orchestrates physiological mammary gland development, breast carcinogenesis and the progression of breast tumours into lethal, treatment-refractory systemic disease. Selective antagonism of ER signalling has been one of the most successful therapeutic approaches in oncology, benefiting patients as both a cancer preventative measure and a cancer treatment strategy. However, resistance to anti-oestrogen therapy is a major clinical challenge. Over the past decade, we have gained an understanding of how breast cancers evolve under the pressure of anti-oestrogen therapy. This is best depicted by the case of oestrogen-independent mutations in the gene encoding ER (ESR1), which are virtually absent in primary breast cancer but highly prevalent (20–40%) in anti-oestrogen-treated metastatic disease. These and other findings highlight the ‘evolvability’ of ER+ breast cancer and the need to understand molecular processes by which this evolution occurs. Recent development and approval of next-generation ER antagonists to target ESR1-mutant breast cancer underscores the clinical importance of this evolvability and sets a new paradigm for the treatment of ER+ breast cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brisken, C. & O’Malley, B. Hormone action in the mammary gland. Cold Spring Harb. Perspect. Biol. 2, a003178 (2010).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451 (2019).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022).
Eeckhoute, J., Carroll, J. S., Geistlinger, T. R., Torres-Arzayus, M. I. & Brown, M. A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev. 20, 2513–2526 (2006).
Waks, A. G. & Winer, E. P. Breast cancer treatment: a review. J. Am. Med. Assoc. 321, 288–300 (2019).
Burstein, H. J. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N. Engl. J. Med. 383, 2557–2570 (2020).
Hartkopf, A. D., Grischke, E. M. & Brucker, S. Y. Endocrine-resistant breast cancer: mechanisms and treatment. Breast Care 15, 347–354 (2020).
Johnston, S. R. D. & Dowsett, M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat. Rev. Cancer 3, 821–831 (2003).
Patel, H. K. & Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 186, 1–24 (2018).
Sakamoto, T. et al. Estrogen receptor-mediated effects of tamoxifen on human endometrial cancer cells. Mol. Cell. Endocrinol. 192, 93–104 (2002).
Liu, H., Lee, E. S., Deb Los Reyes, A., Zapf, J. W. & Jordan, V. C. Silencing and reactivation of the selective estrogen receptor modulator-estrogen receptor alpha complex. Cancer Res. 61, 3632–3639 (2001).
Shang, Y. & Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468 (2002).
Jordan, V. C. Tamoxifen: catalyst for the change to targeted therapy. Eur. J. Cancer 44, 30–38 (2008).
Gottardis, M. M. & Jordan, V. C. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 48, 5183–5187 (1988).
Wolf, D. M. & Jordan, V. C. Characterization of tamoxifen stimulated MCF-7 tumor variants grown in athymic mice. Breast Cancer Res. Treat. 31, 117–127 (1994).
Wakeling, A. E. Therapeutic potential of pure antioestrogens in the treatment of breast cancer. J. Steroid Biochem. Mol. Biol. 37, 771–775 (1990).
Wakeling, A. E., Dukes, M. & Bowler, J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 51, 3867–3873 (1991).
Long, X. & Nephew, K. P. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J. Biol. Chem. 281, 9607–9615 (2006).
Wardell, S. E., Marks, J. R. & McDonnell, D. P. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem. Pharmacol. 82, 122–130 (2011).
Guan, J. et al. Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell 178, 949–963.e18 (2019).
Stenoien, D. L. et al. FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat. Cell Biol. 3, 15–23 (2001).
Kato, S. et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270, 1491–1494 (1995).
Sutherland, R. L., Green, M. D., Hall, R. E., Reddel, R. R. & Taylor, I. W. Tamoxifen induces accumulation of MCF 7 human mammary carcinoma cells in the G0/G1 phase of the cell cycle. Eur. J. Cancer Clin. Oncol. 19, 615–621 (1983).
Butler, W. B. & Kelsey, W. H. Effects of tamoxifen and 4-hydroxytamoxifen on synchronized cultures of the human breast cancer cell line MCF-7. Breast Cancer Res. Treat. 11, 37–43 (1988).
Watts, C. K. W., Sweeney, K. J. E., Warlters, A., Musgrove, E. A. & Sutherland, R. L. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 31, 95–105 (1994).
Musgrove, E. A. et al. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol. Cell Biol. 13, 3577–3587 (1993).
Doisneau-Sixou, S. F. et al. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer 10, 179–186 (2003).
Davies, C. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378, 771–784 (2011).
Elledge, R. M. et al. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int. J. Cancer 89, 111–117 (2000).
Yamashita, H. et al. Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer 13, 74–83 (2006).
Najjar, S. & Allison, K. H. Updates on breast biomarkers. Virchows Arch. 480, 163–176 (2022).
Robertson, J. F. et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J. Clin. Oncol. 27, 4530–4535 (2009).
Bonneterre, J. et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J. Clin. Oncol. 18, 3748–3757 (2000).
Nabholtz, J. M., Bonneterre, J., Buzdar, A., Robertson, J. F. & Thürlimann, B. Anastrozole (Arimidex) versus tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: survival analysis and updated safety results. Eur. J. Cancer 39, 1684–1689 (2003).
Mauri, D., Pavlidis, N., Polyzos, N. P. & Ioannidis, J. P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J. Natl Cancer Inst. 98, 1285–1291 (2006).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Watts, C. K. & King, R. J. Overexpression of estrogen receptor in HTB 96 human osteosarcoma cells results in estrogen-induced growth inhibition and receptor cross talk. J. Bone Min. Res. 9, 1251–1258 (1994).
Schiff, R. et al. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin. Cancer Res. 10, 331s–336s (2004).
Musheyev, D. & Alayev, A. Endocrine therapy resistance: what we know and future directions. Explor. Target. Antitumor Ther. 3, 480–496 (2022).
Zhang, Q. X., Borg, A., Wolf, D. M., Oesterreich, S. & Fuqua, S. A. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 57, 1244–1249 (1997). Following the initial identification of a case in 1997, a series of publications (Toy et al., Robinson et al., Merenbakh-Lamin et al., Li et al. and Jeselsohn et al.) in the early 2010s identified a collection of activating ESR1 mutations to be recurrent, acquired mechanisms of resistance to anti-oestrogen therapy in metastatic ER+ breast cancer.
Toy, W. et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 45, 1439–1445 (2013). This study characterizes the activity and drug sensitivity of various ESR1 mutations as well as the therapeutic potential of oral SERDs in antagonizing ESR1 mutants.
Robinson, D. R. et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 45, 1446–1451 (2013).
Merenbakh-Lamin, K. et al. D538G mutation in estrogen receptor-α: a novel mechanism for acquired endocrine resistance in breast cancer. Cancer Res. 73, 6856–6864 (2013).
Li, S. et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 4, 1116–1130 (2013).
Jeselsohn, R. et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin. Cancer Res. 20, 1757–1767 (2014).
Toy, W. et al. Activating ESR1 mutations differentially affect the efficacy of ER antagonists. Cancer Discov. 7, 277–287 (2017).
Koboldt, D. C. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Chandarlapaty, S. et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2, 1310–1315 (2016).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e6 (2018). This study dissects the genomic data of more than 1,500 ER+ breast tumours and incorporates clinical treatment and outcomes to define acquired alterations that may contribute to resistance to hormonal therapy.
Fribbens, C. et al. Plasma ESR1 mutations and the treatment of estrogen receptor–positive advanced breast cancer. J. Clin. Oncol. 34, 2961–2968 (2016).
Schiavon, G. et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 7, 313ra182 (2015). This study highlights the clinical acquisition of ESR1 mutants in patients with ER+ breast cancer using serial sampling of circulating tumour DNA.
Kuang, Y. et al. Unraveling the clinicopathological features driving the emergence of ESR1 mutations in metastatic breast cancer. npj Breast Cancer 4, 22 (2018).
Fribbens, C. V. et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA (ctDNA) in metastatic breast cancer. J. Clin. Oncol. 35, 1015–1015 (2017).
Katzenellenbogen, J. A., Mayne, C. G., Katzenellenbogen, B. S., Greene, G. L. & Chandarlapaty, S. Structural underpinnings of oestrogen receptor mutations in endocrine therapy resistance. Nat. Rev. Cancer 18, 377–388 (2018).
Fanning, S. W. et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife 5, e12792 (2016).
Gates, L. A. et al. Proteomic profiling identifies key coactivators utilized by mutant ERα proteins as potential new therapeutic targets. Oncogene 37, 4581–4598 (2018).
Jeselsohn, R., Buchwalter, G., De Angelis, C., Brown, M. & Schiff, R. ESR1 mutations — a mechanism for acquired endocrine resistance in breast cancer. Nat. Rev. Clin. Oncol. 12, 573–583 (2015).
Gu, G. et al. Hormonal modulation of ESR1 mutant metastasis. Oncogene 40, 997–1011 (2021).
Li, Z. et al. Hotspot ESR1 mutations are multimodal and contextual modulators of breast cancer metastasis. Cancer Res. 82, 1321–1339 (2022).
Harrod, A. et al. Genomic modelling of the ESR1 Y537S mutation for evaluating function and new therapeutic approaches for metastatic breast cancer. Oncogene 36, 2286–2296 (2017).
Bahreini, A. et al. Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res. 19, 60 (2017).
Martin, L. A. et al. Discovery of naturally occurring ESR1 mutations in breast cancer cell lines modelling endocrine resistance. Nat. Commun. 8, 1865 (2017).
Liang, J. et al. Giredestrant reverses progesterone hypersensitivity driven by estrogen receptor mutations in breast cancer. Sci. Transl. Med. 14, eabo5959 (2022).
Najim, O. et al. The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: a systematic review and meta-analysis of randomized and non-randomized trials. Biochim. Biophys. Acta Rev. Cancer 1872, 188315 (2019).
Carlson, K. E., Choi, I., Gee, A., Katzenellenbogen, B. S. & Katzenellenbogen, J. A. Altered ligand binding properties and enhanced stability of a constitutively active estrogen receptor: evidence that an open pocket conformation is required for ligand interaction. Biochemistry 36, 14897–14905 (1997).
Zhao, Y. et al. Structurally novel antiestrogens elicit differential responses from constitutively active mutant estrogen receptors in breast cancer cells and tumors. Cancer Res. 77, 5602–5613 (2017).
van Kruchten, M. et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 5, 72–81 (2015).
Ferraro, E., Walsh, E. M., Tao, J. J., Chandarlapaty, S. & Jhaveri, K. Accelerating drug development in breast cancer: new frontiers for ER inhibition. Cancer Treat. Rev. 109, 102432 (2022).
Lei, J. T. et al. Functional annotation of ESR1 gene fusions in estrogen receptor-positive breast cancer. Cell Rep. 24, 1434–1444.e7 (2018).
Hartmaier, R. J. et al. Recurrent hyperactive ESR1 fusion proteins in endocrine therapy-resistant breast cancer. Ann. Oncol. 29, 872–880 (2018).
Giltnane, J. M. et al. Genomic profiling of ER(+) breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci. Transl. Med. 9, eaai7993 (2017).
Lei, J. T., Gou, X. & Ellis, M. J. ESR1 fusions drive endocrine therapy resistance and metastasis in breast cancer. Mol. Cell Oncol. 5, e1526005 (2018).
Lonard, D. M. & O’Malley B. W. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol. Cell 27, 691–700 (2007).
Osborne, C. K. et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl Cancer Inst. 95, 353–361 (2003).
Myers, E. et al. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br. J. Cancer 91, 1687–1693 (2004).
McBryan, J. et al. Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1. Cancer Res. 72, 548–559 (2012).
Keeton, E. K. & Brown, M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-α and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol. Endocrinol. 19, 1543–1554 (2005).
Girault, I. et al. Expression analysis of estrogen receptor alpha coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin. Cancer Res. 9, 1259–1266 (2003).
Prat, A. & Perou, C. M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 5, 5–23 (2011).
Knowlden, J. M. et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144, 1032–1044 (2003).
Dowsett, M. et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. J. Clin. Oncol. 26, 1059–1065 (2008).
Nayar, U. et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat. Genet. 51, 207–216 (2019). This study identifies HER2 activating mutations in metastatic biopsies of patients with ER+ breast cancer resistant to hormonal therapy.
Croessmann, S. et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin. Cancer Res. 25, 277–289 (2019).
Smyth, L. M. et al. Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic. Cancer Discov. 10, 198–213 (2020).
Mao, P. et al. Acquired FGFR and FGF alterations confer resistance to estrogen receptor (ER) targeted therapy in ER(+) metastatic breast cancer. Clin. Cancer Res. 26, 5974–5989 (2020).
Lupien, M. et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 24, 2219–2227 (2010).
Pearson, A. et al. Inactivating NF1 mutations are enriched in advanced breast cancer and contribute to endocrine therapy resistance. Clin. Cancer Res. 26, 608–622 (2020).
Sokol, E. S. et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann. Oncol. 30, 115–123 (2019).
Arruabarrena-Aristorena, A. et al. FOXA1 mutations reveal distinct chromatin profiles and influence therapeutic response in breast cancer. Cancer Cell 38, 534–550.e9 (2020).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Seachrist, D. D., Anstine, L. J. & Keri, R. A. FOXA1: a pioneer of nuclear receptor action in breast cancer. Cancers 13, 5205 (2021).
Quintanal-Villalonga, Á. et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 17, 360–371 (2020).
Chan, J. M. et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science 377, 1180–1191 (2022).
Hoefnagel, L. D. C. et al. Prognostic value of estrogen receptor α and progesterone receptor conversion in distant breast cancer metastases. Cancer 118, 4929–4935 (2012).
Gutierrez, M. C. et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J. Clin. Oncol. 23, 2469–2476 (2005).
Drury, S. C. et al. Changes in breast cancer biomarkers in the IGF1R/PI3K pathway in recurrent breast cancer after tamoxifen treatment. Endocr. Relat. Cancer 18, 565–577 (2011).
Ottaviano, Y. L. et al. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 54, 2552–2555 (1994).
Yang, X. et al. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 60, 6890–6894 (2000).
Vesuna, F. et al. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-α. Oncogene 31, 3223–3234 (2012).
Wang, L. & Sharma, A. The quest for orally available selective estrogen receptor degraders (SERDs). ChemMedChem 15, 2072–2097 (2020).
Hernando, C. et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: present and future from a clinical perspective. Int. J. Mol. Sci. 22, 7812 (2021).
Ozyurt, R. & Ozpolat, B. Molecular mechanisms of anti-estrogen therapy resistance and novel targeted therapies. Cancers 14, 5206 (2022).
Willson, T. M. et al. 3-[4-(1,2-Diphenylbut-1-enyl)phenyl]acrylic acid: a non-steroidal estrogen with functional selectivity for bone over uterus in rats. J. Med. Chem. 37, 1550–1552 (1994).
Wu, Y. L. et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol. Cell 18, 413–424 (2005).
Bentrem, D. et al. Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen. Endocrinology 142, 838–846 (2001).
Lloyd, M. R., Wander, S. A., Hamilton, E., Razavi, P. & Bardia, A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther. Adv. Med. Oncol. 14, 17588359221113694 (2022).
Wardell, S. E., Nelson, E. R., Chao, C. A., Alley, H. M. & McDonnell, D. P. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr. Relat. Cancer 22, 713–724 (2015).
Garner, F., Shomali, M., Paquin, D., Lyttle, C. R. & Hattersley, G. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs 26, 948–956 (2015).
Bihani, T. et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER(+) breast cancer patient-derived xenograft models. Clin. Cancer Res. 23, 4793–4804 (2017).
Shomali, M. et al. SAR439859, a novel selective estrogen receptor degrader (SERD), demonstrates effective and broad antitumor activity in wild-type and mutant ER-positive breast cancer models. Mol. Cancer Ther. 20, 250–262 (2021).
Liang, J. et al. GDC-9545 (giredestrant): a potent and orally bioavailable selective estrogen receptor antagonist and degrader with an exceptional preclinical profile for ER+ breast cancer. J. Med. Chem. 64, 11841–11856 (2021).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT04059484 (2019).
Tolaney, S. M. et al. 212MO AMEERA-3, a phase II study of amcenestrant (AMC) versus endocrine treatment of physician’s choice (TPC) in patients (pts) with endocrine-resistant ER+/HER-advanced breast cancer (aBC). Ann. Oncol. 33, S634–S635 (2022).
Wang, Y. & Tang, S.-C. The race to develop oral SERDs and other novel estrogen receptor inhibitors: recent clinical trial results and impact on treatment options. Cancer Metastasis Rev. 41, 975–990 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT04478266 (2020).
Bardia, A. et al. AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2-advanced breast cancer. Ther. Adv. Med. Oncol. 14, 17588359221083956 (2022).
Bardia, A. et al. EMERALD: phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 15, 3209–3218 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT03778931 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT04214288 (2020).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT04576455 (2020).
Chen, Y. C. et al. Latest generation estrogen receptor degraders for the treatment of hormone receptor-positive breast cancer. Exp. Opin. Investig. Drugs 31, 515–529 (2022).
Martin Jimenez, M. et al. 211MO giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2– locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann. Oncol. 33, S633–S634 (2022).
Oliveira, M. et al. Abstract GS3-02: GS3-02 camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial. Cancer Res. 83, GS3-02 (2023).
Fasching, P. A. et al. Neoadjuvant giredestrant (GDC-9545) plus palbociclib (P) versus anastrozole (A) plus P in postmenopausal women with estrogen receptor–positive, HER2-negative, untreated early breast cancer (ER+/HER2–eBC): final analysis of the randomized, open-label, international phase 2 coopERA BC study. J. Clin. Oncol. 40, 589–589 (2022).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Flanagan, J. J. & Neklesa, T. K. Targeting nuclear receptors with PROTAC degraders. Mol. Cell. Endocrinol. 493, 110452 (2019).
Hamilton, E. P. et al. ARV-471, an estrogen receptor (ER) PROTAC degrader, combined with palbociclib in advanced ER+/human epidermal growth factor receptor 2-negative (HER2-) breast cancer: phase 1b cohort (part C) of a phase 1/2 study. J. Clin. Oncol. 40, TPS1120 (2022).
Puyang, X. et al. Discovery of selective estrogen receptor covalent antagonists for the treatment of ERα(WT) and ERα(MUT). Cancer Discov. 8, 1176–1193 (2018).
Furman, C. et al. Covalent ERα antagonist H3B-6545 demonstrates encouraging preclinical activity in therapy-resistant breast cancer. Mol. Cancer Ther. 21, 890–902 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT03250676 (2017).
Hamilton, E. P. et al. Abstract P1-17-10: H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer — a phase II study. Cancer Res. 82, P1-17-10–P11-17-10 (2022).
Hamilton, E. P. et al. Phase I/II study of H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer. J. Clin. Oncol. 39, 1018–1018 (2021).
Pernas, S., Tolaney, S. M., Winer, E. P. & Goel, S. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther. Adv. Med. Oncol. 10, 1758835918786451 (2018).
Gil-Gil, M. et al. The role of CDK4/6 inhibitors in early breast cancer. Breast 58, 160–169 (2021).
Fassl, A., Geng, Y. & Sicinski, P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science 375, eabc1495 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT01942135 (2013).
Li, Z. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell 34, 893–905.e8 (2018).
Yang, C. et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 36, 2255–2264 (2017).
Cornell, L., Wander, S. A., Visal, T., Wagle, N. & Shapiro, G. I. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 26, 2667–2680.e7 (2019).
Li, Q. et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 12, 356–371 (2021).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Turner, N. et al. Abstract GS3-04: GS3-04 capivasertib and fulvestrant for patients with aromatase inhibitor-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: results from the phase III CAPItello-291 trial. Cancer Res. 83, GS3-04 (2023).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 366, 520–529 (2012).
Turner, N. et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 70, 2085–2094 (2010).
Spoerke, J. M. et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat. Commun. 7, 11579 (2016).
Mohammed, H. et al. Progesterone receptor modulates ERα action in breast cancer. Nature 523, 313–317 (2015). This article highlights the role of PR in modulating ER function and behaviour.
Singhal, H. et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci. Adv. 2, e1501924 (2016).
Cottu, P. H. et al. Phase I study of onapristone, a type I antiprogestin, in female patients with previously treated recurrent or metastatic progesterone receptor-expressing cancers. PLoS ONE 13, e0204973 (2018).
Nishino, T., Ishibashi, K., Hirtreiter, C. & Nishino, Y. Potentiation of the antitumor effect of tamoxifen by combination with the antiprogestin onapristone. J. Steroid Biochem. Mol. Biol. 116, 187–190 (2009).
Carroll, J. S., Hickey, T. E., Tarulli, G. A., Williams, M. & Tilley, W. D. Deciphering the divergent roles of progestogens in breast cancer. Nat. Rev. Cancer 17, 54–64 (2017).
Gonzalez, L. O. et al. Androgen receptor expresion in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer 8, 149 (2008).
Collins, L. C. et al. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod. Pathol. 24, 924–931 (2011).
Niemeier, L. A., Dabbs, D. J., Beriwal, S., Striebel, J. M. & Bhargava, R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 23, 205–212 (2010).
D’Amato, N. C. et al. Cooperative dynamics of AR and ER activity in breast cancer. Mol. Cancer Res. 14, 1054–1067 (2016).
Cochrane, D. R. et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 16, R7 (2014).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT02007512 (2013).
Krop, I. et al. A randomized placebo controlled phase II trial evaluating exemestane with or without enzalutamide in patients with hormone receptor-positive breast cancer. Clin. Cancer Res. 26, 6149–6157 (2020).
Hickey, T. E. et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat. Med. 27, 310–320 (2021). This study dissects the role of AR activation in modulating resistance to hormonal therapy in breast cancer.
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT03734029 (2018).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT03901339 (2019).
Rugo, H. S. et al. Primary results from TROPiCS-02: a randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor-positive/HER2-negative (HR+/HER2−) advanced breast cancer. J. Clin. Oncol. 40, LBA1001 (2022).
Stanton, S. E., Adams, S. & Disis, M. L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2, 1354–1360 (2016).
Zhang, M. et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 8, 31347–31354 (2017).
Segovia-Mendoza, M. & Morales-Montor, J. Immune tumor microenvironment in breast cancer and the participation of estrogen and its receptors in cancer physiopathology. Front. Immunol. 10, 348 (2019).
Vathiotis, I. A. et al. Immune checkpoint blockade in hormone receptor-positive breast cancer: resistance mechanisms and future perspectives. Clin. Breast Cancer 22, 642–649 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://classic.clinicaltrials.gov/show/NCT04161755 (2019).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
Muller, M. et al. The immune landscape of human pancreatic ductal carcinoma: key players, clinical implications, and challenges. Cancers 14, 995 (2022).
Li, X. et al. Immune checkpoint blockade in pancreatic cancer: trudging through the immune desert. Semin. Cancer Biol. 86, 14–27 (2022).
Ullman, N. A., Burchard, P. R., Dunne, R. F. & Linehan, D. C. Immunologic strategies in pancreatic cancer: making cold tumors hot. J. Clin. Oncol. 40, 2789–2805 (2022).
Russo, M. & Bardelli, A. Lesion-directed therapies and monitoring tumor evolution using liquid biopsies. Cold Spring Harb. Perspect. Med. 7, a029587 (2017).
Sant, M., Bernat-Peguera, A., Felip, E. & Margelí, M. Role of ctDNA in breast cancer. Cancers 14, 310 (2022).
Rao, S. et al. Transcription factor-nucleosome dynamics from plasma cfDNA identifies ER-driven states in breast cancer. Sci. Adv. 8, eabm4358 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
J.L. and C.M. are both Genentech/Roche employees and own shares of Roche. C.M. is a named co-inventor on patent 11081236 entitled ‘Diagnostic and therapeutic methods for the treatment of breast cancer’. S.C. reports research grants from NIH/NCI and Breast Cancer Research Foundation during the conduct of the study; personal fees from Novartis, AstraZeneca, Nuvalent, Boxer Capital, Effector and Neogenomics; equity/leadership in Totus Medicines and Odyssey Biosciences and grants from Daiichi Sankyo and AstraZeneca outside the submitted work; and a patent for CDK4/CDK6 degraders is also pending. M.W. has no competing interest.
Peer review
Peer review information
Nature Reviews Cancer thanks Jason Carroll and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- ADCs
-
A class of drugs that combine monoclonal antibodies with cytotoxic agents to specifically target cells expressing the antigens recognized by those antibodies.
- Aromatase inhibitors
-
A class of drugs that block the synthesis of oestrogens by the aromatase enzyme in non-ovarian tissues.
- LBD
-
The C-terminal ligand-binding domain of ER, responsible for the binding of oestrogen ligand.
- Liquid biopsies
-
A technology that allows sampling of body fluids such as blood for molecular profiling, allowing for a less-invasive way of tumour monitoring, detection and characterization than traditional tissue-based biopsies.
- SERCA
-
A class of anti-oestrogen agents that bind covalently to the LBD of ER and prevent recruitment of co-activators.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Will, M., Liang, J., Metcalfe, C. et al. Therapeutic resistance to anti-oestrogen therapy in breast cancer. Nat Rev Cancer 23, 673–685 (2023). https://doi.org/10.1038/s41568-023-00604-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00604-3
This article is cited by
-
Circulating tumor DNA validity and potential uses in metastatic breast cancer
npj Breast Cancer (2024)