Abstract
The human epidermal growth factor receptor 2 (HER2) is overexpressed in 13–22% of breast cancers (BC). Approximately 60–70% of HER2+ BC co-express hormone receptors (HRs). HR/HER2 co-expression modulates response to both anti-HER2–directed and endocrine therapy due to “crosstalk” between the estrogen receptor (ER) and HER2 pathways. Combined HER2/ER blockade may be an effective treatment strategy for patients with HR+/HER2+ BC in the appropriate clinical setting(s). In this review, we provide an overview of crosstalk between the ER and HER2 pathways, summarize data from recently published and ongoing clinical trials, and discuss clinical implications for targeted treatment of HR+/HER2+ BC.
Similar content being viewed by others
Introduction
In population-based studies, human epidermal growth factor receptor 2 (HER2) is overexpressed in ~13–22% of all patients with breast cancer, frequently as a consequence of amplification of the ERBB2 gene (i.e., clinical “HER2+”)1,2,3, and confers aggressive clinical behavior (particularly a more rapid rate of cell proliferation and propensity for metastases), resulting in poor clinical prognosis1,2,3,4,5. Concomitant overexpression of hormone receptors (HRs) and HER2 is also common, with HR-positivity occurring in ~60–70% of patients with HER2+ breast cancer, albeit typically at lower expression levels than in the case of HER2-negative disease2,4,6,7,8. Thus, overall, HR-positive (HR+), HER2+ tumors account for about 10% of all breast cancers9. Co-expression of these receptors alters the clinical behavior of tumors10,11 and modulates response to both HER2-directed and endocrine therapies (ETs)12,13,14,15,16,17.
Prior to the advent of HER2-targeting therapeutics, patients with HR+/HER2+ breast cancer had significantly worse prognosis compared with HR+/HER2− breast tumors. Clinical trials in the neoadjuvant setting have shown that patients with HR+ tumors have a significantly lower likelihood of achieving a pathologic complete response (pCR) when treated with HER2-targeted therapy plus chemotherapy than those with HR− tumors15,16,18,19,20,21,22, whereas in the advanced/metastatic disease setting, those with HER2+ tumors are significantly less responsive to ET than HER2− tumors17. In patients with estrogen receptor–positive (ER+) breast cancer, mortality from anti-estrogen–resistant tumors, in part due to aberrant intracellular signal transduction events as a consequence of constitutive activation of the HER2 kinase in HER2+ disease, accounts for a disproportionate fraction of breast cancer mortality each year, despite initial efficacy with selective ER modulators, selective ER downregulators, or estrogen suppressive therapies23. As for breast cancers that are both ER+ and HER2+, inhibition of HER2 alone enables potential compensatory escape pathways due to the bi-directional signaling (i.e., crosstalk) between these receptor signaling pathways, which can result in resistance to HER2-directed agents and eventual tumor growth8,24,25,26,27,28. In addition, a significant and inverse association has also been observed between quantitative measurements of ERBB2 gene copy number and ER/progesterone receptor (PR) protein expression levels in breast cancer, which may further explain the relative resistance of HR+/HER2+ tumors to ETs8. However, although HR expression is quantitatively lower in HER2+ tumors, that is not to say that steroid HRs are inactive. The constitutive activation of HER2 due to overexpression results in activation of downstream signaling cascades that can lead to post-translational modification (phosphorylation) of the ER, rendering ER constitutively active, indeed even ligand-independent29. However, despite substantial crosstalk between HER2 and ER and PR signaling pathways, the presence of HR expression in patients with HER2+ early-stage breast cancer remains a predictive biomarker for response to ET30,31, and both ERs and HER2 receptors prevail as drivers of tumor growth.
As ER-HER2 crosstalk has been implicated in the development of resistance to both endocrine and HER2-directed agents32,33,34, published preclinical and clinical research suggest a potentially greater benefit for dual- versus single-receptor targeting of HR+/HER2+ breast cancer7,23,35,36. Benefits of such combinations have also suggested potential efficacy in treatment regimens without chemotherapy, which may reduce treatment burden and adverse events (AEs) as well as improve patient quality of life37,38. In this article, we provide an overview of preclinical evidence of crosstalk between the ER and HER2 cell signaling pathways, discuss the implications for targeted treatment of HR+/HER2+ breast cancer, and review new clinical evidence from randomized trials demonstrating enhanced antitumor activity with dual ER plus HER2 targeting in breast cancers.
Dual ER and HER2 signaling: preclinical evidence
A wealth of preclinical evidence highlights the role of ER and HER2 crosstalk in the development of resistance to both endocrine and anti-HER2 therapies, thus supporting the rationale for combined receptor blockade targeting the ER and HER2 as a treatment approach in breast cancer32,33,34. ER and HER2 are mediators of two key pathways implicated in the pathogenesis of breast cancer39. Bi-directional signaling, or crosstalk, between the ER and epidermal growth factor receptor (EGFR)/HER2 signaling pathways, summarized in Fig. 1, has been implicated in tumor growth and survival as well as the development of resistance to ET in HR+/HER2+ tumors.
a HER2 overactivation results in downregulation of ER-regulated transcription and resistance to endocrine therapy. b Blockade of HER2 leads to activation of ER gene transcription via signaling crosstalk pathways as a compensatory mechanism for tumor growth and survival. c Inhibition of HER2 and ER pathways are both required to achieve effective HER2+/HR+ antitumor activity. Gray shading of the PI3K/AKT/mTOR and RAS/RAF/MEK/ERK signaling pathways in panels a and b indicate downregulation of these pathways. AI aromatase inhibitor, ER estrogen receptor, ERK extracellular signal-regulated kinase, HER2 human epidermal growth factor receptor 2, HR hormone receptor, MEK mitrogen-activated protein kinase kinase, mTOR mammalian target of rapamycin, P13K phosphatidylinositol 3-kinase, RAF rapidly accelerated fibrosarcoma, SERD selective estrogen receptor degrader, SERM selective estrogen receptor modulator, TKI tyrosine kinase inhibitor.
The classical function of ER is its nuclear or genomic activity, whereby ER-regulated gene transcription of ER-regulated genes promotes cell proliferation and survival32,33,40. In HR+ tumors, aberrant signaling of the ER pathway results in upregulation of ER–regulated gene transcription and subsequent tumor growth. In HR+/HER2+ tumors, bi-directional crosstalk between the ER pathway and HER2 signaling via the downstream RAS and phosphatidylinositol 3-kinase (PI3K) pathways, and/or downregulation of HR expression, can lead to the loss of sensitivity to ET. Hyperactive signaling of the HER2 pathway, resulting from ERBB2 gene amplification, protein overexpression, or even activating mutations (for example in the ERBB2 sequence encoding the kinase domain), activates downstream kinases including Akt and mitogen-activated protein kinases (MAPKs)26. This, in turn, results in attenuation of the expression of ER messenger RNA (mRNA) and protein and subsequently downregulates ER-regulated gene transcription, resulting in a decrease in sensitivity to ET (Fig. 1a). Preclinical cell line models of ER+ breast cancer have revealed that HER2 overexpression or HER family activation via addition of recombinant heregulin β1—a HER family ligand—resulted in attenuated ER expression, with no impact on intrinsic estradiol-binding affinity to the ER by Scatchard analysis36. This mechanism presumably accounts for the observation that in a large cohort of patients with primary breast cancer (N = 894), ERBB2 gene copy number (and resulting HER2 protein overexpression) was inversely correlated with ER and PR protein expression quantified by enzyme immunoassay or a radioligand-binding assay (by Scatchard analysis), thus validating seminal preclinical experimental predictions using a large number of actual human breast cancer clinical specimens8.
Therapeutic blockade of HER2 signaling in HER2+ breast cancer can lead to the upregulation/reactivation of ER-regulated gene transcription or increased ER dependency as shown in Fig. 1b. Bi-directional signaling crosstalk can function as a compensatory mechanism for tumor growth and survival, resulting in acquired treatment resistance40. In a cell line model of HER2-overexpressing breast cancer, chronic exposure to the tyrosine kinase inhibitor (TKI) lapatinib (a dual-targeted quinazoline small-molecule inhibitor that reversibly binds to the cytoplasmic ATP-binding sites of EGFR/HER1 and HER2 receptors, thereby blocking tyrosine kinase enzymatic activity)41 led to development of acquired resistance mediated by increased ER signaling and a switch to co-dependency on ER and HER242. Importantly, in clinical/translational investigations, these authors also observed increased ER signaling in tumor biopsies from lapatinib-treated patients with HER2-overexpressing breast cancer42. Reactivation of ER signaling was also evident following the initial administration of lapatinib in some HER2+ breast cancer cell lines, which was followed by reversion to dependence on HER pathways during prolonged exposure to lapatinib43. In addition, crosstalk between ER and HER2 has also been shown to upregulate MYC-mediated glutamine metabolism, thus promoting cell proliferation and leading to aromatase inhibitor (AI)-resistant breast cancer cells44. The mechanism proposed for this finding is based upon the fact that: 1) the MYC oncogene is an estrogen-dependent gene, transcriptionally regulated by ER in AI-resistant cells45; 2) upregulation of MYC was shown to be HER2-dependent (specifically via MAPK signaling and constitutive activation of ER); 3) MYC enhances glutamine uptake from the extracellular space in AI-resistant breast cancer cells through upregulation of the glutamine importer, solute carrier family SLC1A5; and 4) glutaminase, the enzyme responsible for converting glutamine into glutamate, is also regulated by MYC44. Taken together, this work demonstrates that MYC is upregulated by the crosstalk between ER and HER2 in AI-resistant breast cancer cells and that MYC-mediated glutamine metabolism is associated with AI resistance of breast cancer44.
These findings further support the hypothesis of bi-directional crosstalk between the ER and HER2 pathways in breast cancer, which can lead to the activation of an alternative “escape” survival pathway (e.g., ER signaling) that gives rise to the development of acquired treatment resistance. More extensive blockade of multiple pathways, such as the use of combination therapies for dual blockade of the HER2 and ER pathways in HR+/HER2+ tumors (Fig. 1c), may be necessary to overcome treatment resistance and sustain antitumor activity.
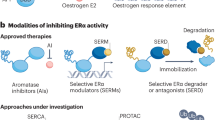
Accordingly, further investigation and research have been conducted to evaluate the benefit of extended dual ER/HER2 blockade and HER2 receptor inhibition. To date, dual blockade of ER/HER2 signaling using combinations of agents that directly target ER (e.g., selective estrogen receptor degraders [SERDs], selective estrogen receptor modulators [SERMs], AIs) and HER2 (e.g., monoclonal antibodies [mAbs] or small-molecule TKIs) have proven to be highly effective in preclinical models (cataloged in Table 1)23,41,42,46,47,48,49,50,51,52,53,54,55.
Pietras and colleagues36 were the first to demonstrate that combined receptor blockade using an anti-HER2 antibody and tamoxifen resulted in greater combined efficacy compared to either the anti-HER2 antibody or anti-estrogen–alone controls against HER2-overexpressing human breast cancer cells co-expressing ER. Working independently some years later, Xia and colleagues42 reported in vitro experiments in HR+/HER2-overexpressing breast cancer cell lines and tumor xenograft models confirming the rationale for dual ER/HER2 blockade, this time with lapatinib plus fulvestrant to enhance efficacy (vs. monotherapy with either agent alone as controls), using a different HER2-targeting agent and different model systems, yet completely consistent with the earlier findings published by Pietras36. Combination therapy with a HER2-targeted agent and ET gave rise to more complete tumor regression than HER2-targeted therapy alone in ER+/HER2+ breast cancer cell line and xenograft models43,56,57,58. One recent in vitro study demonstrated that tamoxifen- and fulvestrant-resistant breast cancer cell lines were sensitive to treatment with lapatinib or afatinib (a second-generation anilinoquinazoline that irreversibly binds to the intracellular tyrosine kinase domains of EGFR, HER2, and HER4 receptors), and gradual reactivation of ERα sensitivity was observed with lapatinib therapy34. Synergistic effects were also evident in this study following combination treatment with afatinib plus an anti-endocrine agent or lapatinib plus tamoxifen or fulvestrant34.
It has been suggested that dual blockade of HER2 receptors or blockade of multiple HER receptors may offer enhanced antitumor efficacy43,56. Pertuzumab targets HER2 by blocking ligand-dependent HER2-HER3 heterodimerization and reverses HER3-mediated resistance pathways59. It has been shown that in HR+/HER2+ breast cancer cell xenografts, the combination of HER1 (EGFR) and HER2 blockade with gefitinib, trastuzumab, and pertuzumab in combination with estrogen withdrawal was more effective in suppressing tumor growth than any of these agents alone56. In a separate study, the combination of trastuzumab, pertuzumab, and the HER3-targeted monoclonal antibody patritumab suppressed tumor progression more effectively than trastuzumab alone in a similar breast cancer xenograft model60.
Subsequently, novel compounds that irreversibly bind multiple HER receptors have been developed in recent years for the treatment of cancer. One such compound is the oral, irreversible, pan-HER TKI neratinib, which reduces phosphorylation and activation of downstream signaling pathways by covalently binding to a conserved cysteine residue within the ATP-binding pockets of HER1, HER2, and HER461. Initial in vitro experiments demonstrated antitumor activity in both HER2-overexpressing breast cancer and EGFR-overexpressing epidermal carcinoma cell lines, with neratinib inhibiting downstream signal transduction events and cell cycle regulatory pathway, and reducing cell proliferation62.
Recent studies have also evaluated neratinib in combination with other targeted agents to overcome treatment resistance in preclinical models of breast cancer23,52,54. Dual blockade of ER and HER2 with neratinib plus fulvestrant was associated with significant inhibition of tumor growth and prolonged survival in HR+/HER2+ breast tumor xenografts unresponsive to ET compared with single-agent neratinib or controls52. In ER+ breast cancer cell lines and xenografts resistant to anti-estrogen therapies, treatment with neratinib restored fulvestrant sensitivity in cell lines harboring ERBB2 kinase domain-activating mutations (L755S and V777L), and dual blockade of ER and HER2 with neratinib plus fulvestrant was required to inhibit the growth of these ER+/ERBB2-mutant cell lines23. In addition, the neratinib plus everolimus and neratinib plus fulvestrant combinations were equally effective at suppressing tumor progression in mice bearing ERBB2 mutant xenografts and superior to the single-agents–alone controls23, emphasizing the importance of targeting both the ER and HER2 pathways in ER+/ERBB2-mutant tumors. Furthermore, treatment with neratinib overcame endocrine resistance in ER+/ERBB2-mutant breast cancer cell lines, derived from metastatic biopsies from patients with ER+ metastatic breast cancer who developed endocrine resistance to tamoxifen or fulvestrant, thus suggesting enhanced tumor growth inhibition when combining neratinib with fulvestrant54,63.
In a human-in-mouse model of ER+/HER2+ breast cancer, adjuvant paclitaxel plus trastuzumab ± pertuzumab for 4 weeks, followed by “extended adjuvant therapy” with fulvestrant plus neratinib for an additional 4 weeks, was associated with maintenance of a complete response (CR), whereas subjects treated with extended fulvestrant alone relapsed rapidly51. The combination of neratinib plus fulvestrant was also shown to potently inhibit cancer cell growth; downregulate ER reporter activity, P-AKT, and P-ERK; and reduce cyclin D1 mRNA and protein levels in ER+/HER2+ breast cancer cell lines51. Evaluation of neratinib and everolimus in a panel of ER+ breast cancer in vitro and in vivo models of acquired AI resistance demonstrated that the combination of either agent with ET enhanced reductions in cell proliferation and tumor volume, and the greatest effect was observed with the triple combination of everolimus, neratinib, and ET53. Achieving durable clinical outcomes in patients with ER+/HER2+ breast cancer may therefore require extended HER2 blockade to overcome reactivation of the HER2 pathway during ER blockade. Further mechanistic insight into perturbation of mammalian target of rapamycin (mTOR) and HER family kinases comes from analysis of gene expression in xenografts following combination therapy with everolimus, neratinib, and ET reduced expression of the EGFR/ERK gene set, which suggests that the EGF/EGFR feedback loop associated with mTOR inhibition was negated53.
In another novel approach, resistance to HER2-targeted therapy may be overcome by the addition of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors through suppression of Rb phosphorylation and TSC2 phosphorylation that attenuated mTOR complex 1 (mTORC1) activity64. This can reduce feedback inhibition of EGFR-family kinases found upstream, allowing for continued treatment of tumors via the EGFR/HER2 blockade64. Consequently, inhibition of both EGFR/HER2 and CDK4/6 blockades further suppresses TSC2 phosphorylation, thereby also suppressing mTORC1/S6K/S6RP activity64. Combinations of the HER2-targeted small-molecule inhibitor tucatinib, the CDK4/6 inhibitor palbociclib, and fulvestrant were investigated in three HR+/HER2+ human breast tumor cell lines55. The tucatinib plus fulvestrant and tucatinib plus palbociclib doublets were synergistic in all three cell lines; the addition of fulvestrant to tucatinib plus palbociclib further improved efficacy in all three cell lines.
Overall, these preclinical laboratory research findings demonstrate that bi-directional crosstalk between the ER and HER2 pathways in breast cancer may lead to the activation of alternative “escape” survival pathways through ER signaling and, eventually, treatment resistance and tumor growth. Therefore, dual blockade of ER and HER2 signaling pathways in HR+ tumors may result in enhanced and sustained antitumor activity. In addition, it has been hypothesized that blockade of multiple HER family receptors may be more effective than blockade of HER2 kinase alone and use of the pan-HER TKI neratinib as part of combination therapy may re-sensitize ER pathways to ET. Such results warranted further investigation in the safety and efficacy of dual ER/HER2 blockade in the clinical setting.
Dual ER and HER2 targeting: clinical data
Based on the promising preclinical data described above, several clinical trials have evaluated the effectiveness of combining endocrine and HER2-targeted therapies in the neoadjuvant, extended adjuvant, and advanced/metastatic breast cancer settings (Table 2)7,35,37,38,41,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97.
Early-stage breast cancer
Neoadjuvant studies testing combined receptor blockade targeting both HER2 and ER
Demonstration of meaningful clinical benefit from a combined receptor blockade strategy targeting HER2 and the ER has been challenging in the neoadjuvant setting, perhaps limited by the constraint of the limited number of therapeutic treatment cycles given in a preoperative setting in neoadjuvant study designs. Moreover, such trials frequently employ pCR as a primary clinical endpoint, whereas in adjuvant trial designs (with much longer duration of combined receptor blockade in the post-operative setting), time-to-event primary clinical endpoints (e.g., invasive disease-free survival [iDFS]) can be utilized.
In the randomized, phase III NSABP B-52 trial (NCT02003209), 315 patients with locally advanced, HR+/HER2+ invasive breast cancer received a 6-cycle regimen of neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) with or without estrogen deprivation (goserelin plus an AI) before undergoing surgery76. The primary endpoint of NSABP B-52 (pCR) was not met; no statistically significant difference in pCR (breast and lymph nodes) was observed between TCHP plus estrogen deprivation versus TCHP alone (46.1% vs. 40.9%; P = 0.36)76. However, with this trial design, the addition of chemotherapy to the dual HER2 antibody regimen may complicate assessment of the combined receptor blockade strategy of targeting HER2 and ER. For example, any advantage of combined receptor blockade against ER and HER2 could theoretically be offset by antagonism between chemotherapy and anti-estrogen treatment98,99,100,101. Moreover, the number of neoadjuvant cycles was limited to six in this trial. Longer exposure to combined receptor blockade strategies may be needed to observe beneficial clinical efficacy outcomes from this strategy. Importantly, no new safety signals emerged in NSABP B-52, with AEs reflecting expected additive toxicities from component agents of TCHP and ET. Grade 3/4 AEs included diarrhea (23%/<1% vs. 21%/0%), vomiting (8%/<1% vs. 5%/0%), and febrile neutropenia (5%/<1% vs. 7%/1%) for TCHP versus TCHP plus estrogen deprivation, respectively76.
In another neoadjuvant randomized clinical trial, the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer (WSG-ADAPT-HER2+) trial (NCT01817452), compared pCR rates of the HER2-directed antibody-drug conjugate ado-trastuzumab emtansine (T-DM1) versus trastuzumab with ET in early HER2+/HR+ breast cancer91. In this trial, 375 patients with early HER2+/HR+ breast cancer were randomized to receive a 12-week treatment of T-DM1 with or without ET or to trastuzumab with ET. The protocol-defined primary endpoint was pCR (defined as ypT0/is/ypN0). Previously, Rimawi et al. 71 had administered 12 weeks of trastuzumab, lapatinib, and ET with letrozole with or without a luteinizing hormone–releasing hormone agonist (dependent on menopausal status) to 64 patients. pCR (ypT0/is/ypN0) was 22% overall, 18% in ER+ disease, and 28% in ER− disease. Notably, the near-pCR percentage differed substantially between HR+ and HR− subtypes (54% vs. 40% with ypT1a/b or ypN0/is, respectively). In a subsequent trial with more prolonged (up to 24 weeks) preoperative therapy, the same researchers observed 33% pCR (breast only, ypT0/is) in ER+ disease versus 18% in ER−, following neoadjuvant treatment with lapatinib plus trastuzumab, with ET94. Building upon these results, this time with an antibody-drug conjugate (ADC) backbone (T-DM1) in the WSG-ADAPT HER+ trial, a pCR was observed in 41.0% of T-DM1–treated patients and 41.5% of T-DM1 plus ET-treated patients versus 15.1% of patients treated with trastuzumab and ET (P < 0.001)91. As in the case of the NSABP B52 trial, the authors acknowledge that one handicap of the WSG-ADAPT-HER2+ trial design is the use of just four preoperative cycles of T-DM1 ± ET, which may be insufficient to demonstrate a significant improvement in pCR by the addition of ET. In addition, we note that the derivative of maytansine (DM1) payload of T-DM1 is a microtubule-interacting chemotherapeutic payload, which in theory could also have antagonistic interactions with ET, thus negating potential synergistic interactions between ET and the trastuzumab antibody ADC backbone. In terms of mechanism of action, it is hypothesized that ADCs with cytotoxic payloads will follow all of the same principles as cytotoxic chemotherapeutics, perhaps including antagonism with anti-estrogens102. In terms of safety in WSG-ADAPT-HER2+, T-DM1 was associated with a significantly higher prevalence of grade 1 and 2 AEs, especially thrombocytopenia, nausea, and elevation of liver enzymes. Overall toxicity was low; seventeen therapy-related serious AEs (T-DM1 arms vs. trastuzumab plus ET; 5.3% vs. 3.1%, respectively) were reported91.
In another clinical trial that evaluated duration of neoadjuvant therapy in the context of HER2/HR combined receptor blockade, Masuda and colleagues93 designed a randomized, phase II, five-arm trial (UMIN-CTR identifier: UMIN000007576) to evaluate the efficacy and safety of lapatinib and trastuzumab (6 weeks) followed by lapatinib and trastuzumab plus weekly paclitaxel (12 weeks) with or without prolongation of anti-HER2 therapy prior to chemotherapy (18 vs. 6 weeks), and with or without ET in patients with HER2+ and/or ER+ disease. The primary endpoint was comprehensive pCR (CpCR) rate (CpCR included residual ductal carcinoma in situ of the breast). Although pCR with pN0 was achieved in 42.2% of 212 patients (ER−, 57.6%; ER+, 30.3%), there were no statistical differences based on duration of lapatinib and trastuzumab plus ET treatment (6 vs. 18 weeks)93. There were no major safety concerns associated with prolonging the anti-HER2 treatment or adding ET. Therefore, further research is required to support the benefit of longer-duration combination therapy with HER2-targeted agents and estrogen deprivation in maximizing clinical efficacy in the neoadjuvant setting. To this end, Prat Aparicio and colleagues have tested the hypothesis that biomarker selection of HER2+/HR+ patients may identify a subgroup with superior outcomes following combined receptor blockade (NCT01973660). Indeed, Prat Aparicio et al. 92 reported that after 18 weeks of trastuzumab plus lapatinib and ET, the overall pCR rate was just 18%, but with biomarker selection using the 50-gene PAM50 subtype classifier, the pCR rate was 31.6% in the HER2-enriched intrinsic subtype versus 5.3% for non-HER2 subtypes within the subset of HER2+/HR+ disease92. Indeed, further work by Pernas and Prat103 has shown that increased pCR rates in the PAM50 HER2-enriched gene expression phenotype are independent of HR expression103. Taken together, these observations hold promise that biomarker selection may be a path forward for patient identification for de-escalation treatment strategies utilizing combined receptor blockade, potentially including non-chemotherapy regimens, for carefully selected patients with early-stage HER2+. However, at the time of this writing, neither the National Comprehensive Cancer Center Network (NCCN) guidelines nor the American Society of Clinical Oncology (ASCO) guidelines have as yet recognized the PAM50 assay as a biomarker for patient selection for neoadjuvant therapy considerations in early-stage HER2+ disease. Interestingly, we note that Prat and colleagues104 have recently reported development and validation of a new assay (HER2DX) for predicting pathological response and survival outcome in early-stage HER2+ breast cancer104. HER2DX is a supervised learning algorithm that generates a single score based on tumor size, nodal status, and four-gene expression signature that tracks immune infiltration, cell proliferation, luminal differentiation, and HER2 expression. Using both training and validation clinical cohorts, the investigators have shown that HER2DX variables were significantly (P = 0.002) associated with good risk outcomes (i.e., immune and luminal) and poor risk outcomes (i.e., proliferation and tumor and nodal stage), with the 5-year DFS in the low-risk group being 97.4%. For a neoadjuvant training cohort, HER2DX variables were associated with pCR (i.e., immune, proliferation, and HER2 amplicon) and non-pCR (i.e., luminal and tumor and nodal staging), with continuous HER2DX pCR likelihood score significantly associated with pCR (P < 0.0001). However, we note that HER2DX has not yet been tested or validated as a predictor of clinical efficacy from a combined receptor (HER2/ER) blockade treatment strategy104.
Finally, as noted in the preclinical section above, convergence of HER2 and ER signals on RB1 suggests that a combined pharmacological intervention with individual drugs directed to all three targets (HER2, ER, and RB1) could be synergistic. In the open-label NA-PHER2 trial (NCT02530424), the CDK4/6 inhibitor palbociclib was added to trastuzumab, pertuzumab, and fulvestrant in the neoadjuvant setting and resulted in an objective clinical response in 29 of 30 patients (97%, before surgery)69. The most frequent grade 3 AEs in NA-PHER2 were neutropenia (29%), diarrhea (14%), and stomatitis; increased alanine aminotransferase levels; and hypersensitivity reactions (3% of each event). No grade 4 or serious AEs were recorded in the trial, and there were no deaths. Additionally, the combination had a significant effect on the expression of Ki67 at 2 weeks following treatment initiation; and at surgery, eight (27%; 95% CI, 12–46%) patients had a pCR in breast and axillary nodes69. Thus, in addition to biomarker selection strategies such as PAM50, more complete coverage of HR-HER2 crosstalk and associated downstream signaling (in this case, by the addition of CDK4/6 inhibition) may offer future clinical consideration of chemotherapy-free regimens as neoadjuvant treatment for HR+/HER2+ early breast cancer. In summary, the need for these newer approaches is underscored by results from the neoadjuvant WSG-TP-II trial results. In the prospective WSG TP-II phase II trial (NCT03272477), 207 patients with centrally confirmed HR+/HER2+ early breast cancer were randomized to 12 weeks of standard ET (n = 100) versus paclitaxel 80 mg/m2 weekly (n = 107) plus trastuzumab and pertuzumab intravenously every 3 weeks; all patients received dual HER2-antibody blockade in the adjuvant setting. The primary endpoint was pCR (ypT0/is/ypN0), which was observed in 24% (95% CI, 16–34%) in patients with ET plus the dual anti-HER2 antibodies versus 57% (95% CI, 47–67%) in patients receiving paclitaxel chemotherapy plus trastuzumab and pertuzumab (odds ratio, 0.24; 95% CI, 0–0.46; P < 0.001). Neoadjuvant treatment was well tolerated in both trial arms and completed per protocol in 93 and 92 patients in the ET plus pertuzumab plus trastuzumab and the paclitaxel plus pertuzumab plus trastuzumab) arms, respectively. Only nine and thirteen serious AEs, respectively, were reported in each group during neoadjuvant therapy90. Thus with the chemotherapy-based neoadjuvant regimen yielding significantly superior pCR, and the expectation that pCR in HER2+ early breast cancer will correlate with long-term time-to-event clinical outcomes (i.e., iDFS and overall survival [OS])105,106,107, the current standard of care for HER2+/HR+ early breast cancer at this time (for the vast majority of patients and outside the context of participation in a clinical trial) remains a combination of chemotherapy plus dual anti-HER2 antibodies.
Combined receptor blockade targeting HER2 and ER in the adjuvant and extended adjuvant setting
The most common setting in which HER2-blockade and ET are combined is in the adjuvant setting. Because the optimal adjuvant ET for HER2+/HR+ patients remains unclear, investigators in the Short-HER trial (853 patients with HR+ breast cancer were included) evaluated the impact of ET type on DFS in patients with HER2+/HR+ breast cancer (NCT00629278). After a median follow-up of 8.7 years, patients who received AIs had a significantly better DFS versus patients on tamoxifen or tamoxifen followed by an AI (7-year DFS, 87.3% vs. 81.7%; log-rank P = 0.017; hazard ratio, 1.46, 95% CI, 1.05–2.03). In multi-variate analyses including menopausal status, stage, and treatment arm, the type of ET maintained a significant association with DFS. Additionally, the use of gonadotropin-releasing hormone was associated with numerically improved DFS in premenopausal patients (86.6% vs. 81.6%; log-rank P = 0.168; hazard ratio, 0.70; 95% CI, 0.43–1.16). Although there are no randomized trials of ET versus not in the adjuvant setting for HER2+ early-stage breast cancer in the post-trastuzumab era, the observation that the specific type of ET in the Short-HER trial is significantly associated with long-term time-to-event clinical outcomes (i.e., DFS) strongly suggests that ET is impactful (compared to omission of anti-estrogen treatment) in patients with HER2+ early-stage disease in the adjuvant setting88. Indeed, the combined receptor blockade strategy targeting HER2 and ER is now being investigated in a non-chemotherapy (de-escalation trial design) adjuvant treatment in an ongoing phase II trial of adjuvant ET, pertuzumab, and trastuzumab (administered subcutaneously with recombinant hyaluronidase) in patients (N = 375) with anatomic stage I HR+/HER2+ breast cancer (ADEPT; NCT04569747).
One strategy to overcome resistance to HER2-targeted therapy is to extend the duration of adjuvant therapy, with the addition of an irreversible pan-HER TKI, neratinib, taken for 1 year. In the randomized, placebo-controlled, phase III ExteNET trial (NCT00878709), the effect of neratinib (240 mg/day) was evaluated in patients (n = 2840) with stage I-III HER2+ breast cancer who had completed neoadjuvant and adjuvant chemotherapy plus trastuzumab up to 2 years prior to randomization; concurrent adjuvant ET was recommended for patients with HR+ disease7. The inclusion criteria were subsequently amended to recruit higher-risk patients (defined as patients with stage II-III node-positive disease who had completed trastuzumab therapy up to 1 year prior to randomization)7. At the 2-year follow-up, iDFS rate was 93.9% in patients receiving neratinib versus 91.6% in those receiving placebo (stratified hazard ratio, 0.67; 95% CI, 0.50–0.91; P = 0.0091)7. This benefit was maintained at the 5-year analysis of ExteNET, with 5-year iDFS rates favoring neratinib (90.2% vs. 87.7%; stratified hazard ratio, 0.73; 95% CI, 0.57–0.92; P = 0.0083) after a median follow-up of 5.2 years79. A prespecified subgroup analysis suggested that the iDFS benefit of neratinib was more pronounced in patients with HR+ tumors at 2 years (hazard ratio, 0.51; 95% CI, 0.33–0.77; P = 0.0013) and at 5 years (hazard ratio, 0.60; 95% CI, 0.43–0.83)7,79 versus HR− tumors at the 2-year (hazard ratio, 0.93; 95% CI, 0.60–1.43; P = 0.74) and 5-year time points (hazard ratio, 0.95; 95% CI, 0.66–1.35)7,79. After a median follow-up of 8 years, reduced OS hazard ratios (0.95–0.78) upon completion of neratinib therapy further supported the benefit of neratinib treatment. Such benefit was also observed across subgroups with HR+ tumors108. It is postulated that the enhanced efficacy of neratinib in HR+/HER2+ breast cancer in the ExteNET trial may be explained by interruption of crosstalk between the ER and HER2 pathways given that >90% of patients with HR+ disease in ExteNET received concurrent adjuvant ET along with extended adjuvant neratinib. In support of this hypothesis, the ExteNET investigators have published a subset analysis on “patients of clinical interest” in ExteNET who: (1) were HR+, (2) were enrolled within 1 year of completion of adjuvant trastuzumab treatment, 3) had received prior neoadjuvant therapy, and 4) had not achieved a pCR. In this small subset (N = 295), 8-year OS rates were 91.3% (95% CI, 84.4–95.2%) in the neratinib group versus 82.2% (95% CI, 75.1–87.4%) for placebo, corresponding to a 9.1% absolute benefit in OS (hazard ratio, 0.47; 95% CI, 0.23–0.92)89. Rightly, no P-values were published for this retrospective, highly selected exploratory subset with modest sample size. Moreover, due to constraints in the trial design (in particular, no neratinib-alone control arm without ET), it is not scientifically possible to draw definitive conclusions regarding mechanistic insight for clinical outcome observations in the HR+ subgroup(s) of ExteNET. It is important to note that due to ethical considerations of withholding ET, inclusion of such control groups is not clinically feasible in many of the clinical trials conducted to date testing the combined receptor blockade hypothesis. Thus, it is not possible to state definitively that the ExteNET trial results prove the combined receptor blockade (HER2/ER) hypothesis. Instead, it is only possible to state that the trial results are consistent with the hypothesis. In the United States, neratinib is approved in the extended adjuvant setting in HER2+ early breast cancer irrespective of HR status, based upon the intent-to-treat principle; however, the European Medicines Agency has approved neratinib only for HR+/HER2+ patients109. In the ExteNET trial, diarrhea was observed in 95% of patients, with grade 3 diarrhea observed in 40% of patients. In addition, a considerable proportion of patients experienced nausea, abdominal pain, fatigue, and vomiting (NERLYNX® (neratinib) tablets, for oral use [package insert]. Los Angeles, CA: Puma Biotechnology, Inc.; 2022). It is important to note that neratinib-associated diarrhea may be mitigated simply by gradually increasing the dose over time (120 mg/day Days 1–7, 160 mg/day Days 8–14, and 240 mg/day thereafter and loperamide administered as needed), as demonstrated in the CONTROL clinical trial (NCT02400476)110.
Combined receptor blockade targeting HER2 and ER in advanced (locally recurrent, surgically unresectable or metastatic) breast cancer
Because HER2-positivity in HR+ breast cancer is associated with ET resistance, potentially due to the crosstalk mechanism(s), including attenuation of ER and PR expression8, hormone sensitivity may theoretically be restored through HER2 blockade111. Early randomized phase III trials demonstrated a clinical benefit for combining an AI (anastrozole or letrozole) with trastuzumab or lapatinib in patients with ER+/HER2+ metastatic breast cancer65,66,67. In the randomized phase III TAnDEM trial (NCT00022672), the safety and efficacy of anastrozole plus trastuzumab was evaluated in patients with HR+/HER2+ metastatic breast cancer. Results showed that patients in the trastuzumab plus anastrozole arm experienced significant progression-free survival (PFS) improvements compared with patients receiving anastrozole alone (hazard ratio, 0.63; 95% CI, 0.47–0.84; median PFS, 4.8 vs. 2.4 months; log-rank P = 0.0016)67. In TAnDEM, grade 3 and 4 AEs occurred in 23% and 5% of patients, respectively, in the trastuzumab plus anastrozole arm, and 15% and 1% of patients, respectively, in the anastrozole-alone arm; there was one patient who experienced New York Heart Association class II congestive heart failure in the combination arm67. However, interpretation of these results is limited by the lack of a trastuzumab-alone control arm. Nevertheless, the benefit from the addition of a HER2-inhibiting agent to an AI was confirmed with the open-label, randomized, phase III eLEcTRA trial (NCT00171847), in which numerically greater improvements in time to progression (14.1 vs. 3.3 months), objective response rate (ORR; 27% vs. 13%), and clinical benefit rate (CBR; 65% vs. 39%) were observed with letrozole plus trastuzumab versus letrozole alone73. In eLEcTRA, most AEs were classified as Common Terminology Criteria grade 1 or 2. Cardiac events were comparable in all arms (8.6–9.7%), with no life-threatening (grade 4) events reported73.
The combination of letrozole plus lapatinib was evaluated in the phase III EGF 30008 trial (NCT00073528) of patients with HR+ metastatic breast cancer. In 219 HER2+ patients, combination therapy improved median PFS (the trial primary endpoint) to 8.2 months compared with 3 months for letrozole alone (hazard ratio, 0.71; 95% CI, 0.53–0.96; P = 0.019)65. Overall response rate and CBR rate were also significantly improved with letrozole plus lapatinib (Table 2), garnering US Food and Drug Administration approval for the combination in 2010. Grade 3 or 4 AEs were more common in the lapatinib-letrozole arm versus letrozole-placebo arm (diarrhea, 10% vs. 1%; rash, 1% vs. 0%, respectively), but they were considered manageable65. The trial also included a large cohort (N = 952) of HR+/HER2− patients to determine whether the EGFR kinase inhibitory effects of lapatinib (along with HER2 kinase inhibition in a HER2− population) would also synergize with aromatase inhibition. This effect was not observed, with no improvement in PFS and no statistically significant difference in response rates65. Thus, combined receptor blockade targeting EGFR and HER2 and ER in HER2−/HR+ patients was unsuccessful in a large prospective randomized phase III clinical trial.
Dual HER2 inhibition with synergistic combination of lapatinib and trastuzumab was studied in combination with an AI, without chemotherapy, in the phase III ALTERNATIVE trial (NCT01160211) of patients with HR+/HER2+ metastatic breast cancer who had been treated with prior trastuzumab and ET112. Median PFS was significantly improved by 38% with lapatinib, trastuzumab, and AI (11 months) versus trastuzumab and AI (5.6 months; hazard ratio, 0.62; 95% CI, 0.45–0.88; P = 0.0063)95. This effect on PFS was observed across predefined subgroups of patients with measurable disease, patients treated with AIs, and patients who had previously received trastuzumab. Overall response rate and CBR also favored the three-drug combination regimen95. In ALTERNATIVE, common AEs (≥15%) with lapatinib plus trastuzumab plus AI, trastuzumab plus AI, and lapatinib plus AI were diarrhea (69%, 9%, and 51%, respectively), rash (36%, 2%, and 28%, respectively), nausea (22%, 9%, and 22%, respectively), and paronychia (30%, 0%, and 15%, respectively), most of which were grade 1 or 2. The incidence of serious AEs was similar across groups, and AEs leading to discontinuation were actually lower with lapatinib plus trastuzumab plus AI95.
The combination of neratinib plus ET has also been assessed in patients with ERBB2-mutated, non-amplified metastatic breast cancers63,96,113,114,115. Such mutations most frequently involve the kinase domain and are constitutively activating. The SUMMIT trial (NCT01953926) is an open-label phase II multi-histology “basket” trial evaluating the efficacy of neratinib in patients with a variety of tumors harboring ERBB2 or ERBB3 gene mutations, including breast, lung, bladder, biliary, cervical, and colorectal cancers113,114. The breast cancer cohort of SUMMIT comprised heavily pretreated patients (n = 81) with metastatic ERBB2-mutant breast cancer who were treated with neratinib monotherapy (240 mg/day; n = 34, including 23 with ER+ breast cancer) or neratinib (240 mg/day) plus fulvestrant (500 mg on Days 1 and 15 of Month 1, then on Day 1 every 4 weeks; n = 47)79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96. Promising antitumor activity was observed, with a confirmed ORR (primary endpoint) of 17% (95% CI, 5–39%) for patients with ER+ tumors in the neratinib monotherapy group and 30% (95% CI, 17–45%) for neratinib plus fulvestrant; CBRs were 30% (95% CI, 13–53%) and 47% (95% CI, 32–62%), respectively96,113,114. As the SUMMIT trial data met the original objective of evaluating the use of neratinib in ERBB2-mutant tumors, trial enrollment has recently concluded. The five most common AEs (all grades) reported in SUMMIT were diarrhea (90.2%, necessitating high-dose loperamide prophylaxis), nausea (72.5%), vomiting (52.9%), fatigue (43.1%), and constipation (41.2%)87. In another trial (part 1 of the open-label MutHER trial (NCT01670877), 16 patients with heavily pretreated metastatic breast cancer (15 of whom had ER+/HER2+ tumors) received neratinib monotherapy63. A total of five patients (31%) experienced clinical benefit. In part 2 of the trial, 31 patients with HR+/ERBB2-mutated metastatic breast cancer received neratinib plus fulvestrant, with a CBR of 40% (95% CI, 19–64%) in patients previously treated with fulvestrant and 30% (95% CI, 7–65%) in fulvestrant-naive patients97. Median PFS was 24 weeks (95% CI, 16-31) and 20 weeks (95% CI, 8-not assessed) in fulvestrant-exposed and fulvestrant-naive patients, respectively. The phase II MutHER trial has recently been updated83. The investigators evaluated the efficacy of neratinib plus fulvestrant in patients with ER+/ERBB2-mutant, ERBB2 non-amplified metastatic breast cancer in a fulvestrant-treated (n = 24) or fulvestrant-naive cohort (n = 11). Patients with ER−/ERBB2 mutant metastatic breast cancer received neratinib monotherapy in a small exploratory cohort (n = 5). The CBR was 38% (95% CI, 18–62%), 30% (95% CI, 7–65%), and 25% (95% CI, 1–81%) in the fulvestrant-treated, fulvestrant-naive, and ER− cohorts, respectively. Anecdotally, adding trastuzumab at progression in five patients resulted in three objective partial clinical responses and one stable disease of ≥24 weeks duration. CBR appeared positively associated with lobular histology and negatively associated with ERBB2 L755 alterations. Additional acquired ERBB2 mutations were detected in five of 23 patients at progression83. Taken together, these data suggest that the concept of combined receptor blockade targeting HER2 and ER extends beyond clinical HER2+ tumors (defined as HER2 gene amplification and/or 3+ immunohistochemical [IHC] protein overexpression) to include ERBB2-mutant biology, the latter very likely mimicking constitutive activation of the HER2 kinase, as is seen in cases of aberrant HER2 overexpression. The safety profile of neratinib or neratinib plus fulvestrant was consistent with prior trials. Across all cohorts, the most common AEs considered related to the trial were diarrhea (85%, with high-dose loperamide prophylaxis), nausea (53%), fatigue (50%), anorexia (35%), and aspartate aminotransferase level increase (28%). Neratinib was dose-reduced in six (15%) patients who received neratinib 200 mg daily plus fulvestrant due to nausea/vomiting (n = 2), diarrhea (n = 3), or elevated liver enzyme levels (n = 1). One patient had a second dose reduction to 160 mg daily due to diarrhea. No patients discontinued therapy due to AEs83.
In terms of other agents, previous findings have shown that dual CDK4 and CDK6 inhibitors could sensitize HER2-targeted therapies and delay tumor recurrence in HER2+ breast cancer models81. In a phase Ib/II trial (NCT03054363), the combination of tucatinib plus palbociclib plus letrozole is under investigation for the treatment of patients with HR+/HER2+ metastatic breast cancer80. The interim analysis results, after a median follow-up of 6 months in 40 patients, showed that the combination was well tolerated with manageable and expected AEs. The most common grade ≥3 AEs were neutropenia (60%), leukopenia (24%), diarrhea (19%), fatigue (14%), and infections (14%). The median PFS was 8.7 months, with 10.1 months for patients without brain metastasis and 6.0 months for those with brain metastasis116. Another CDK4/6 inhibitor, SHR6390, is being investigated in a phase Ib/II trial (NCT03772353) in combination with pyrotinib and letrozole in patients with HR+/HER2+ metastatic breast cancer. In the 15 patients enrolled to date, 10 patients had achieved a confirmed partial response and four patients had stable disease. Enrollment is ongoing and further results, including safety signals, will be reported81. In a randomized, open-label, phase II trial (MonarcHER; NCT02675231), the CDK4/6 inhibitor abemaciclib was assessed in patients with HR+/HER2+ advanced breast cancer (recurrent locally advanced, unresectable, or metastatic disease). Patients were randomized 1:1:1 to one of three treatment arms: oral abemaciclib 150 mg BID, IV trastuzumab (8 mg/kg on cycle 1 followed by 6 mg/kg thereafter) and IM fulvestrant 500 mg (arm A); abemaciclib plus trastuzumab (arm B); or standard of-care single agent physician’s choice chemotherapy plus trastuzumab (arm C). Results showed that after a median follow-up of 52.9 months,157 mortality events had occurred across the arms: 63%, 68% and 67% of patients in arms A, B, and C, respectively. Median OS was 31.1 months in arm A, 29.2 months in arm B, and 20.7 months in arm C (arm A vs. arm C: hazard ratio, 0.75; 95% confidence interval [CI] 0.47–1.21; P = 0.243; arm B vs. arm C: hazard ratio, 0.73; 95% CI 0.46–1.15; P = 0.177)117. Earlier published results showed a significant PFS benefit in patients who received abemaciclib, trastuzumab, and fulvestrant (8.3 months; 95% CI, 5.9–12.6) compared with patients who received standard-of-care chemotherapy and trastuzumab (5.7 months; 5.4–7.0; hazard ratio, 0.67 [95% CI, 0.45–1.00]; P = 0.051)37. Abemaciclib was generally well tolerated in monarcHER; however, the incidence of thrombocytopenia was higher than that previously reported, perhaps because >96% of the patients in monarcHER had been treated with prior T-DM137. This concept will be tested further in early-stage breast cancer in the ongoing eMonarcHER trial: a randomized, double blind, placebo-controlled phase III trial (N = 2450) of abemaciclib plus standard adjuvant endocrine therapy in participants with high-risk, node-positive, HR+/HER2+ early breast cancer who have completed adjuvant HER2-targeted therapy (NCT047523320). In the SOLTI-1303 PATRICIA trial (NCT02448420; N = 72), palbociclib was assessed in combination with trastuzumab with or without ET in patients with HER2+ advanced breast cancer. The median number of prior lines of systemic therapy for metastatic disease was three. Interestingly, among HR+/HER2+ patients in PATRICIA, PAM50 intrinsic subtype was significantly associated with PFS in patients with ER+ disease (P = 0.001 by log-rank test). Median PFS was 10.6 months (95% CI, 4.1–14.8) in Luminal B, 8.2 months (95% CI, 2.2–24.1) in Luminal A, 3.8 months (95% CI, 2.1–10.9) in HER2-E, and 6.0 months (95% CI, 1.7–11.2) in normal-like. In univariate analysis, patients with luminal disease had better PFS than patients with non-luminal tumors (median PFS 10.6 vs. 4.2 months; hazard ratio, 0.32; 95% CI, 0.25–0.98; P = 0.0020). In PATRICIA, 64.8% of patients reported a grade 3 or higher hematologic treatment-related AE, with 63.4% and 11.3% reporting grade 3 or higher neutropenia and thrombocytopenia, respectively. Three patients (4.2%) reported febrile neutropenia. Grade 3 or higher non-hematologic treatment-related AEs occurred in 7% of patients, most commonly grade 3 asthenia in two patients. No clinically significant cardiovascular toxicity was observed86. Finally, the PATINA phase III trial (NCT02947685) will evaluate the combination of palbociclib, anti-HER2 therapy, and ET in patients with HR+/HER2+ metastatic breast cancer as a maintenance strategy following four to eight cycles of induction treatment with taxane chemotherapy plus anti-HER2 antibodies pertuzumab plus trastuzumab118. It is hoped that the results of these trials may translate the preclinical hypothesis that integration of CDK4/6 inhibition along with anti-HER2 agents and anti-ER will result in a therapeutic advantage in the clinical domain and, in some cases, offer non-chemotherapeutic regimens as effective alternative treatment options.
Findings from these clinical trials indicate that dual blockade of ER and HER2 is an effective treatment approach across the continuum of HR+/HER2+ breast cancer disease settings (Fig. 2)7,38,66,67,68,73. These combination treatment strategies can improve clinical outcomes in HR+/HER2+ or ERBB2-mutant breast cancer, potentially overcoming resistance to ET arising from crosstalk between the ER and HER2 signaling pathways.
Results from other trials suggest that bi-directional signaling pathways are also evident between the ER and downstream PI3K/AKT/mTOR signaling pathways, which may play an important role in ER+ breast cancers119,120. The PI3K/AKT/mTOR signaling pathway drives many cellular processes, including proliferation, growth, and survival. Aberrant signaling of this pathway has been implicated in the tumorigenesis of ER+ breast cancer121,122,123 and development of endocrine resistance124,125,126. Inhibition of the PI3K/AKT/mTOR signaling pathway has been shown to augment the response of ER+ breast cancer to ET, indicating that dual targeting of the ER and PI3K/AKT/mTOR pathways may be another rational treatment approach119,120. In contrast, combinations of anti-HER2 therapy and mTOR inhibition have yielded only minimal clinical activity in the metastatic setting, at the expense of enhanced toxicity (as reviewed by Holloway and Marignani127 and Fujimoto et al. 128). A two-part phase III trial (a safety run-in cohort, followed by a randomized placebo-controlled cohort) is ongoing (EPIK-B2; NCT04208178) to evaluate the efficacy and safety of alpelisib, a PI3K inhibitor, in combination with dual HER2 inhibition with trastuzumab and pertuzumab in patients with HER2+ (any HR status) advanced breast cancer in the “maintenance” setting (a similar trial design as in the PATINA clinical trial, cf. Above) following completion of taxane chemotherapy combined with dual HER2 antibody therapy129.
Finally, to underscore the potential clinical utility of combined receptor blockade, in the SYSUCC-002 clinical trial, Hua and colleagues85 tested whether trastuzumab plus ET is non-inferior to trastuzumab plus chemotherapy in an open-label, non-inferiority, phase III, randomized, controlled trial (NCT01950182) in the first-line metastatic disease setting, at nine hospitals in China. Patients in SYSUCC-002 were randomly assigned (1:1) to receive trastuzumab plus ET (per investigator’s choice of ER modulators or AI, with or without concurrent ovarian suppression) or chemotherapy (per investigator’s choice of taxanes, capecitabine, or vinorelbine). The primary endpoint was PFS with a hazard ratio non-inferiority upper margin of 1.35. In 392 patients, after a median follow-up of 30.2 months, the median PFS was 19.2 months (95% CI, 16.7–21.7) in the ET group and 14.8 months (12.8–16.8) in the chemotherapy group (hazard ratio, 0.88; 95% CI, 0.71–1.09; non-inferiority P <0.0001). Not surprisingly, toxicity was observed significantly more frequently in the chemotherapy group compared with the ET group. Most AEs in the ET group were grade 1 to 2. The most common AEs were joint pain (16.8%), muscle pain (16.3%), and fatigue (15.8%). The most frequently reported AEs in the chemotherapy group were alopecia (63.8%), leukopenia (50.0%), and nausea (47.5%). Patients in the ET group had a significantly lower prevalence of AEs of grade 3 to 4 compared with those in the chemotherapy group (3.1% vs. 51.0%; P < 0.01). This provocative result suggests that, as in the case of HER2−/HR+ metastatic breast cancer, in which usually multiple endocrine manipulations are utilized prior to consideration of palliative chemotherapy regimens, the same principle may apply even in the context of HER2+/HR+ disease. However, the SYSUCC-002 trial has limitations in that the hazard ratio upper bound defining non-inferiority was rather modest (1.35) and the trial did not incorporate pertuzumab in the first-line HER2+ metastatic setting, which is the current standard of care (based upon a large and highly significant OS advantage in first-line HER2+ metastatic disease in the CLEOPATRA trial84) and only a minority of patients in SYSUCC-002 were assigned to taxane chemotherapy in the control arm. Thus, overall, such intriguing results from SYSUCC-002 notwithstanding, further clinical trials are warranted to optimize patient selection, identify novel biomarkers, and improve those currently available to guide therapy, optimize existing combinations, and investigate novel targeted therapies that have the potential to overcome endocrine and anti-HER2 treatment resistance in the metastatic setting40.
Summary
Preclinical studies performed since 1995 have provided compelling evidence of a bi-directional molecular crosstalk between the ER and HER2 cellular signaling pathways that promote tumor growth and progression36. The use of combined anti-HER2 therapy and ET needs to be addressed because it is an underestimated (and underutilized) option in both settings of early breast cancer and metastatic breast cancer. Further support for crosstalk came from studies that showed treatment strategies targeting a single pathway (ER or HER2) resulted in the upregulation of the other pathway, ultimately resulting in resistance to therapy. These findings led to assessment of simultaneous ER and HER2 blockade in ER+/HER2+ breast cancer models, in which enhanced antitumor activity was observed, compared with single-target blockade controls36,42.
In the clinical setting, various combinations targeting the ER and HER2 signaling pathways have been evaluated, and the evidence to date suggests that this approach produces enhanced and sustained antitumor activity compared with targeting either pathway alone (with the aforementioned caveat that many trial designs lacked single-agent–alone control groups for either HER2- or ER-targeting agents). Clinical trials have shown an enhanced benefit with combined endocrine and HER2-directed therapies in patients with HR+/HER2+ or ERBB2-mutated breast cancers. Additional research is needed to identify patient subpopulations that will derive optimal clinical benefit from currently available treatment approaches, as well as new combination treatment strategies and novel agents with downstream effects that may improve clinical outcomes69,92. Moreover, a revolution in the field of HER2-targeting has recently happened, with the emergence of the targetability of “HER2-low” (tumors with HER2 immunohistochemical staining of 1+ or 2+ without evidence of ERBB2 gene amplification by in situ hybridization) breast cancer with fam-trastuzumab deruxtecan (an antibody-drug conjugate composed of a humanized anti-HER2 monoclonal antibody and a topoisomerase I inhibitor payload). In the randomized phase III DESTINY-Breast04 trial (NCT03734029), patients with one or two prior lines of chemotherapy for advanced/metastatic disease were randomly assigned (2:1) to receive trastuzumab deruxtecan or the physician’s choice of chemotherapy. A total of 494 of 557 randomly assigned patients (88.7%) had HR+ disease. The median PFS (primary endpoint) in this cohort was 10.1 months in the trastuzumab deruxtecan group and 5.4 months in the physician’s choice group (hazard ratio for disease progression or death, 0.51; P < 0.001), and OS was 23.9 months and 17.5 months, respectively (hazard ratio for death, 0.64; P = 0.003)130. Interestingly, in contrast to the quantitatively inverse correlation between ERBB2 gene amplification and ER/PR protein expression published by Konecny et al. 8, ER and HER2 expression is positively correlated in HER2− tumors131. In a recent report, HR expression was significantly more common among HER2-low compared with HER2-IHC zero breast cancer (89.9% vs. 80.9%; P < 0.001)132. These findings may be important to consider in future clinical trials of anti-HER2 and anti-ET in HER2− (including HER2-low) disease. For example, one such trial is the ongoing DESTINY-Breast08 trial (NCT04556773) of fam-trastuzumab deruxtecan plus ET with either anastrozole or fulvestrant133.
In conclusion, sustained inhibition of either HER2 or ER can result in functioning of the other pathway as a key means of escape/survival in ER+/HER2+ breast cancer cells. Combined receptor (HER2 plus ET) blockade may help overcome the resistance to therapy in this disease population8,24,25,26,27,28, potentially reduce the use of chemotherapy69, and hopefully improve survival results89,117 while maintaining clinical feasibility in terms of safety and tolerability. Further research in patient selection, predictive biomarkers92, treatment sequencing7,118,129, and combination therapies118,119,120,121,122,123,124,125,126,127,128,129, as well as novel targeted therapies, are needed in this population to improve patient outcomes.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Caldarella, A. et al. Female breast cancer status according to ER, PR and HER2 expression: a population based analysis. Pathol. Oncol. Res. 17, 753–758 (2011).
Howlader, N. et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055 (2014).
Purdie, C. A. et al. Increased mortality in HER2 positive, oestrogen receptor positive invasive breast cancer: a population-based study. Brit. J. Cancer 103, 475–481 (2010).
Gong, Y., Liu, Y. R., Ji, P., Hu, X. & Shao, Z. M. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci. Rep. 7, 45411 (2017).
Noone, A. M. et al. SEER Cancer Statistics Review 1975–2015 (National Cancer Institute, 2018).
Vaz-Luis, I. et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 14, R129 (2012).
Chan, A. et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 17, 367–377 (2016).
Konecny, G. et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J. Natl. Cancer Inst. 95, 142–153 (2003).
Kay, C. et al. Current trends in the treatment of HR+/HER2+ breast cancer. Future Oncol. 17, 1665–1681 (2021).
Alqaisi, A. et al. Impact of estrogen receptor (ER) and human epidermal growth factor receptor-2 (HER2) co-expression on breast cancer disease characteristics: implications for tumor biology and research. Breast Cancer Res. Treat. 148, 437–444 (2014).
Garcia Fernandez, A. et al. Survival and clinicopathological characteristics of breast cancer patient according to different tumour subtypes as determined by hormone receptor and Her2 immunohistochemistry. a single institution survey spanning 1998 to 2010. Breast 21, 366–373 (2012).
Montemurro, F. et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer 118, 17–26 (2012).
Bhargava, R. et al. Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod. Pathol. 24, 367–374 (2011).
Baselga, J. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379, 633–640 (2012).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet. Oncol. 13, 25–32 (2012).
Zhao, B., Zhao, H. & Zhao, J. Impact of hormone receptor status on the efficacy of HER2-targeted treatment. Endocr. Relat. Cancer 25, 687–697 (2018).
De Laurentiis, M. et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin. Cancer Res. 11, 4741–4748 (2005).
Gianni, L. et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER. Lancet 375, 377–384 (2010).
von Minckwitz, G. et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J. Clin. Oncol. 28, 2015–2023 (2010).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284 (2013).
Untch, M. et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J. Clin. Oncol. 28, 2024–2031 (2010).
Untch, M. et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 13, 135–144 (2012).
Croessmann, S. et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-18-1544 (2018).
Oh, A. S. et al. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol. Endocrinol. 15, 1344–1359 (2001).
Creighton, C. J. et al. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 66, 3903–3911 (2006).
Guo, S. & Sonenshein, G. E. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the HER-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol. Cell Biol. 24, 8681–8690 (2004).
Cotrim, C. Z. et al. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene 32, 2390–2402 (2013).
O’Regan, R. Complexities of Targeting HER2 in Estrogen Receptor–Positive Breast Cancers. https://ascopost.com/issues/january-25-2015/complexities-of-targeting-her2-in-estrogen-receptor-positive-breast-cancers/ (2015).
Barone, I., Brusco, L. & Fuqua, S. A. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin. Cancer Res. 16, 2702–2708 (2010).
Lim, E., Palmieri, C. & Tilley, W. D. Renewed interest in the progesterone receptor in breast cancer. Brit. J. Cancer 115, 909–911 (2016).
Campbell, E. J. et al. The combined endocrine receptor in breast cancer, a novel approach to traditional hormone receptor interpretation and a better discriminator of outcome than ER and PR alone. Brit. J. Cancer 115, 967–973 (2016).
Arpino, G., Wiechmann, L., Osborne, C. K. & Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocrine Rev. 29, 217–233 (2008).
Montemurro, F., Di Cosimo, S. & Arpino, G. Human epidermal growth factor receptor 2 (HER2)-positive and hormone receptor-positive breast cancer: new insights into molecular interactions and clinical implications. Ann. Oncol. 24, 2715–2724 (2013).
Montaser, R. Z. & Coley, H. M. Crosstalk between ERalpha and receptor tyrosine kinase signalling and implications for the development of anti-endocrine resistance. Cancers https://doi.org/10.3390/cancers10060209 (2018).
Guarneri, V. et al. Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J. Clin. Oncol. 32, 1050–1057 (2014).
Pietras, R. J. et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10, 2435–2446 (1995).
Tolaney, S. M. et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 21, 763–775 (2020).
Rimawi, M. et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II trial. J. Clin. Oncol. 36, 2826–2835 (2018).
Mehta, A. & Tripathy, D. Co-targeting estrogen receptor and HER2 pathways in breast cancer. Breast 23, 2–9 (2014).
Giuliano, M., Trivedi, M. V. & Schiff, R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel) 8, 256–262 (2013).
Rusnak, D. W. et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 1, 85–94 (2001).
Xia, W. et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc. Natl Acad. Sci. USA 103, 7795–7800 (2006).
Wang, Y. C. et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers-role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 13, R121 (2011).
Chen, Z., Wang, Y., Warden, C. & Chen, S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 149, 118–127 (2015).
Wang, C. et al. Estrogen induces c-myc gene expression via an upstream enhancer activated by the estrogen receptor and the AP-1 transcription factor. Mol. Endocrinol. 25, 1527–1538 (2011).
Cavaliere, C. et al. Combined inhibitory effect of formestane and herceptin on a subpopulation of CD44+/CD24low breast cancer cells. Cancer Sci. 101, 1661–1669 (2010).
Chan, J. C., Ong, P. S., Lim, P., Teng, P. X. & Chan, E. C. Synergistic disruption of ERalpha/HER2 crosstalk by endoxifen and lapatinib in breast cancer cells. Cancer Chemother. Pharmacol. 79, 117–130 (2017).
Collins, D. et al. Direct estrogen receptor (ER) / HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS One 12, e0177331 (2017).
Li, Z. et al. Activated estrogen receptor-mitogen-activated protein kinases cross talk confer acquired resistance to lapatinib. Thorac. Cancer 6, 695–703 (2015).
McDermott, M. S. J. et al. Dual inhibition of IGF1R and ER enhances response to trastuzumab in HER2 positive breast cancer cells. Int. J. Oncol. 50, 2221–2228 (2017).
Sudhan, D. R. et al. Extended adjuvant therapy with neratinib plus fulvestrant blocks ER/HER2 crosstalk and maintains complete responses of ER+/HER2+ breast cancers: implications to the ExteNET trial. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-18-1131 (2018).
Scaltriti, M. et al. Neratinib induces estrogen receptor function and sensitizes HER2-mutant breast cancer to anti-endocrine therapy. Euro. J. Cancer 69, S125 (2016).
Ribas, R. et al. Targeting tumour re-wiring by triple blockade of mTORC1, epidermal growth factor, and oestrogen receptor signalling pathways in endocrine-resistant breast cancer. Breast Cancer Res. 20, 44 (2018).
Nayar, U. et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat. Genet. 51, 207–216 (2019).
Shagisultanova, E., Borakove, M., Kabos, P. & Borges, V. Abstract P4-07-07: Triple targeted combination therapy with tucatinib, palbociclib and fulvestrant is active in hormone receptor and HER2-positive human breast tumor cell lines. Cancer Res. 79, P4-07-07-P04-07-07 (2019).
Arpino, G. et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J. Natl Cancer Inst. 99, 694–705 (2007).
Rimawi, M. F. et al. Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin. Cancer Res. 17, 1351–1361 (2011).
Chakraborty, A. K., Mehra, R. & Digiovanna, M. P. Co-targeting ER and HER family receptors induces apoptosis in HER2-normal or overexpressing breast cancer models. Anticancer Res. 35, 1243–1250 (2015).
Phillips, G. D. et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin. Cancer Res. 20, 456–468 (2014).
Watanabe, S. et al. Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer. Cancer Med. 8, 1258–1268 (2019).
Feldinger, K. & Kong, A. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer (Dove Med. Press) 7, 147–162 (2015).
Rabindran, S. K. et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 64, 3958–3965 (2004).
Ma, C. X. et al. Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer. Clin. Cancer Res. 23, 5687–5695 (2017).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016).
Johnston, S. et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 27, 5538–5546 (2009).
Schwartzberg, L. S. et al. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. Oncologist 15, 122–129 (2010).
Kaufman, B. et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J. Clin. Oncol. 27, 5529–5537 (2009).
Johnston, S. R. D. et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: ALTERNATIVE. J. Clin. Oncol. 36, 741–748 (2018).
Gianni, L. et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 19, 249–256 (2018).
Marcom, P. K. et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res. Treat. 102, 43–49 (2007).
Rimawi, M. F. et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J. Clin. Oncol. 31, 1726–1731 (2013).
Villanueva, C. et al. Phase II study assessing lapatinib added to letrozole in patients with progressive disease under aromatase inhibitor in metastatic breast cancer-Study BES 06. Target Oncol. 8, 137–143 (2013).
Huober, J. et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast 21, 27–33 (2012).
Chu, Q. S. et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clinical Cancer Res. 14, 4484–4490 (2008).
Koeberle, D. et al. Combination of trastuzumab and letrozole after resistance to sequential trastuzumab and aromatase inhibitor monotherapies in patients with estrogen receptor-positive, HER-2-positive advanced breast cancer: a proof-of-concept trial (SAKK 23/03). Endocr. Relat. Cancer 18, 257–264 (2011).
Rimawi, M. et al. Abstract S3-06: A phase III trial evaluating pCR in patients with HR+, HER2-positive breast cancer treated with neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) +/- estrogen deprivation: NRG oncology/NSABP B-52. Cancer Res. 77, S3-06-S03-06 (2017).
Fumoleau, P. et al. A phase I pharmacokinetics study of lapatinib and tamoxifen in metastatic breast cancer (EORTC 10053 Lapatam study. Breast 23, 663–669 (2014).
Park, J. H. et al. Phase II trial of neoadjuvant letrozole and lapatinib in Asian postmenopausal women with estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2)-positive breast cancer [Neo-ALL-IN]: highlighting the TILs, ER expressional change after neoadjuvant treatment, and FES-PET as potential significant biomarkers. Cancer Chemother. Pharmacol. 78, 685–695 (2016).
Martin, M. et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 1688–1700 (2017).
Shagisultanova, E. et al. Tucatinib, palbociclib, and letrozole in HR+/HER2+ metastatic breast cancer: report of phase IB safety cohort. J. Clin. Oncol. 37, 1029 (2019).
Zhang, J. et al. Cyclin-dependent kinase 4/6 (CDK4/6) inhibitor SHR6390 combined with pyrotinib and letrozole in patients with human epidermal growth factor receptor 2-positive (HER2+), hormone receptor positive (HR+) metastatic breast cancer (MBC): phase Ib study results. J. Clin. Oncol. 39, 1035 (2021).
Tolaney, S. M. et al. eMonarcHER: A phase 3 study of abemaciclib plus standard adjuvant endocrine therapy in patients with HR+, HER2+, node-positive, high-risk early breast cancer. J. Clin. Oncol. 39, TPS596 (2021).
Ma, C. X. et al. The phase II MutHER study of neratinib alone and in combination with fulvestrant in HER2-mutated, non-amplified metastatic breast cancer. Clin. Cancer Res. 28, 1258–1267 (2022).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21, 519–530 (2020).
Hua, X. et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor-positive and HER2-positive metastatic breast cancer (SYSUCC-002). Clin. Cancer Res. 28, 637–645 (2022).
Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin. Cancer Res. 26, 5820–5829 (2020).
Jhaveri, K. L. et al. Neratinib plus fulvestrant plus trastzuzumab (N+F+T) for hormone receptor-positive (HR+), HER2-negative, HER2-mutant metastatic breast cancer (MBC): outcomes and biomarker analysis from the SUMMIT trial. J. Clin. Oncol. 40, 1028 (2022).
Dieci, M. V. et al. Type of endocrine therapy and DFS in patients with early HER2+/HR+ BC: analysis from the phase III randomized ShortHER trial. J. Clin. Oncol. 40, 547 (2022).
Chan, A. et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin. Breast Cancer 21, 80–91 e87 (2021).
Gluz, O. et al. De-escalated chemotherapy versus endocrine therapy plus pertuzumab+ trastuzumab for HR+/HER2+ early breast cancer (BC): first efficacy results from the neoadjuvant WSG-TP-II study. J. Clin. Oncol. 38, 515 (2020).
Harbeck, N. et al. De-escalation strategies in Human Epidermal Growth Factor Receptor 2 (HER2)-positive early Breast Cancer (BC): final analysis of the West German Study Group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without Endocrine Therapy (ET) versus trastuzumab plus ET. J. Clin. Oncol. 35, 3046–3054 (2017).
Prat Aparicio, A. et al. Abstract S3-03: PAM50 intrinsic subtype as a predictor of pathological complete response following neoadjuvant dual HER2 blockade without chemotherapy in HER2-positive breast cancer: first results of the PAMELA clinical trial. Cancer Res. 77, S3–S03 (2017).
Masuda, N. et al. Efficacy and safety of trastuzumab, lapatinib, and paclitaxel neoadjuvant treatment with or without prolonged exposure to anti-HER2 therapy, and with or without hormone therapy for HER2-positive primary breast cancer: a randomised, five-arm, multicentre, open-label phase II trial. Breast Cancer (Dove Med. Press) 25, 407–415 (2018).
Rimawi, M. F. et al. TBCRC023: A randomized phase II neoadjuvant trial of lapatinib plus trastuzumab without chemotherapy for 12 versus 24 weeks in patients with HER2-positive breast cancer. Clin. Cancer Res. 26, 821–827 (2020).
Johnston, S. R. D. et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: updated results of ALTERNATIVE. J. Clin. Oncol. 39, 79–89 (2021).
Smyth, L. M. et al. Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic breast cancer. Cancer Discov. 10, 198–213 (2020).
Ma, C. X. et al. Abstract CT026: A phase II trial of neratinib (NER) or NER plus fulvestrant (FUL) (N+F) in HER2 mutant, non-amplified (HER2mut) metastatic breast cancer (MBC): part II of MutHER. Cancer Res. 81, CT026 (2021).
Osborne, C. K., Kitten, L. & Arteaga, C. L. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J. Clin. Oncol. 7, 710–717 (1989).
Fisher, B. et al. Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer: 5-year results from the National Surgical Adjuvant Breast and Bowel Project Trial. J. Clin. Oncol. 4, 459–471 (1986).
Albain, K. S. et al. Abstract 143: Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results from intergroup trial 0100 (SWOG-8814). Proc. Am. Soc. Clin. Onc. 21, 37a (2002).
Albain, K. S. et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet 374, 2055–2063 (2009).
Pegram, M. D., Miles, D., Tsui, C. K. & Zong, Y. HER2-Overexpressing/amplified breast cancer as a testing ground for antibody-drug conjugate drug development in solid tumors. Clin. Cancer Res. 26, 775–786 (2020).
Pernas, S. et al. PAM50 Subtypes in baseline and residual tumors following neoadjuvant trastuzumab-based chemotherapy in HER2-positive breast cancer: a consecutive-series from a single institution. Front. Oncol. 9, 707 (2019).
Prat, A. et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 75, 103801 (2022).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Guarneri, V. et al. Survival after neoadjuvant therapy with trastuzumab-lapatinib and chemotherapy in patients with HER2-positive early breast cancer: a meta-analysis of randomized trials. ESMO Open 7, 100433 (2022).
Broglio, K. R. et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2, 751–760 (2016).
Moy, B. et al. Association between treatment duration and overall survival in early-stage HER2+ breast cancer patients receiving extended adjuvant therapy with neratinib in the ExteNET trial. J. Clin. Oncol. 39, 540 (2021).
European Medicines Agency. Nerlynx Neratinib. https://www.ema.europa.eu/en/medicines/human/EPAR/nerlynx (2021).
Barcenas, C. H. et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann. Oncol. 31, 1223–1230 (2020).
Martínez-Sáez, O. & Prat, A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol. Practice, OP 21, 00172 (2021).
Konecny, G. E. et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 66, 1630–1639 (2006).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Hyman, D. et al. Abstract PD2-08: Neratinib + fulvestrant in ERBB2-mutant, HER2–non-amplified, estrogen receptor (ER)-positive, metastatic breast cancer (MBC): preliminary analysis from the phase II SUMMIT trial. Cancer Res. 77, PD2–08 (2017).
Turner, N. et al. Abstract OT1-06-03: The plasmaMATCH trial: a multiple parallel cohort, open-label, multi-centre phase II clinical trial of ctDNA screening to direct targeted therapies in patients with advanced breast cancer (CRUK/15/010). Cancer Res. 78, OT1-06-03 (2018).
Shagisultanova, E. et al. Interim safety and efficacy analysis of phase IB/II clinical trial of tucatinib, palbociclib and letrozole in patients with hormone receptor and HER2-positive metastatic breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS20-PS10-03 (2021).
André, F. et al. Final overall survival (OS) for abemaciclib plus trastuzumab +/− fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): a randomized, open-label, phase II trial. Ann. Oncol. 33, S808–S869 (2022).
ClinicalTrials.gov. Randomized, Open Label, Clinical Study of the Targeted Therapy, Palbociclib, To Treat Metastatic Breast Cancer (PATINA). https://clinicaltrials.gov/ct2/show/NCT02947685 (2021).
Ciruelos Gil, E. M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat. Rev. 40, 862–871 (2014).
Vasan, N., Toska, E. & Scaltriti, M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann. Oncol. 30, x3–x11 (2019).
Perez-Tenorio, G. et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin. Cancer Res. 13, 3577–3584 (2007).
Stemke-Hale, K. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 68, 6084–6091 (2008).
Ellis, M. J. et al. Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and response to neoadjuvant endocrine therapy for estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 119, 379–390 (2010).
Miller, T. W., Rexer, B. N., Garrett, J. T. & Arteaga, C. L. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 13, 224 (2011).
Barone, I. et al. Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway. Cancer Res. 69, 4724–4732 (2009).
Cavazzoni, A. et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 323, 77–87 (2012).
Holloway, R. W. & Marignani, P. A. Targeting mTOR and glycolysis in HER2-positive breast cancer. Cancers (Basel) https://doi.org/10.3390/cancers13122922 (2021).
Fujimoto, Y. et al. Combination treatment with a PI3K/Akt/mTOR pathway inhibitor overcomes resistance to anti-HER2 therapy in PIK3CA-mutant HER2-positive breast cancer cells. Sci. Rep. 10, 21762 (2020).
ClinicalTrials.gov. Study of Alpelisib (BYL719) in Combination With Trastuzumab And Pertuzumab as Maintenance Therapy in Patients With HER2-Positive Advanced Breast Cancer With a PIK3CA Mutation. https://clinicaltrials.gov/ct2/show/NCT04208178 (2021).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Pinhel, I. et al. ER and HER2 expression are positively correlated in HER2 non-overexpressing breast cancer. Breast Cancer Res. 14, R46 (2012).
Tarantino, P. et al. Prognostic and biologic significance of HER2-low expression in early breast cancer. Ann Oncol. 33, S123–S147 (2022).
Andre, F. et al. Dose-finding and -expansion studies of trastuzumab deruxtecan in combination with other anti-cancer agents in patients (pts) with advanced/metastatic HER2+ (DESTINY-Breast07 [DB-07]) and HER2-low (DESTINY-Breast08 [DB-08]) breast cancer (BC). J. Clin. Oncol. 40, 3025 (2022).
Acknowledgements
Medical writing and editorial assistance were provided by Lumanity Communications Inc. with financial support by Puma Biotechnology, Inc.
Author information
Authors and Affiliations
Contributions
MP, CJ, and SRDJ all contributed equally to the inception, writing, and review of this review article. MP has also served as the corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: MP has served as a consultant for Roche/Genentech, AstraZeneca, Novartis, Pfizer, Seattle Genetics. CJ has served as a consultant for Roche, AstraZeneca, Pfizer, Celgene, Exact Sciences, Lilly, Pierre Fabre, and Sanofi Genzyme; has presented on behalf of Roche, AstraZeneca, Pfizer, Celgene, Exact Sciences, Novartis, Lilly, and medupdate GmbH; and has received research funding from Exact Sciences and Roche. SRDJ has served as a consultant for Lilly, Puma Biotechnology, Pfizer, Novartis, and Sanofi Genzyme; has presented on behalf of Pfizer, Novartis, Eisai, Lilly, AstraZeneca, Roche/Genentech, and Sanofi Genzyme; and has received research funding from Lilly, Pfizer, AstraZeneca, Novartis, Roche/Genentech, and Puma Biotechnology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pegram, M., Jackisch, C. & Johnston, S.R.D. Estrogen/HER2 receptor crosstalk in breast cancer: combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. npj Breast Cancer 9, 45 (2023). https://doi.org/10.1038/s41523-023-00533-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00533-2
This article is cited by
-
Endosomal recycling inhibitors downregulate estrogen receptor-alpha and synergise with endocrine therapies
Breast Cancer Research and Treatment (2024)