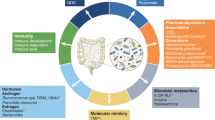
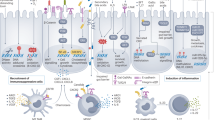
Abstract
Cancer cells originate from a series of acquired genetic mutations that can drive their uncontrolled cell proliferation and immune evasion. Environmental factors, including the microorganisms that colonize the human body, can shift the metabolism, growth pattern and function of neoplastic cells and shape the tumour microenvironment. Dysbiosis of the gut microbiome is now recognized as a hallmark of cancer by the scientific community. However, only a few microorganisms have been identified that directly initiate tumorigenesis or skew the immune system to generate a tumour-permissive milieu. Over the past two decades, research on the human microbiome and its functionalities within and across individuals has revealed microbiota-focused strategies for health and disease. Here, we review the evolving understanding of the mechanisms by which the microbiota acts in cancer initiation, promotion and progression. We explore the roles of bacteria in gastrointestinal tract malignancies and cancers of the lung, breast and prostate. Finally, we discuss the promises and limitations of targeting or harnessing bacteria in personalized cancer prevention, diagnostics and treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Poore, G. D. et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020). This study shows that the tumour microbiome composition and the immune system play a crucial prognosis role in PDAC where higher alpha-diversity and specific microbiome signatures are linked to long-term PDAC survival.
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020). This study analyses the intra-tumoural microbiome of 1,526 tumours across seven cancer types, revealing that each tumour type has a distinct microbiome composition as well as correlations between the intra-tumoural bacteria, their predicted functions, tumour types and subtypes, factors such as patients’ smoking status and response to immunotherapy.
Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 17, 742–753 (2019).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Cao, Y. et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 67, 672–678 (2018).
Velicer, C. M. et al. Antibiotic use in relation to the risk of breast cancer. J. Am. Med. Assoc. 291, 827–835 (2004).
Yonekura, S. et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 12, 1128–1151 (2022).
Plummer, M. et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health 4, e609–e616 (2016).
de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health 8, e180–e190 (2020).
Garrett, W. S. Cancer and the microbiota. Science 348, 80–86 (2015).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Parsonnet, J. et al. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 330, 1267–1271 (1994).
Geis, A. L. et al. Regulatory T-cell response to enterotoxigenic Bacteroides fragilis colonization triggers IL17-dependent colon carcinogenesis. Cancer Discov. 5, 1098–1109 (2015).
Nougayrède, J.-P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013). This paper demonstrates that F. nucleatum plays a significant role in promoting intestinal tumorigenesis in humans and mice by driving a pro-inflammatory tumour milieu and MDSC infiltration.
Sepe, L. P. et al. Genotoxic effect of Salmonella paratyphi A infection on human primary gallbladder cells. mBio 11, e01911–e01920 (2020).
Parhi, L. et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 11, 3259 (2020).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e12 (2019). This study identifies a microbiome signature predictive of long-term survival in patients with pancreatic cancer and demonstrates that modulating the tumour microbiome through faecal microbiota transplantation can affect tumour growth and immune infiltration.
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). Together with Viaud et al. (2013), this study uncovers for the first time the significant involvement of microbiota in regulating the response to immunotherapy and chemotherapy, establishing that a healthy gut microbiota activates tumour-associated myeloid cells to produce pro-inflammatory cytokines and ROS.
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). This study uncovers the role of the microbiome in response to the immune checkpoint blockade anti-CTLA4, finding that specific gut microbiota members, such as B. fragilis, modulate the anticancer immune response induced by anti-CTLA4 therapy in both mice and humans.
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). Together with Gopalakrishnan et al. (2018) and Routy et al. (Science, 2018), this study discovers that faecal microbiota transplantation from patients with cancer who responded to immune checkpoint blockade can restore the antitumour effects of PD-1 blockade in patients who are non-responders.
Stein-Thoeringer, C. K. et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19–CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021). This study finds that higher dietary fibre intake is associated with improved response to immune checkpoint blockade treatment in patients with melanoma, with preclinical models showing impaired treatment response in mice receiving a low-fibre diet.
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Matson, V. & Gajewski, T. F. Dietary modulation of the gut microbiome as an immunoregulatory intervention. Cancer Cell 40, 246–248 (2022).
Walboomers, J. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019). This study describes how fungi translocation from the gut to the pancreas promotes oncogenesis, uncovering the essential role of the mannose-binding lectin-complement cascade pathway activation in driving pancreatic cancer progression.
Dohlman, A. B. et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 185, 3807–3822.e12 (2022).
Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012).
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M. & Owen, L. J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191 (2015).
Clay, S. L., Fonseca-Pereira, D. & Garrett, W. S. Colorectal cancer: the facts in the case of the microbiota. J. Clin. Invest. 132, e155101 (2022).
Warren, J. R. & Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet Lond. Engl. 1, 1273–1275 (1983).
Wang, F., Meng, W., Wang, B. & Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202 (2014).
Van Rossum, T., Ferretti, P., Maistrenko, O. M. & Bork, P. Diversity within species: interpreting strains in microbiomes. Nat. Rev. Microbiol. 18, 491–506 (2020).
Cover, T. L., Lacy, D. B. & Ohi, M. D. The Helicobacter pylori Cag type IV secretion system. Trends Microbiol. 28, 682–695 (2020).
Parsonnet, J. Clinician-discoverers — Marshall, Warren, and H. pylori. N. Engl. J. Med. 353, 2421–2423 (2005).
Howden, C. W. & Hunt, R. H. Relationship between gastric secretion and infection. Gut 28, 96–107 (1987).
Mobley, H. L., Cortesia, M. J., Rosenthal, L. E. & Jones, B. D. Characterization of urease from Campylobacter pylori. J. Clin. Microbiol. 26, 831–836 (1988).
Fallone, C. A. et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151, 51–69.e14 (2016).
Cheung, K. S. et al. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 67, 28–35 (2018).
Nehra, A. K., Alexander, J. A., Loftus, C. G. & Nehra, V. Proton pump inhibitors: review of emerging concerns. Mayo Clin. Proc. 93, 240–246 (2018).
Yang, Y.-X., Lewis, J. D., Epstein, S. & Metz, D. C. Long-term proton pump inhibitor therapy and risk of hip fracture. J. Am. Med. Assoc. 296, 2947–2953 (2006).
Janarthanan, S., Ditah, I., Adler, D. G. & Ehrinpreis, M. N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am. J. Gastroenterol. 107, 1001–1010 (2012).
Laheij, R. J. F. et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. J. Am. Med. Assoc. 292, 1955–1960 (2004).
Sherwood, M. W. et al. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: a systematic review. J. Am. Heart Assoc. 4, e002245 (2015).
Königer, V. et al. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat. Microbiol. 2, 16188 (2016). This study identifies HopQ as the adhesin of H. pylori enabling translocation of the pathogenicity factor into host cells, and thus contributing to H. pylori-induced peptic ulcer disease and gastric adenocarcinoma.
Maeda, S. et al. Distinct mechanism of Helicobacter pylori-mediated NF-κB activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276, 44856–44864 (2001).
Viala, J. et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5, 1166–1174 (2004).
Suzuki, N. et al. Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci. Rep. 5, 10024 (2015).
Zavros, Y. & Merchant, J. L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 19, 451–467 (2022).
Stolte, M. et al. Helicobacter and gastric MALT lymphoma. Gut 50 (Suppl. 3), III19–III24 (2002).
Lin, W.-C. et al. Translocation of Helicobacter pylori CagA into human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 70, 5740–5748 (2010).
Ruskoné-Fourmestraux, A. et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 60, 747–758 (2011).
Park, H. S., Kim, Y. J., Yang, W. I., Suh, C. O. & Lee, Y. C. Treatment outcome of localized Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J. Gastroenterol. 16, 2158–2162 (2010).
Zullo, A. et al. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. J. Clin. Gastroenterol. 47, 824–827 (2013).
Islami, F. & Kamangar, F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev. Res. 1, 329–338 (2008).
Holleczek, B., Schöttker, B. & Brenner, H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: results from the prospective population-based ESTHER cohort study. Int. J. Cancer 146, 2773–2783 (2020).
Chiba, N. cagA-seropositive strains of Helicobacter pylori increase the risk for gastric cancer more than the presence of H pylori alone. Can. J. Gastroenterol. J. Can. Gastroenterol. 18, 341–343 (2004).
Ding, S.-Z. Global whole family based — Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J. Gastroenterol. 26, 995–1004 (2020).
Argueta, E. A. & Moss, S. F. The prevention of gastric cancer by Helicobacter pylori eradication. Curr. Opin. Gastroenterol. 37, 625–630 (2021).
Roa, J. C. et al. Gallbladder cancer. Nat. Rev. Dis. Prim. 8, 69 (2022).
Randi, G., Franceschi, S. & La Vecchia, C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int. J. Cancer 118, 1591–1602 (2006).
Shukla, R. et al. Roles of Salmonella typhi and Salmonella paratyphi in gallbladder cancer development. Asian Pac. J. Cancer Prev. 22, 509–516 (2021).
Di Domenico, E. G., Cavallo, I., Pontone, M., Toma, L. & Ensoli, F. Biofilm producing Salmonella Typhi: chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 18, E1887 (2017).
Liu, X., Lu, R., Wu, S. & Sun, J. Salmonella regulation of intestinal stem cells through the Wnt/β-catenin pathway. FEBS Lett. 584, 911–916 (2010).
Song, J., Gao, X. & Galán, J. E. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 499, 350–354 (2013).
Spanò, S., Ugalde, J. E. & Galán, J. E. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 3, 30–38 (2008).
Haghjoo, E. & Galán, J. E. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl Acad. Sci. USA 101, 4614–4619 (2004).
McCann, N., Scott, P., Parry, C. M. & Brown, M. Antimicrobial agents for the treatment of enteric fever chronic carriage: a systematic review. PLoS ONE 17, e0272043 (2022).
Sekirov, I., Russell, S. L., Antunes, L. C. M. & Finlay, B. B. Gut microbiota in health and disease. Physiol. Rev. 90, 859–904 (2010).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020). This study finds that exposing human intestinal organoids to genotoxic pks+ E. coli results in a distinct mutational signature that was also found in a subset of human CRC genomes.
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Wu, S. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022 (2009). The study investigates the role of ETBF in promoting colon tumour formation and that ETBF triggers colitis and strongly induces colonic tumours in mice by activating the Stat3 and TH17 cell-dependent immune pathway, providing new insights into human colon carcinogenesis.
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Abed, J. et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 20, 215–225 (2016).
Cuevas-Ramos, G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl Acad. Sci. USA 107, 11537–11542 (2010).
Drewes, J. L. et al. Human colon cancer-derived Clostridioides difficile strains drive colonic tumorigenesis in mice. Cancer Discov. 12, 1873–1885 (2022).
Alcock, F. & Palmer, T. Activation of a bacterial killing machine. PLoS Genet. 17, e1009261 (2021).
Garrett, W. S. The gut microbiota and colon cancer. Science 364, 1133–1135 (2019).
Zagato, E. et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 5, 511–524 (2020).
Nelson, D. P. & Mata, L. J. Bacterial flora associated with the human gastrointestinal mucosa. Gastroenterology 58, 56–61 (1970).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Denamur, E., Clermont, O., Bonacorsi, S. & Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 19, 37–54 (2021).
Vizcaino, M. I. & Crawford, J. M. The colibactin warhead crosslinks DNA. Nat. Chem. 7, 411–417 (2015).
Bossuet-Greif, N. et al. The colibactin genotoxin generates DNA interstrand cross-links in infected cells. mBio 9, e02393-17 (2018).
Wilson, M. R. et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785 (2019).
Putze, J. et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 77, 4696–4703 (2009).
Dougherty, M. W. & Jobin, C. Shining a light on colibactin biology. Toxins 13, 346 (2021).
Fu, D., Calvo, J. A. & Samson, L. D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer 12, 104–120 (2012).
Tripathi, P. et al. ClbS is a cyclopropane hydrolase that confers colibactin resistance. J. Am. Chem. Soc. 139, 17719–17722 (2017).
Sadecki, P. W. et al. Evolution of polymyxin resistance regulates colibactin production in Escherichia coli. ACS Chem. Biol. 16, 1243–1254 (2021).
Rehm, N. et al. Two polyketides intertwined in complex regulation: posttranscriptional CsrA-mediated control of colibactin and yersiniabactin synthesis in Escherichia coli. mBio 13, e0381421 (2022).
Faïs, T., Delmas, J., Barnich, N., Bonnet, R. & Dalmasso, G. Colibactin: more than a new bacterial toxin. Toxins 10, E151 (2018).
Wallenstein, A. et al. ClbR is the key transcriptional activator of colibactin gene expression in Escherichia coli. mSphere 5, e00591-20 (2020).
Volpe, M. R. et al. A small molecule inhibitor prevents gut bacterial genotoxin production. Nat. Chem. Biol. 19, 159–167 (2023).
He, Z. et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut 68, 289–300 (2019).
Man, S. M. The clinical importance of emerging Campylobacter species. Nat. Rev. Gastroenterol. Hepatol. 8, 669–685 (2011).
Warren, R. L. et al. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 1, 16 (2013).
Endo, A., Pärtty, A., Kalliomäki, M., Isolauri, E. & Salminen, S. Long-term monitoring of the human intestinal microbiota from the 2nd week to 13 years of age. Anaerobe 28, 149–156 (2014).
Carrow, H. C., Batachari, L. E. & Chu, H. Strain diversity in the microbiome: lessons from Bacteroides fragilis. PLoS Pathog. 16, e1009056 (2020).
Sears, C. L., Geis, A. L. & Housseau, F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J. Clin. Invest. 124, 4166–4172 (2014).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Boleij, A. et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60, 208–215 (2015).
Toprak, N. U. et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12, 782–786 (2006).
Wu, S., Morin, P. J., Maouyo, D. & Sears, C. L. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 124, 392–400 (2003).
Wu, S., Rhee, K.-J., Zhang, M., Franco, A. & Sears, C. L. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and γ-secretase-dependent E-cadherin cleavage. J. Cell Sci. 120, 1944–1952 (2007).
Wu, S. et al. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect. Immun. 72, 5832–5839 (2004).
Cheng, W. T., Kantilal, H. K. & Davamani, F. The mechanism of Bacteroides fragilis toxin contributes to colon cancer formation. Malays. J. Med. Sci. 27, 9–21 (2020).
Liu, Q.-Q. et al. Enterotoxigenic Bacteroides fragilis induces the stemness in colorectal cancer via upregulating histone demethylase JMJD2B. Gut Microbes 12, 1788900 (2020).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018). This study shows that mice co-colonized with the bacterial biofilms found in patients with adenomatous polyposis exhibit increased inflammation and DNA damage in the colon, faster tumour onset and increased mortality compared with mice with only one bacterial strain.
Pagès, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353, 2654–2666 (2005).
Sinicrope, F. A. et al. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137, 1270–1279 (2009).
Allen, J. et al. Colon tumors in enterotoxigenic Bacteroides fragilis (ETBF)-colonized mice do not display a unique mutational signature but instead possess host-dependent alterations in the APC gene. Microbiol. Spectr. 10, e0105522 (2022).
Yu, J. et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78 (2017).
Nakatsu, G. et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 6, 8727 (2015).
Degirolamo, C., Modica, S., Palasciano, G. & Moschetta, A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol. Med. 17, 564–572 (2011).
Attard, G., Cooper, C. S. & de Bono, J. S. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell 16, 458–462 (2009).
Tsoi, H. et al. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 152, 1419–1433.e5 (2017).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330 (2019).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Gabrilovich, D. I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 5, 3–8 (2017).
Huang, B., Song, B. & Xu, C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat. Metab. 2, 132–141 (2020).
McDonald, L. C. et al. Clinical Practice Guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 66, e1–e48 (2018).
Farrow, M. A. et al. Clostridium difficile toxin B-induced necrosis is mediated by the host epithelial cell NADPH oxidase complex. Proc. Natl Acad. Sci. USA 110, 18674–18679 (2013).
Bullman, S. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017).
Fukugaiti, M. H. et al. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 46, 1135–1140 (2015).
Magat, E. M. et al. Clostridioides difficile antibody response of colorectal cancer patients versus clinically healthy individuals. Biosci. Microbiota Food Health 39, 123–127 (2020).
Cao, Y. et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 378, eabm3233 (2022). This study identifies a new family of genotoxins called indolimines produced by M. morganii, a gut bacterium associated with CRC.
Brennan, C. A. & Garrett, W. S. Fusobacterium nucleatum — symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166 (2019).
Han, Y. W. et al. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect. Immun. 72, 2272–2279 (2004).
Hajishengallis, G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44 (2015).
Coppenhagen-Glazer, S. et al. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 83, 1104–1113 (2015).
Park, J., Shokeen, B., Haake, S. K. & Lux, R. Characterization of Fusobacterium nucleatum ATCC 23726 adhesins involved in strain-specific attachment to Porphyromonas gingivalis. Int. J. Oral. Sci. 8, 138–144 (2016).
Liu, P.-F. et al. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: implication for treatment of periodontal infection and halitosis. Vaccine 28, 3496–3505 (2010).
Kaplan, C. W., Lux, R., Haake, S. K. & Shi, W. The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol. Microbiol. 71, 35–47 (2009).
Wu, T. et al. Cellular components mediating coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. 94, 1432–1438 (2015).
Yamamura, K. et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 22, 5574–5581 (2016).
Al-Hebshi, N. N. et al. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 7, 1834 (2017).
Zhao, H. et al. Variations in oral microbiota associated with oral cancer. Sci. Rep. 7, 11773 (2017).
Audirac-Chalifour, A. et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS ONE 11, e0153274 (2016).
Kostic, A. D. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 (2012).
Castellarin, M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012).
Abed, J. et al. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell. Infect. Microbiol. 10, 400 (2020).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019).
Kölbl, A. C., Jeschke, U., Friese, K. & Andergassen, U. The role of TF- and Tn-antigens in breast cancer metastasis. Histol. Histopathol. 31, 613–621 (2016).
Patil, S. A. et al. Overexpression of α2,3sialyl T-antigen in breast cancer determined by miniaturized glycosyltransferase assays and confirmed using tissue microarray immunohistochemical analysis. Glycoconj. J. 31, 509–521 (2014).
McCoy, A. N. et al. Fusobacterium is associated with colorectal adenomas. PLoS ONE 8, e53653 (2013).
Bertocchi, A. et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39, 708–724.e11 (2021).
Yang, Y. et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating Toll-like receptor 4 signaling to nuclear factor-κb, and up-regulating expression of microRNA-21. Gastroenterology 152, 851–866.e24 (2017).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563.e16 (2017).
Loesche, W. J. & Gibbons, R. J. Amino acid fermentation by Fusobacterium nucleatum. Arch. Oral. Biol. 13, 191–202 (1968).
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P. & Macfarlane, G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Macia, L. et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6, 6734 (2015).
Chun, E. et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 51, 871–884.e6 (2019).
Lavoie, S. et al. Expression of free fatty acid receptor 2 by dendritic cells prevents their expression of interleukin 27 and is required for maintenance of mucosal barrier and immune response against colorectal tumors in mice. Gastroenterology 158, 1359–1372.e9 (2020).
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Brennan, C. A. et al. Fusobacterium nucleatum drives a pro-inflammatory intestinal microenvironment through metabolite receptor-dependent modulation of IL-17 expression. Gut Microbes 13, 1987780 (2021).
Ternes, D. et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 4, 458–475 (2022). This study uncovers a novel pro-tumorigenic function of the CRC-associated bacterium F. nucleatum by producing formate, a metabolite that triggers AhR signalling further increasing cancer stemness, tumour invasion and TH17 cell expansion.
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
Stanford, E. A. et al. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 14, 20 (2016).
Queen, J. et al. Comparative analysis of colon cancer-derived Fusobacterium nucleatum subspecies: inflammation and colon tumorigenesis in murine models. mBio 13, e0299121 (2022).
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Mitsuhashi, K. et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6, 7209–7220 (2015).
Farrell, J. J. et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Saunders, C. W., Scheynius, A. & Heitman, J. Malassezia fungi are specialized to live on skin and associated with dandruff, eczema, and other skin diseases. PLoS Pathog. 8, e1002701 (2012).
Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Invest. 127, 780–789 (2017).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5 (2018).
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398, 535–554 (2021).
Dickson, R. P., Erb-Downward, J. R., Martinez, F. J. & Huffnagle, G. B. The microbiome and the respiratory tract. Annu. Rev. Physiol. 78, 481–504 (2016).
Willis, A. L., Calton, J. B., Carr, T. F., Chiu, A. G. & Chang, E. H. Dead or alive: deoxyribonuclease I sensitive bacteria and implications for the sinus microbiome. Am. J. Rhinol. Allergy 30, 94–98 (2016).
Segal, L. N. et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 1, 19 (2013).
Remot, A. et al. Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J. 11, 1061–1074 (2017).
Hsu-Kim, C., Hoag, J. B., Cheng, G.-S. & Lund, M. E. The microbiology of postobstructive pneumonia in lung cancer patients. J. Bronchol. Interv. Pulmonol. 20, 266–270 (2013).
Littman, A. J., Jackson, L. A. & Vaughan, T. L. Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol. Biomark. Prev. 14, 773–778 (2005).
Tsay, J.-C. J. et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am. J. Respir. Crit. Care Med. 198, 1188–1198 (2018).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013.e16 (2019).
Gadelle, D., Raibaud, P. & Sacquet, E. β-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl. Environ. Microbiol. 49, 682–685 (1985).
Dabek, M., McCrae, S. I., Stevens, V. J., Duncan, S. H. & Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495 (2008).
Kwa, M., Plottel, C. S., Blaser, M. J. & Adams, S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl. Cancer Inst. 108, djw029 (2016).
Flores, R. et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med. 10, 253 (2012).
Fu, A. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 185, 1356–1372.e26 (2022). This work shows that the tumour-resident intracellular microbiota promotes metastases.
Mikó, E. et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim. Biophys. Acta Bioenerg. 1859, 958–974 (2018).
Thirunavukkarasan, M. et al. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells. PLoS ONE 12, e0186334 (2017).
Ridlon, J. M., Kang, D.-J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Kang, D. Clostridium scindens baiCD and baiH genes encode stereo-specific 7α/7β-hydroxy-3-oxo-Δ4-cholenoic acid oxidoreductases. Biochim. Biophys. Acta 1781, 16–25 (2008).
Pols, T. W. H., Noriega, L. G., Nomura, M., Auwerx, J. & Schoonjans, K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig. Dis. 29, 37–44 (2011).
Terrisse, S. et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021).
Abed, J. et al. Tumor targeting by fusobacterium nucleatum: a pilot study and future perspectives. Front. Cell. Infect. Microbiol. 7, 295 (2017).
Patel, A. R. & Klein, E. A. Risk factors for prostate cancer. Nat. Clin. Pract. Urol. 6, 87–95 (2009).
Chan, J. M., Gann, P. H. & Giovannucci, E. L. Role of diet in prostate cancer development and progression. J. Clin. Oncol. 23, 8152–8160 (2005).
Feng, Y. et al. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genomics 20, 146 (2019).
Cavarretta, I. et al. The microbiome of the prostate tumor microenvironment. Eur. Urol. 72, 625–631 (2017).
Liss, M. A. et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur. Urol. 74, 575–582 (2018).
Golombos, D. M. et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology 111, 122–128 (2018).
Pernigoni, N. et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 374, 216–224 (2021). This study shows that the gut microbiota contributes to the onset of castration-resistant prostate cancer by promoting the expansion of species capable of converting androgen precursors into active androgens.
Terrisse, S. et al. Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J. Immunother. Cancer 10, e004191 (2022).
Matsushita, M. et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 81, 4014–4026 (2021).
Daisley, B. A. et al. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat. Commun. 11, 4822 (2020).
Terrisse, S., Zitvogel, L. & Kroemer, G. Effects of the intestinal microbiota on prostate cancer treatment by androgen deprivation therapy. Microb. Cell Graz Austria 9, 202–206 (2022).
Navarro, M., Nicolas, A., Ferrandez, A. & Lanas, A. Colorectal cancer population screening programs worldwide in 2016: an update. World J. Gastroenterol. 23, 3632 (2017).
Wolf, A. M. D. et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. Ca. Cancer J. Clin. 68, 250–281 (2018).
Kartal, E. et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 71, 1359–1372 (2022).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
McNeil, J. J. et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 379, 1519–1528 (2018).
Chan, A. T., Ogino, S. & Fuchs, C. S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 356, 2131–2142 (2007).
Chan, A. T. et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prev. Res. 5, 164–178 (2012).
Guo, C.-G. et al. Aspirin use and risk of colorectal cancer among older adults. JAMA Oncol. 7, 428 (2021).
Prizment, A. E. et al. Randomised clinical study: oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment. Pharmacol. Ther. 52, 976–987 (2020).
Brennan, C. A. et al. Aspirin modulation of the colorectal cancer-associated microbe Fusobacterium nucleatum. mBio 12, e00547-21 (2021).
Wang, W. H. et al. Aspirin inhibits the growth of Helicobacter pylori and enhances its susceptibility to antimicrobial agents. Gut 52, 490–495 (2003).
Cederlund, H. & Mårdh, P. A. Antibacterial activities of non-antibiotic drugs. J. Antimicrob. Chemother. 32, 355–365 (1993).
Afzal, M. & Shafeeq, S. Impact of aspirin on the transcriptome of Streptococcus pneumoniae D39. Genom. Data 12, 38–40 (2017).
Kunin, C. M., Hua, T. H., Guerrant, R. L. & Bakaletz, L. O. Effect of salicylate, bismuth, osmolytes, and tetracycline resistance on expression of fimbriae by Escherichia coli. Infect. Immun. 62, 2178–2186 (1994).
Rosner, J. L., Chai, T. J. & Foulds, J. Regulation of ompF porin expression by salicylate in Escherichia coli. J. Bacteriol. 173, 5631–5638 (1991).
Kupferwasser, L. I. et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 112, 222–233 (2003).
Sonnenburg, E. D. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Giovannucci, E. & Willett, W. C. Dietary factors and risk of colon cancer. Ann. Med. 26, 443–452 (1994).
Bouvard, V. et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 16, 1599–1600 (2015).
Gurjao, C. et al. Discovery and features of an alkylating signature in colorectal cancer. Cancer Discov. 11, 2446–2455 (2021).
Mehta, R. S. et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 3, 921–927 (2017).
Łusiak-Szelachowska, M., Weber-Dąbrowska, B., Jończyk-Matysiak, E., Wojciechowska, R. & Górski, A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 9, 44 (2017).
Sulakvelidze, A., Alavidze, Z. & Morris, J. G. Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659 (2001).
d’Herelle, F. Bacteriophage as a treatment in acute medical and surgical infections. Bull. N. Y. Acad. Med. 7, 329–348 (1931).
Cieplak, T., Soffer, N., Sulakvelidze, A. & Nielsen, D. S. A bacteriophage cocktail targeting Escherichia coli reduces E. coli in simulated gut conditions, while preserving a non-targeted representative commensal normal microbiota. Gut Microbes 9, 391–399 (2018).
Cuomo, P. et al. An innovative approach to control H. pylori-induced persistent inflammation and colonization. Microorganisms 8, E1214 (2020).
Zheng, D.-W. et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 3, 717–728 (2019).
Zhou, S., Gravekamp, C., Bermudes, D. & Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018).
Lynch, J. P., Goers, L. & Lesser, C. F. Emerging strategies for engineering Escherichia coli Nissle 1917-based therapeutics. Trends Pharmacol. Sci. 43, 772–786 (2022).
Nißle, A. Weiteres über Grundlagen und Praxis der mutaflorbehandlung [German]. Dtsch. Med. Wochenschr. 51, 1809–1813 (1925).
Zhou, Y. & Han, Y. Engineered bacteria as drug delivery vehicles: principles and prospects. Eng. Microbiol. 2, 100034 (2022).
Ho, C. L. et al. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2, 27–37 (2018).
Leventhal, D. S. et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 11, 2739 (2020).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12, eaax0876 (2020).
Sonnenborn, U. & Schulze, J. The non-pathogenic Escherichia coli strain Nissle 1917 — features of a versatile probiotic. Microb. Ecol. Health Dis. 21, 122–158 (2009).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Chowdhury, S. et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 25, 1057–1063 (2019).
Geiger, R. et al. l-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Yu, W. et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32, 466–477 (2021).
Kim, O. Y. et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat. Commun. 8, 626 (2017).
Cheng, L. & Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399 (2022).
Chronopoulos, A. & Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 39, 6951–6960 (2020).
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M. & Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019). This study shows that a consortium of 11 bacterial strains from human faeces induces IFNγ-producing CD8+ T cells in the intestine without causing inflammation and could therefore potentially be used as biotherapeutics to enhance host immunity against infections and cancer.
Luckheeram, R. V., Zhou, R., Verma, A. D. & Xia, B. CD4+ T cells: differentiation and functions. Clin. Dev. Immunol. 2012, 925135 (2012).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Wang, K. et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity 41, 1052–1063 (2014).
Calcinotto, A. et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 9, 4832 (2018).
Atarashi, K. et al. TH17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Tan, T. G. et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal TH17 cells in mice. Proc. Natl Acad. Sci. USA 113, E8141–E8150 (2016).
Overacre-Delgoffe, A. E. et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 54, 2812–2824.e4 (2021).
Chen, M.-L. et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc. Natl Acad. Sci. USA 102, 419–424 (2005).
Daillère, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019).
Yu, A. I. et al. Gut microbiota modulate CD8 T cell responses to influence colitis-associated tumorigenesis. Cell Rep. 31, 107471 (2020).
Nakatsuji, T. et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci. Adv. 4, eaao4502 (2018).
Mrázek, J. et al. Melanoma-related changes in skin microbiome. Folia Microbiol. 64, 435–442 (2019).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155 (2018).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Chehoud, C. et al. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc. Natl Acad. Sci. USA 110, 15061–15066 (2013).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Belkaid, Y. & Segre, J. A. Dialogue between skin microbiota and immunity. Science 346, 954–959 (2014).
Chen, Y. E., Fischbach, M. A. & Belkaid, Y. Skin microbiota–host interactions. Nature 553, 427–436 (2018).
Markle, J. G. M. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013). This study shows that early-life microbial exposures can affect the progression of type 1 diabetes in male mice by regulating sex hormone levels.
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Vemuri, R. et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin. Immunopathol. 41, 265–275 (2019).
Schwabe, R. F. & Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013).
Plottel, C. S. & Blaser, M. J. Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011).
Colldén, H. et al. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiol. Endocrinol. Metab. 317, E1182–E1192 (2019).
Pala, L. et al. Sex differences in efficacy and toxicity of systemic cancer treatments: role of the microbiome. J. Clin. Oncol. 37, 439 (2019).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752.e20 (2021).
Rao, A., Barkley, D., França, G. S. & Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 596, 211–220 (2021).
Dar, D., Dar, N., Cai, L. & Newman, D. K. Spatial transcriptomics of planktonic and sessile bacterial populations at single-cell resolution. Science 373, eabi4882 (2021).
Shi, H. et al. Highly multiplexed spatial mapping of microbial communities. Nature 588, 676–681 (2020).
Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Acknowledgements
This work is supported by National Institutes of Health (NIH) grant R01CA154426 and the Cancer Research UK Grand Challenge Initiative C10674/A27140 to W.S.G. G.E.T. is the recipient of a European Molecular Biology Organization (EMBO) Postdoctoral Fellowship (ALTF 1020-2021). The authors thank all Garrett laboratory members for helpful discussions and contributions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
W.S.G. is on the scientific advisory board of Freya Biosciences, Scipher Medicine and Senda Biosciences, all outside the current work. W.S.G.’s laboratory receives funding from Merck and Astellas. G.E.T. declares no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Maria Rescigno, Bertrand Routy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- γ-H2AX
-
A sensitive marker of DNA damage, as phosphorylation of H2AX is required for the assembly of the DNA double-strand repair machinery.
- Apc Min/+ mouse model
-
Mice carrying a heterogeneous mutation in the commonly mutated (more than 80% of patients with colon cancer) adenomatous polyposis coli (APC) gene that develop spontaneous intestinal adenomas.
- Azoxymethane (AOM)–dextran sodium sulfate (DSS) mouse model
-
A chemically inducible mouse model of colitis-associated colon cancer, where mice are treated with AOM, a jet fuel-derived mutagenic agent that damages the DNA of colonic epithelial cells, followed by three cycles of mucosal disruptant DSS.
- Bacterial species
-
Bacteria sharing common genomic features and exhibiting a high degree of similarity in phenotype.
- Bacterial strain
-
A genetic variant of a particular species of bacteria.
- Bacteriophages
-
Viruses that infect and replicate within bacteria.
- Biogeography
-
Localization at particular body sites.
- Caecal microbiota transplant
-
(CMT). The transfer of caecal contents and microorganisms therein from a donor to a recipient host.
- Faecal microbiota transplant
-
(FMT). The transfer of the microorganisms from the stool of a donor to a recipient.
- Familial adenomatous polyposis
-
(FAP). A rare, autosomal dominant syndrome, involving the adenomatous polyposis coli (APC) gene, that predisposes an individual to tumours of the colon and rectum.
- Genotoxic
-
A property that induces genetic damage (DNA mutation) within a cell.
- Gnotobiotics
-
A specialized microbially controlled animal husbandry practice enabling experiments in which animals can be kept completely devoid of microorganisms or with defined microbial communities.
- Gram-negative
-
A description of a bacterium that harbours an outer lipid membrane and does not retain crystal violet staining (Gram staining).
- Gram-positive
-
A description of a bacterium that does not harbour an outer lipid membrane and thus retains crystal violet staining (Gram staining).
- Microbiome
-
The collection of microorganisms (archaea, bacteria, fungi, protists and viruses) that inhabit a specific environment.
- Mutational signature
-
The combination of mutations emerging from DNA damage and repair processes.
- Oncomicrobe
-
Microorganisms with established features that influence cancer susceptibility and therapeutic response.
- Symbionts
-
Organisms living in a neutral or beneficial way with their host.
- Theranostic
-
The combination of therapeutics and diagnostics.
- Type 3 secretion system
-
A bacterial complex or injectisome widely used by gram-negative bacteria to inject their effector molecules or toxins into host cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Tekle, G., Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat Rev Cancer 23, 600–618 (2023). https://doi.org/10.1038/s41568-023-00594-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00594-2
This article is cited by
-
The microbiome as a determinant of racial and ethnic cancer disparities
Nature Reviews Cancer (2024)
-
New pathogen on the block
Nature Reviews Cancer (2024)