Abstract
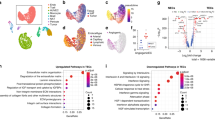
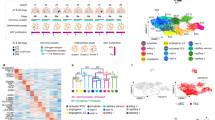
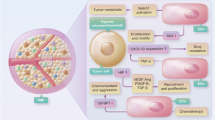
Anti-angiogenic therapies (AATs) are used to treat different types of cancers. However, their success is limited owing to insufficient efficacy and resistance. Recently, single-cell omics studies of tumour endothelial cells (TECs) have provided new mechanistic insight. Here, we overview the heterogeneity of human TECs of all tumour types studied to date, at the single-cell level. Notably, most human tumour types contain varying numbers but only a small population of angiogenic TECs, the presumed targets of AATs, possibly contributing to the limited efficacy of and resistance to AATs. In general, TECs are heterogeneous within and across all tumour types, but comparing TEC phenotypes across tumours is currently challenging, owing to the lack of a uniform nomenclature for endothelial cells and consistent single-cell analysis protocols, urgently raising the need for a more consistent approach. Nonetheless, across most tumour types, universal TEC markers (ACKR1, PLVAP and IGFBP3) can be identified. Besides angiogenesis, biological processes such as immunomodulation and extracellular matrix organization are among the most commonly predicted enriched signatures of TECs across different tumour types. Although angiogenesis and extracellular matrix targets have been considered for AAT (without the hoped success), the immunomodulatory properties of TECs have not been fully considered as a novel anticancer therapeutic approach. Therefore, we also discuss progress, limitations, solutions and novel targets for AAT development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Markers and functions of TECs were extracted from the original referenced work. The summarized tables for plotting Fig. 2a and the source code used in this paper and written by Q.Z. are available as Supplementary Information.
References
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 (2007).
Potente, M. & Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 18, 477–494 (2017).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science https://doi.org/10.1126/science.aal2379 (2017).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Jayson, G. C., Kerbel, R., Ellis, L. M. & Harris, A. L. Antiangiogenic therapy in oncology: current status and future directions. Lancet 388, 518–529 (2016).
Garcia, J. et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017 (2020).
Yang, Y. et al. Anti-VEGF– and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc. Natl Acad. Sci. USA 110, 12018–12023 (2013).
Cao, Y. VEGF-targeted cancer therapeutics — paradoxical effects in endocrine organs. Nat. Rev. Endocrinol. 10, 530–539 (2014).
Goel, S., Wong, A. H.-K. & Jain, R. K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a006486 (2012).
Cao, Y. Off-tumor target — beneficial site for antiangiogenic cancer therapy. Nat. Rev. Clin. Oncol. 7, 604–608 (2010).
Xue, Y. et al. Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc. Natl Acad. Sci. USA 105, 18513–18518 (2008).
Bergers, G. & Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 (2008).
Gacche, R. N. & Assaraf, Y. G. Redundant angiogenic signaling and tumor drug resistance. Drug. Resist. Updat. 36, 47–76 (2018).
Carmeliet, P., De Smet, F., Loges, S. & Mazzone, M. Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat. Rev. Clin. Oncol. 6, 315–326 (2009).
Jakobsson, L. et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943–953 (2010).
Croix, B. S. et al. Genes expressed in human tumor endothelium. Science 289, 1197–1202 (2000).
Bussolati, B., Deambrosis, I., Russo, S., Deregibus, M. C. & Camussi, G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 17, 1159–1161 (2003).
Ohga, N. et al. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 180, 1294–1307 (2012).
Kikuchi, H. et al. Chemotherapy-induced IL8 upregulates MDR1/ABCB1 in tumor blood vessels and results in unfavorable outcome. Cancer Res. 80, 2996–3008 (2020).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e20 (2020). This work pioneers an atlas of EC heterogeneity in the whole body of healthy mice.
Paik, D. T. et al. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation 142, 1848–1862 (2020).
Jones, R. C. et al. The tabula sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Wang, F. et al. Endothelial cell heterogeneity and microglia regulons revealed by a pig cell landscape at single-cell level. Nat. Commun. 13, 3620 (2022).
Goveia, J. et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell 37, 21–36.e13 (2020). This work for the first time characterizes ECs in depth specifically in lung cancer and validates bioinformatic findings by integrated approaches.
Geldhof, V. et al. Single cell atlas identifies lipid-processing and immunomodulatory endothelial cells in healthy and malignant breast. Nat. Commun. 13, 5511 (2022). This work for the first time systematically analyses EC heterogeneity at single-cell resolution in breast cancer.
Schupp, J. C. et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 144, 286–302 (2021).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Becker, L. M. et al. Deciphering endothelial heterogeneity in health and disease at single cell resolution: progress and perspectives. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvac018 (2022).
Cavalli, M. et al. A multi-omics approach to liver diseases: integration of single nuclei transcriptomics with proteomics and HiCap bulk data in human liver. OMICS 24, 180–194 (2020).
Wang, H. et al. Integrative single-cell transcriptome analysis reveals a subpopulation of fibroblasts associated with favorable prognosis of liver cancer patients. Transl. Oncol. 14, 100981 (2021).
Zhang, Y. et al. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Lett. 470, 84–94 (2020).
Zhao, Q. et al. Heterogeneity and chimerism of endothelial cells revealed by single-cell transcriptome in orthotopic liver tumors. Angiogenesis 23, 581–597 (2020).
Sharma, A. et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell 183, 377–394.e21 (2020). This work identifies a conserved EC population in liver cancer and the fetal liver and demonstrates its important role in contributing to the immunosuppressive TME.
Massalha, H. et al. A single cell atlas of the human liver tumor microenvironment. Mol. Syst. Biol. 16, e9682 (2020).
Aizarani, N. et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 572, 199–204 (2019).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147 (2022).
Xing, X. & Song, J. Identification of the different gene expression characteristics from liver cirrhosis to hepatocellular carcinoma using single-cell sequencing analyses. J. Immunol. Res. 2021, 6619302 (2021).
Ioannidou, S. et al. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc. Natl Acad. Sci. USA 103, 16770–16775 (2006).
Stan, R. V. et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev. Cell 23, 1203–1218 (2012).
Jones, J. H., Friedrich, E., Hong, Z., Minshall, R. D. & Malik, A. B. PV1 in caveolae controls lung endothelial permeability. Am. J. Respir. Cell Mol. Biol. 63, 531–539 (2020).
Rantakari, P. et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 16, 386–396 (2015).
Keuschnigg, J. et al. The prototype endothelial marker PAL-E is a leukocyte trafficking molecule. Blood 114, 478–484 (2009).
Strickland, L. A. et al. Plasmalemmal vesicle-associated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF). J. Pathol. 206, 466–475 (2005).
Li, Z. et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur. Heart J. 40, 2507–2520 (2019).
Wang, Y. et al. Plasmalemma vesicle-associated protein promotes angiogenesis in cholangiocarcinoma via the DKK1/CKAP4/PI3K signaling pathway. Oncogene 40, 4324–4337 (2021).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Wang, Y.-H. et al. Plasmalemmal vesicle associated protein (PLVAP) as a therapeutic target for treatment of hepatocellular carcinoma. BMC Cancer 14, 815 (2014).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/ct2/show/NCT04601428 (2020).
Poisson, J. et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66, 212–227 (2017).
Thomann, S. et al. YAP orchestrates heterotypic endothelial cell communication via HGF/c-MET signaling in liver tumorigenesis. Cancer Res. 80, 5502–5514 (2020).
Browaeys, R., Saelens, W. & Saeys, Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020).
Wang, B. et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 4, 19–29 (2003).
Raza, A., Franklin, M. J. & Dudek, A. Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 85, 593–598 (2010).
Pitulescu, M. E. et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat. Cell Biol. 19, 915–927 (2017).
Lee, H. O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52, 594–603 (2020).
Qian, J. et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 30, 745–762 (2020). This work is an ‘antetype’ of a human tumour EC atlas and compares ECs from three different types of cancer.
Rohlenova, K. et al. Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab. 31, 862–877.e14 (2020).
Thiriot, A. et al. Differential DARC/ACKR1 expression distinguishes venular from non-venular endothelial cells in murine tissues. BMC Biol. 15, 45 (2017).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184, 4734–4752.e20 (2021).
Che, L.-H. et al. A single-cell atlas of liver metastases of colorectal cancer reveals reprogramming of the tumor microenvironment in response to preoperative chemotherapy. Cell Discov. 7, 80 (2021).
Li, X., Kumar, A. & Carmeliet, P. Metabolic pathways fueling the endothelial cell drive. Annu. Rev. Physiol. 81, 483–503 (2019).
Kuipers, E. J. et al. Colorectal cancer. Nat. Rev. Dis. Prim. 1, 15065 (2015).
Lin, W. et al. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions. Genome Med. 12, 80 (2020).
Wang, X. et al. Single-cell RNA-seq reveals the genesis and heterogeneity of tumor microenvironment in pancreatic undifferentiated carcinoma with osteoclast-like giant-cells. Mol. Cancer 21, 133 (2022).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Chen, K. et al. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. eBioMedicine https://doi.org/10.1016/j.ebiom.2021.103315 (2021).
Zhou, Y. et al. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 17, 3760–3775 (2021).
Chen, K. et al. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. Int. J. Biol. Sci. 18, 1220–1237 (2022).
Shiau, C. et al. Treatment-associated remodeling of the pancreatic cancer endothelium at single-cell resolution. Front. Oncol. https://doi.org/10.3389/fonc.2022.929950 (2022).
Abdel-Wahab, R. et al. Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma. J. Hematol. Oncol. 11, 71 (2018).
Mutgan, A. C. et al. Insulin/IGF-driven cancer cell-stroma crosstalk as a novel therapeutic target in pancreatic cancer. Mol. Cancer 17, 66 (2018).
Baxter, R. C. IGF binding proteins in cancer: mechanistic and clinical insights. Nat. Rev. Cancer 14, 329–341 (2014).
Kundranda, M. et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann. Oncol. 31, 79–87 (2020).
Fuchs, C. S. et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial†. Ann. Oncol. 26, 921–927 (2015).
Choi, K. J., Nam, J.-K., Kim, J.-H., Choi, S.-H. & Lee, Y.-J. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp. Mol. Med. 52, 781–792 (2020).
Choi, S.-H. et al. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6 + cancer cell and macrophage polarization. Nat. Commun. 9, 5108 (2018).
Schlesinger, Y. et al. Single-cell transcriptomes of pancreatic preinvasive lesions and cancer reveal acinar metaplastic cells’ heterogeneity. Nat. Commun. 11, 4516 (2020).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 (2020).
Fox, J. G. & Wang, T. C. Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 117, 60–69 (2007).
Yin, H. et al. A dynamic transcriptome map of different tissue microenvironment cells identified during gastric cancer development using single-cell RNA sequencing. Front. Immunol. https://doi.org/10.3389/fimmu.2021.728169 (2021). This work is a good example of how one could take advantage of publicly available data and gain insights into the dynamic changes of ECs during cancer development.
Jiang, H. et al. Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA Sequencing. Clin. Transl. Med. 12, e730 (2022).
Jeong, H. Y. et al. Spatially distinct reprogramming of the tumor microenvironment based on tumor invasion in diffuse-type gastric cancers. Clin. Cancer Res. 27, 6529–6542 (2021).
Li, Y. et al. Single-cell landscape reveals active cell subtypes and their interaction in the tumor microenvironment of gastric cancer. Theranostics 12, 3818–3833 (2022).
Sun, K. et al. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat. Commun. 13, 4943 (2022).
Li, X. et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics 12, 620–638 (2022).
Yu, X. et al. Tumor vessel normalization and immunotherapy in gastric cancer. Ther. Adv. Med. Oncol. 14, 17588359221110176 (2022).
Zhang, X. et al. Dissecting esophageal squamous-cell carcinoma ecosystem by single-cell transcriptomic analysis. Nat. Commun. 12, 5291 (2021).
Nagl, L., Horvath, L., Pircher, A. & Wolf, D. Tumor endothelial cells (TECs) as potential immune directors of the tumor microenvironment — new findings and future perspectives. Front. Cell Dev. Biol. 8, 766–766 (2020).
Amersfoort, J., Eelen, G. & Carmeliet, P. Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00694-4 (2022). This review article comprehensively revisits the immunomodulatory function of ECs in different organs and diseases.
Saharinen, P., Eklund, L. & Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discov. 16, 635–661 (2017).
Chen, Y. T. et al. Platelet-derived growth factor receptor signaling activates pericyte–myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80, 1170–1181 (2011).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT04879368 (2021).
Chen, Z. et al. Dissecting the single-cell transcriptome network in patients with esophageal squamous cell carcinoma receiving operative paclitaxel plus platinum chemotherapy. Oncogenesis 10, 71–71 (2021).
Huinen, Z. R., Huijbers, E. J. M., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents — overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/s41571-021-00496-y (2021). This is a comprehensive review article of the clinical applications of AAT and immunotherapy combinations.
Ma, J. & Waxman, D. J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 7, 3670–3684 (2008).
Kapoor, H., Lohani, K. R., Lee, T. H., Agrawal, D. K. & Mittal, S. K. Animal models of Barrett’s esophagus and esophageal adenocarcinoma — past, present, and future. Clin. Transl. Sci. 8, 841–847 (2015).
Nowicki-Osuch, K. et al. Molecular phenotyping reveals the identity of Barrett’s esophagus and its malignant transition. Science 373, 760–767 (2021).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Peleli, M., Moustakas, A. & Papapetropoulos, A. Endothelial–tumor cell interaction in brain and CNS malignancies. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21197371 (2020).
Xie, Y. et al. Key molecular alterations in endothelial cells in human glioblastoma uncovered through single-cell RNA sequencing. JCI Insight https://doi.org/10.1172/jci.insight.150861 (2021). This work comprehensively characterizes EC heterogeneity in glioblastoma and demonstrates distinct TEC phenotypes in the tumour core.
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05496595 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04396717 (2022).
Bosma, E. K., van Noorden, C. J. F., Schlingemann, R. O. & Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood–brain and blood–retinal barriers: potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 15, 24 (2018).
Soda, Y. et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA 108, 4274–4280 (2011).
Wei, X. et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer 20, 7 (2021).
Wälchli, T. et al. Shaping the brain vasculature in development and disease in the single-cell era. Nat. Rev. Neurosci. 24, 271–298 (2023).
Jain, S., Chalif, E. J. & Aghi, M. K. Interactions between anti-angiogenic therapy and immunotherapy in Glioblastoma. Front. Oncol. https://doi.org/10.3389/fonc.2021.812916 (2022).
Zhang, M. et al. Anti-vascular endothelial growth factor therapy in breast cancer: molecular pathway, potential targets, and current treatment strategies. Cancer Lett. 520, 422–433 (2021).
Cejuela, M., Martin-Castillo, B., Menendez, J. A. & Pernas, S. Metformin and breast cancer: where are we now? Int. J. Mol. Sci. 23, 2705 (2022).
Sun, Z. et al. Single-cell RNA sequencing reveals gene expression signatures of breast cancer-associated endothelial cells. Oncotarget 9, 10945–10961 (2018).
Wang, W., Wang, L., She, J. & Zhu, J. Examining heterogeneity of stromal cells in tumor microenvironment based on pan-cancer single-cell RNA sequencing data. Cancer Biol. Med. 19, 30–42 (2021).
Wu, S. Z. et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 53, 1334–1347 (2021).
Hua, Y. et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1 + T lymphocyte niches through a feed-forward loop. Cancer Cell https://doi.org/10.1016/j.ccell.2022.11.002 (2022).
Li, Q. et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to PD1 blockade. Clin. Cancer Res. 26, 1712–1724 (2020).
Heidegger, I. et al. Comprehensive characterization of the prostate tumor microenvironment identifies CXCR4/CXCL12 crosstalk as a novel antiangiogenic therapeutic target in prostate cancer. Mol. Cancer 21, 132 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05465590 (2022).
Chen, S. et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 23, 87–98 (2021).
Ioannidou, E. et al. Angiogenesis and anti-angiogenic treatment in prostate cancer: mechanisms of action and molecular targets. Int. J. Mol. Sci. 22, 9926 (2021).
Kelly, W. K. et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 30, 1534–1540 (2012).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05000294 (2021).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05489211 (2022).
Kim, N. et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 11, 2285 (2020).
Lambrechts, D. et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24, 1277–1289 (2018).
Lu, T. et al. Single-cell transcriptome atlas of lung adenocarcinoma featured with ground glass nodules. Cell Discov. 6, 69 (2020).
Ma, K. Y. et al. Single-cell RNA sequencing of lung adenocarcinoma reveals heterogeneity of immune response-related genes. JCI Insight https://doi.org/10.1172/jci.insight.121387 (2019).
Zhang, F. et al. Dynamics of peripheral T cell clones during PD-1 blockade in non-small cell lung cancer. Cancer Immunol. Immunother. 69, 2599–2611 (2020).
Zhong, R. et al. Immune cell infiltration features and related marker genes in lung cancer based on single-cell RNA-seq. Clin. Transl. Oncol. 23, 405–417 (2021).
Raemer, P. C. et al. Endothelial progenitor cells possess monocyte-like antigen-presenting and T-cell-co-stimulatory capacity. Transplantation 87, 340–349 (2009).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01332721 (2011).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/ct2/show/NCT03780010 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/ct2/show/NCT03181308 (2017).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/ct2/show/NCT05401110 (2022).
Girard, J.-P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Bischoff, P. et al. Single-cell RNA sequencing reveals distinct tumor microenvironmental patterns in lung adenocarcinoma. Oncogene 40, 6748–6758 (2021).
Moran, T. et al. Activity of dalotuzumab, a selective anti-IGF1R antibody, in combination with erlotinib in unselected patients with non-small-cell lung cancer: a phase I/II randomized trial. Exp. Hematol. Oncol. 3, 1 (2014).
Huang, C. H. et al. Impact study: MK-0646 (dalotuzumab), insulin growth factor 1 receptor antibody combined with pemetrexed and cisplatin in stage IV metastatic non-squamous lung cancer. Front. Oncol. https://doi.org/10.3389/fonc.2015.00301 (2016).
Langer, C. J. et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 32, 2059–2066 (2014).
Molema, G., Zijlstra, J. G., van Meurs, M. & Kamps, J. A. A. M. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury. Nat. Rev. Nephrol. 18, 95–112 (2022).
Dumas, S. J. et al. Single-cell RNA sequencing reveals renal endothelium heterogeneity and metabolic adaptation to water deprivation. J. Am. Soc. Nephrol. 31, 118–138 (2020).
Zhang, Y. et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proc. Natl Acad. Sci. USA 118, e2103240118 (2021). This work adopts ambitious strategies combining scRNA-seq data of RCC and patient survival and response to an immunotherapy to discover the prognostic and predictive values of PLVAP+ EC signatures.
Young, M. D. et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361, 594–599 (2018).
US National Library of Medicine. ClinicalTrials.gov, https://ClinicalTrials.gov/ct2/show/NCT04205227 (2019).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Shi, Y. et al. Decoding the multicellular ecosystem of vena caval tumor thrombus in clear cell renal cell carcinoma by single-cell RNA sequencing. Genome Biol. 23, 87 (2022).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 175, 984–997.e24 (2018).
Li, J. et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin. Cancer Res. 28, 2131–2146 (2022).
Ji, A. L. et al. Multimodal analysis of composition and spatial architecture in human squamous cell carcinoma. Cell 182, 497–514.e22 (2020).
Durante, M. A. et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 11, 496 (2020).
Liu, X. et al. Single-cell RNA-sequencing reveals lineage-specific regulatory changes of fibroblasts and vascular endothelial cells in keloids. J. Invest. Dermatol. 142, 124–135.e111 (2022).
Brosseau, J. P. et al. Human cutaneous neurofibroma matrisome revealed by single-cell RNA sequencing. Acta Neuropathol. Commun. 9, 11 (2021).
Miao, Y. et al. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell 177, 1172–1186.e1114 (2019).
Chen, G. S. et al. CXC chemokine receptor CXCR4 expression enhances tumorigenesis and angiogenesis of basal cell carcinoma. Br. J. Dermatol. 154, 910–918 (2006).
Saxena, R., Wang, Y. & Mier, J. W. CXCR4 inhibition modulates the tumor microenvironment and retards the growth of B16-OVA melanoma and Renca tumors. Melanoma Res. 30, 14–25 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/ct2/show/NCT02823405 (2016).
Andtbacka, R. H. I. et al. Mavorixafor, an orally bioavailable CXCR4 antagonist, increases immune cell infiltration and inflammatory status of tumor microenvironment in patients with melanoma. Cancer Res. Commun. 2, 904–913 (2022).
Pu, W. et al. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat. Commun. 12, 6058 (2021).
Melaccio, A. et al. Prognostic and therapeutic role of angiogenic microenvironment in thyroid cancer. Cancers 13, 2775 (2021).
Pan, J. et al. Papillary thyroid carcinoma landscape and its immunological link with hashimoto thyroiditis at single-cell resolution. Front. Cell Dev. Biol. 9, 758339 (2021).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Yang, Z. et al. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. 12, 51 (2021).
Lee, Y. H. et al. Is male gender a prognostic factor for papillary thyroid microcarcinoma. Ann. Surg. Oncol. 24, 1958–1964 (2017).
Peng, M. et al. Single‐cell transcriptomic landscape reveals the differences in cell differentiation and immune microenvironment of papillary thyroid carcinoma between genders. Cell Biosci. 11, 39 (2021).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
GRANATA, R. et al. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J. Thromb. Haemost. 5, 835–845 (2007).
Kim, J.-H. et al. Antiangiogenic antitumor activities of IGFBP-3 are mediated by IGF-independent suppression of Erk1/2 activation and Egr-1-mediated transcriptional events. Blood 118, 2622–2631 (2011).
Dallinga, M. G. et al. IGF-binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol. Biol. Rep. 47, 2561–2572 (2020).
Anwar, A., Zahid, A. A., Scheidegger, K. J., Brink, M. & Delafontaine, P. Tumor necrosis factor-α regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation 105, 1220–1225 (2002).
Horton, L. W., Yu, Y., Zaja-Milatovic, S., Strieter, R. M. & Richmond, A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 67, 9791–9799 (2007).
Jenkins, B. D. et al. Atypical chemokine receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African ancestry. Cancer Epidemiol. Biomark. Prev. 28, 690–700 (2019).
Massara, M., Bonavita, O., Mantovani, A., Locati, M. & Bonecchi, R. Atypical chemokine receptors in cancer: friends or foes? J. Leukoc. Biol. 99, 927–933 (2016).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Jiang, Y. et al. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 15, 34 (2022).
Luo, H. et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 13, 6619 (2022).
Emmerich, C. H. et al. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Discov. 20, 64–81 (2021). This work systematically assesses the processes of translational research and provides helpful insights.
Su, Y. et al. Multi-omic single-cell snapshots reveal multiple independent trajectories to drug tolerance in a melanoma cell line. Nat. Commun. 11, 2345 (2020).
Ding, J., Sharon, N. & Bar-Joseph, Z. Temporal modelling using single-cell transcriptomics. Nat. Rev. Genet. 23, 355–368 (2022).
Bassez, A. et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med. 27, 820–832 (2021).
Miao, Z., Humphreys, B. D., McMahon, A. P. & Kim, J. Multi-omics integration in the age of million single-cell data. Nat. Rev. Nephrol. 17, 710–724 (2021).
Erhard, F. et al. scSLAM-seq reveals core features of transcription dynamics in single cells. Nature 571, 419–423 (2019).
Hendriks, G. J. et al. NASC-seq monitors RNA synthesis in single cells. Nat. Commun. 10, 3138 (2019).
Qiu, Q. et al. Massively parallel and time-resolved RNA sequencing in single cells with scNT-seq. Nat. Methods 17, 991–1001 (2020).
Chen, W. et al. Live-seq enables temporal transcriptomic recording of single cells. Nature 608, 733–740 (2022).
Moses, L. & Pachter, L. Museum of spatial transcriptomics. Nat. Methods 19, 534–546 (2022).
Liu, B., Li, Y. & Zhang, L. Analysis and visualization of spatial transcriptomic data. Front. Genet. https://doi.org/10.3389/fgene.2021.785290 (2022).
Xia, C., Fan, J., Emanuel, G., Hao, J. & Zhuang, X. Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc. Natl Acad. Sci. USA 116, 19490–19499 (2019).
Eng, C. L. et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568, 235–239 (2019).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 185, 1777–1792.e21 (2022).
Atta, L. & Fan, J. Computational challenges and opportunities in spatially resolved transcriptomic data analysis. Nat. Commun. 12, 5283 (2021).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Peterson, V. M. et al. Multiplexed quantification of proteins and transcripts in single cells. Nat. Biotechnol. 35, 936–939 (2017).
Katzenelenbogen, Y. et al. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 182, 872–885.e19 (2020).
Liu, Y. et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell 183, 1665–1681.e18 (2020).
Ma, A., McDermaid, A., Xu, J., Chang, Y. & Ma, Q. Integrative methods and practical challenges for single-cell multi-omics. Trends Biotechnol. 38, 1007–1022 (2020).
Ginhoux, F., Yalin, A., Dutertre, C. A. & Amit, I. Single-cell immunology: past, present, and future. Immunity 55, 393–404 (2022).
Gault, J. et al. Combining native and ‘omics’ mass spectrometry to identify endogenous ligands bound to membrane proteins. Nat. Methods 17, 505–508 (2020).
Specht, H. et al. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biol. 22, 50 (2021).
Touat-Hamici, Z. et al. Role of lipid phosphate phosphatase 3 in human aortic endothelial cell function. Cardiovasc. Res. 112, 702–713 (2016).
Luo, X., Chen, X., Wang, L., Yang, B. & Cai, S. Metformin adjunct with antineoplastic agents for the treatment of lung cancer: a meta-analysis of randomized controlled trials and observational cohort studies. Front. Pharmacol. https://doi.org/10.3389/fphar.2021.639016 (2021).
Su, C. et al. Single-cell RNA sequencing in multiple pathologic types of renal cell carcinoma revealed novel potential tumor-specific markers. Front. Oncol. 11, 719564 (2021).
Lex, A., Gehlenborg, N., Strobelt, H., Vuillemot, R. & Pfister, H. UpSet: visualization of intersecting sets. IEEE Trans. Vis. Comput. Graph. 20, 1983–1992 (2014).
Swanson, E. et al. Simultaneous trimodal single-cell measurement of transcripts, epitopes, and chromatin accessibility using TEA-seq. eLife 10, e63632 (2021).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Chen, G., Ning, B. & Shi, T. Single-cell RNA-seq technologies and related computational data analysis. Front. Genet. https://doi.org/10.3389/fgene.2019.00317 (2019).
Haque, A., Engel, J., Teichmann, S. A. & Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 9, 75 (2017).
Hwang, B., Lee, J. H. & Bang, D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 50, 1–14 (2018).
Mereu, E. et al. Benchmarking single-cell RNA-sequencing protocols for cell atlas projects. Nat. Biotechnol. 38, 747–755 (2020).
Luecken, M. D. et al. Benchmarking atlas-level data integration in single-cell genomics. Nat. Methods 19, 41–50 (2022).
Jin, S. et al. Inference and analysis of cell–cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Ubezio, B. et al. Synchronization of endothelial Dll4-Notch dynamics switch blood vessels from branching to expansion. eLife 5, e12167 (2016).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
Lin, C. & Bar-Joseph, Z. Continuous-state HMMs for modeling time-series single-cell RNA-Seq data. Bioinformatics 35, 4707–4715 (2019).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Trimm, E. & Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-022-00770-1 (2022).
Vanlandewijck, M. & Betsholtz, C. in Lymphangiogenesis: Methods and Protocols (eds Guillermo, O. & Mark, L. K.) 309–324 (Springer, 2018).
Sauler, M. et al. Characterization of the COPD alveolar niche using single-cell RNA sequencing. Nat. Commun. 13, 494 (2022).
Vila Ellis, L. et al. Epithelial Vegfa specifies a distinct endothelial population in the mouse lung. Dev. Cell 52, 617–630.e6 (2020).
Gillich, A. et al. Capillary cell-type specialization in the alveolus. Nature 586, 785–789 (2020).
Vidal, R. et al. Transcriptional heterogeneity of fibroblasts is a hallmark of the aging heart. JCI Insight https://doi.org/10.1172/jci.insight.131092 (2019).
Davies, P., Jones, M., Liu, J. & Hebenstreit, D. Anti-bias training for (sc)RNA-seq: experimental and computational approaches to improve precision. Brief. Bioinform. https://doi.org/10.1093/bib/bbab148 (2021).
Kim, J. K., Kolodziejczyk, A. A., Ilicic, T., Teichmann, S. A. & Marioni, J. C. Characterizing noise structure in single-cell RNA-seq distinguishes genuine from technical stochastic allelic expression. Nat. Commun. 6, 8687 (2015).
Kharchenko, P. V., Silberstein, L. & Scadden, D. T. Bayesian approach to single-cell differential expression analysis. Nat. Methods 11, 740–742 (2014).
Tran, H. T. N. et al. A benchmark of batch-effect correction methods for single-cell RNA sequencing data. Genome Biol. 21, 12 (2020).
Kiselev, V. Y., Andrews, T. S. & Hemberg, M. Challenges in unsupervised clustering of single-cell RNA-seq data. Nat. Rev. Genet. 20, 273–282 (2019).
Zhang, M. et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol. 73, 1118–1130 (2020).
Maynard, A. et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell 182, 1232–1251.e22 (2020).
Ilicic, T. et al. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 17, 29 (2016).
Acknowledgements
The work is supported by the Biomedical Science Discovery Program between the KU Leuven Institution and Khalifa University (Project code: 8434000456).
Author information
Authors and Affiliations
Contributions
Q.Z., L.F., M.M., H.A., A.S.N. and F.Y.A. researched data for the article. Q.Z., L.F., M.M., H.A., A.S.N., F.Y.A., H.A.S. and P.C. contributed substantially to discussion of the content and wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Yihai Cao, Ankur Sharma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Carmeliet laboratory: https://carmelietlab.sites.vib.be/en/software-tools/
UpSetR software: https://gehlenborglab.shinyapps.io/upsetr/
Supplementary information
Glossary
- Angiocrine signalling
-
The paracrine or juxtacrine signalling between endothelial cells and the neighbouring cells to regulate tissue growth and repair.
- Angiogenesis
-
The formation of new blood vessels from existing ones.
- Batch effects
-
Systematic variations in experimental measurements that are not caused by the biological factors, but rather by technical factors such as differences in experimental conditions, instruments, reagents or equipment.
- Chromium single-cell-fixed RNA profiling
-
A high-throughput single-cell gene expression profiling technique that uses oligonucleotide-conjugated antibodies to capture and barcode individual cells in fixed tissue samples.
- Cytometry by time of flight
-
(CyTOF). A single-cell analysis technique that combines flow cytometry with mass spectrometry and differentiates the metal isotype-labelled antibodies by the time of flight.
- Doublet cells
-
Two or more aggregated cells that are encapsulated into one reaction volume and tagged by the same barcode during a single-cell RNA sequencing experiment.
- Duplex RNA in situ hybridization
-
A technique used to detect and visualize two RNA molecules simultaneously within a single sample.
- Fenestral diaphragms
-
A thin protein barrier anchored in the fenestrae that is found in endothelial cells containing multiple small circular openings.
- Gene set variation analysis
-
A computational method to calculate the gene enrichment score of a pathway in samples.
- Genome-scale metabolic models
-
A mathematical modelling approach that predicts the metabolic network reconstructions, metabolic pathways and metabolite production rates of an organism.
- Hierarchical clustering
-
A computational method to group similar cells and form a hierarchy of clusters.
- In silico lineage tracing
-
A computational method to determine cell lineage and fate of individual cells on the basis of their gene expression profiles and/or epigenetic markers.
- Kupffer cells
-
Specialized liver macrophages involved in maintaining liver homeostasis.
- Mural cell
-
Specialized cells found in the walls of blood vessels, including vascular smooth muscle cells and pericytes.
- RNA velocity
-
A computational method used to predict the direction and speed of cell differentiation by analysing the spliced and unspliced RNA molecules.
- Shear stress
-
The parallel force applied on the endothelial surface of the blood vessel by flowing blood.
- Vesicular transcytosis
-
The transportation of macromolecules from one side of an epithelial or endothelial cell to the other side through vesicles.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Q., Mousa, M., Nadukkandy, A.S. et al. Understanding tumour endothelial cell heterogeneity and function from single-cell omics. Nat Rev Cancer 23, 544–564 (2023). https://doi.org/10.1038/s41568-023-00591-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00591-5
This article is cited by
-
Single cell atlas of kidney cancer endothelial cells reveals distinct expression profiles and phenotypes
BJC Reports (2024)
-
Rebuilding the microenvironment of primary tumors in humans: a focus on stroma
Experimental & Molecular Medicine (2024)
-
CAFs vs. TECs: when blood feuds fuel cancer progression, dissemination and therapeutic resistance
Cellular Oncology (2024)
-
A high-resolution view of the heterogeneous aging endothelium
Angiogenesis (2024)