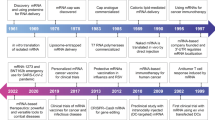
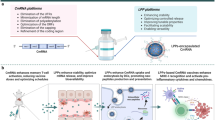
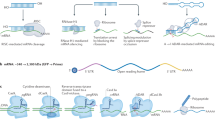
Abstract
Due to the fact that mRNA technology allows the production of diverse vaccines and treatments in a shorter time frame and with reduced expense compared to conventional approaches, there has been a surge in the use of mRNA-based therapeutics in recent years. With the aim of encoding tumour antigens for cancer vaccines, cytokines for immunotherapy, tumour suppressors to inhibit tumour development, chimeric antigen receptors for engineered T cell therapy or genome-editing proteins for gene therapy, many of these therapeutics have shown promising efficacy in preclinical studies, and some have even entered clinical trials. Given the evidence supporting the effectiveness and safety of clinically approved mRNA vaccines, coupled with growing interest in mRNA-based therapeutics, mRNA technology is poised to become one of the major pillars in cancer drug development. In this Review, we present in vitro transcribed mRNA-based therapeutics for cancer treatment, including the characteristics of the various types of synthetic mRNA, the packaging systems for efficient mRNA delivery, preclinical and clinical studies, current challenges and future prospects in the field. We anticipate the translation of promising mRNA-based treatments into clinical applications, to ultimately benefit patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976). This work presents the first report of nucleic acids (DNA isolated from mouse cells) that can be encapsulated and delivered by tiny particles.
Dimitriadis, G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 274, 923–924 (1978).
Ostro, M. J., Giacomoni, D., Lavelle, D., Paxton, W. & Dray, S. Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line. Nature 274, 921–923 (1978).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Dolgin, E. The tangled history of mRNA vaccines. Nature 597, 318–324 (2021). This review presents the discovery of mRNA, the in vitro synthesis of mRNA to the in vivo application of mRNA, which gives the reader an overview of the field.
Cobb, M. Who discovered messenger RNA? Curr. Biol. 25, R526–R532 (2015).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02413645 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03076385 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03014089 (2019).
Szebeni, J. et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 17, 337–346 (2022).
Kirtane, A. R. et al. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 16, 369–384 (2021).
Shin, M. D. et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 15, 646–655 (2020).
Huang, X. et al. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 17, 1027–1037 (2022).
Qu, P. et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N. Engl. J. Med. 387, 1329–1331 (2022).
Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics-developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Huang, X. et al. The landscape of mRNA nanomedicine. Nat. Med. 28, 2273–2287 (2022). This review provides the latest advances and innovations in mRNA nanomedicine, in the context of ongoing clinical translation and future directions to improve clinical efficacy.
Xiong, Q., Lee, G. Y., Ding, J., Li, W. & Shi, J. Biomedical applications of mRNA nanomedicine. Nano Res. 11, 5281–5309 (2018).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Xiao, Y. et al. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 51, 3828–3845 (2022). This review provides a summary and discussion of the progress, chemical designs and principles of mRNA technologies.
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Zhang, Y., Sun, C., Wang, C., Jankovic, K. E. & Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 121, 12181–12277 (2021).
Kong, N. et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl Med. 11, eaaw1565 (2019). This work presents a redox-responsive nanoparticle platform for the effective delivery of TP53 mRNA, which can markedly improve the sensitivity of tumour cells to rapamycin (mTOR) inhibitors for potent combinatorial cancer treatment.
Islam, M. A. et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2, 850–864 (2018). This work provides proof-of-principle evidence of the in vivo restoration of mRNA-based tumour suppression.
Sahin, U. & Tureci, O. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36gamma, and OX40L mRNAs. Sci. Transl Med. 11, eaat9143 (2019).
Hotz, C. et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl Med. 13, eabc7804 (2021).
Wang, H. X. et al. CRISPR/Cas9-based genome editing for disease modeling and therapy: challenges and opportunities for nonviral delivery. Chem. Rev. 117, 9874–9906 (2017).
Katti, A., Diaz, B. J., Caragine, C. M., Sanjana, N. E. & Dow, L. E. CRISPR in cancer biology and therapy. Nat. Rev. Cancer 22, 259–279 (2022).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020). This work presents the mRNA nanoparticles binding to T cells to reprogramme T cells in situ to express cancer-specific CARs or TCRs to cause tumour regression.
Zhang, D. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17, 777–787 (2022).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Lorentzen, C. L., Haanen, J. B., Met, O. & Svane, I. M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 23, e450–e458 (2022).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Rohner, E., Yang, R., Foo, K. S., Goedel, A. & Chien, K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Chen, R. et al. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 41, 262–272 (2022).
Muramatsu, H. et al. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 30, 1941–1951 (2022).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Dolgin, E. Startups set off new wave of mRNA therapeutics. Nat. Biotechnol. 39, 1029–1031 (2021).
Abramson, A. et al. Oral mRNA delivery using capsule-mediated gastrointestinal tissue injections. Matter 5, 975–987 (2022).
Kong, N. et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc. Natl Acad. Sci. USA 119, e2112696119 (2022). This work presents a mucoadhesive nanoparticle strategy to restore KDM6A expression in bladder cancer, which is helpful for mechanistic understanding and translational study of bladder-related diseases.
Tao, W. & Peppas, N. A. Robotic pills for gastrointestinal-tract-targeted oral mRNA delivery. Matter 5, 775–777 (2022). This work previews the design of robotic pills for gastrointestinal-tract-targeted oral mRNA delivery, which may open up a new avenue for the oral mRNA medicines.
Melton, D. A. et al. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 12, 7035–7056 (1984).
Schlaeger, T. M. et al. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 33, 58–63 (2015).
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
Kim, J., Eygeris, Y., Gupta, M. & Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 170, 83–112 (2021).
Kim, Y. K. RNA therapy: rich history, various applications and unlimited future prospects. Exp. Mol. Med. 54, 455–465 (2022).
Jemielity, J., Kowalska, J., Rydzik, A. M. & Darzynkiewicz, E. Synthetic mRNA cap analogs with a modified triphosphate bridge – synthesis, applications and prospects. N. J. Chem. 34, 829–844 (2010).
Ziemniak, M., Strenkowska, M., Kowalska, J. & Jemielity, J. Potential therapeutic applications of RNA cap analogs. Future Med. Chem. 5, 1141–1172 (2013).
Martinez-Salas, E., Francisco-Velilla, R., Fernandez-Chamorro, J. & Embarek, A. M. Insights into structural and mechanistic features of viral IRES elements. Front. Microbiol. 8, 2629 (2017).
Zarghampoor, F., Azarpira, N., Khatami, S. R., Behzad-Behbahani, A. & Foroughmand, A. M. Improved translation efficiency of therapeutic mRNA. Gene 707, 231–238 (2019).
Zhang, Z. et al. Genetic analyses support the contribution of mRNA N(6)-methyladenosine (m(6)A) modification to human disease heritability. Nat. Genet. 52, 939–949 (2020).
Alexaki, A. et al. Effects of codon optimization on coagulation factor IX translation and structure: implications for protein and gene therapies. Sci. Rep. 9, 15449 (2019).
Jemielity, J. et al. Novel “anti-reverse” cap analogs with superior translational properties. RNA 9, 1108–1122 (2003).
Rabinovich, P. M. et al. Synthetic messenger RNA as a tool for gene therapy. Hum. Gene Ther. 17, 1027–1035 (2006).
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
Linares-Fernandez, S., Lacroix, C., Exposito, J. Y. & Verrier, B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 26, 311–323 (2020).
Kariko, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
Weng, Y. et al. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 40, 107534 (2020).
Igyarto, B. Z., Jacobsen, S. & Ndeupen, S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr. Opin. Virol. 48, 65–72 (2021).
Tenchov, R., Bird, R., Curtze, A. E. & Zhou, Q. Lipid nanoparticles horizontal line from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15, 16982–17015 (2021).
Lundstrom, K. Self-amplifying RNA viruses as RNA vaccines. Int. J. Mol. Sci. 21, 5130 (2020).
Bloom, K., van den Berg, F. & Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 28, 117–129 (2021).
Balmayor, E. R. Synthetic mRNA - emerging new class of drug for tissue regeneration. Curr. Opin. Biotechnol. 74, 8–14 (2022).
Beissert, T. et al. A trans-amplifying RNA vaccine strategy for induction of potent protective immunity. Mol. Ther. 28, 119–128 (2020).
Fuller, D. H. & Berglund, P. Amplifying RNA vaccine development. N. Engl. J. Med. 382, 2469–2471 (2020).
Chen, L. L. & Yang, L. Regulation of circRNA biogenesis. RNA Biol. 12, 381–388 (2015).
Enuka, Y. et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 44, 1370–1383 (2016).
Kwon, S., Kwon, M., Im, S., Lee, K. & Lee, H. mRNA vaccines: the most recent clinical applications of synthetic mRNA. Arch. Pharm. Res. 45, 245–262 (2022).
Morse, D. E. & Yanofsky, C. Polarity and the degradation of mRNA. Nature 224, 329–331 (1969).
Wang, C. & Liu, H. Factors influencing degradation kinetics of mRNAs and half-lives of microRNAs, circRNAs, lncRNAs in blood in vitro using quantitative PCR. Sci. Rep. 12, 7259 (2022).
Bessis, N., GarciaCozar, F. J. & Boissier, M. C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 11, S10–S17 (2004).
Thomas, C. E., Ehrhardt, A. & Kay, M. A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4, 346–358 (2003).
Meng, C., Chen, Z., Li, G., Welte, T. & Shen, H. Nanoplatforms for mRNA therapeutics. Adv. Ther. 4, 2000099 (2020).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl Acad. Sci. USA 118, e2005191118 (2021).
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
Pastor, F. et al. An RNA toolbox for cancer immunotherapy. Nat. Rev. Drug Discov. 17, 751–767 (2018).
Lang, F., Schrors, B., Lower, M., Tureci, O. & Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 21, 261–282 (2022). This review explains in detail the basic immune mechanisms involved in neoantigen vaccines, providing the readers with a deep and comprehensive understanding of how neoantigen vaccines work, the development process, the areas of application and their promise.
Mitchell, D. A. et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369 (2015).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Conry, R. M. et al. Characterization of a messenger-RNA polynucleotide vaccine vector. Cancer Res. 55, 1397–1400 (1995).
He, Q., Gao, H., Tan, D., Zhang, H. & Wang, J. Z. mRNA cancer vaccines: advances, trends and challenges. Acta Pharm. Sin. B 12, 2969–2989 (2022).
Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J. Exp. Med. 184, 465–472 (1996).
Anguille, S. et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 130, 1713–1721 (2017).
Van Tendeloo, V. F. et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc. Natl Acad. Sci. USA 107, 13824–13829 (2010).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00965224 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01291420 (2023).
Lichtenegger, F. S. et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: results of a phase I trial. Clin. Transl. Immunol. 9, e1117 (2020).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01734304 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01983748 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04157127 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04837547 (2023).
Beck, J. D. et al. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 20, 69 (2021).
Koch, S. D. et al. A randomized, double-blind, placebo-controlled, phase I/II trial of RNActive®-vaccine cv9104 in patients with metastatic castrate-refractory prostate cancer (mcrpc): first results of the phase I part. J. Immunother. Cancer 2, p85 (2014).
Kubler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer 3, 26 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01817738 (2013).
Sebastian, M. et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive(R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 14, 748 (2014).
Papachristofilou, A. et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 7, 38 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01915524 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02410733 (2023).
Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020). This research demonstrates an intravenously administered mRNA vaccine targeting four non-mutated, tumour-associated antigens that are prevalent in melanoma for patients with advanced melanoma.
BioNTech SE. BioNTech receives FDA fast track designation for its FixVac candidate BNT111 in advanced melanoma. GlobeNewswire https://www.globenewswire.com/news-release/2021/11/19/2338113/0/en/BioNTech-Receives-FDA-Fast-Track-Designation-for-its-FixVac-Candidate-BNT111-in-Advanced-Melanoma.html (2021).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04526899 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04534205 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04163094 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04382898 (2023).
Benteyn, D., Heirman, C., Bonehill, A., Thielemans, K. & Breckpot, K. mRNA-based dendritic cell vaccines. Expert Rev. Vaccines 14, 161–176 (2015).
Lecoultre, M., Dutoit, V. & Walker, P. R. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J. Immunother. Cancer 8, e001408 (2020).
Hirosue, S. & Dubrot, J. Modes of antigen presentation by lymph node stromal cells and their immunological implications. Front. Immunol. 6, 446 (2015).
Ruhland, M. K. et al. Visualizing synaptic transfer of tumor antigens among dendritic cells. Cancer Cell 37, 786–799.e5 (2020).
Castle, J. C. et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 72, 1081–1091 (2012).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Zhang, R. et al. DP7-C-modified liposomes enhance immune responses and the antitumor effect of a neoantigen-based mRNA vaccine. J. Control. Release 328, 210–221 (2020).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02035956 (2020).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04486378 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03289962 (2023).
Cafri, G. et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Invest. 130, 5976–5988 (2020).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03480152 (2020).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03897881 (2022). The mRNA-4157/V940 used in this clinical trial gained the approval of breakthrough therapy designation by the FDA for the adjuvant treatment of patients with high-risk melanoma following complete resection.
Hilf, N. et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565, 240–245 (2019).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program. Nature 610, 173–181 (2022).
Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer 21, 481–499 (2021).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Thayer, A. Interleukin-2 wins FDA market clearance. Chem. Eng. N. Arch. 70, 5 (1992).
Naing, A. et al. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell 34, 775–791.e3 (2018).
Zhang, B. et al. Site-specific PEGylation of interleukin-2 enhances immunosuppression via the sustained activation of regulatory T cells. Nat. Biomed. Eng. 5, 1288–1305 (2021).
Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 4, 531–543 (2020).
Qiao, J. et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell 35, 901–915.e4 (2019).
Gorby, C. et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci. Signal. 13, eabc0653 (2020).
Trotta, E. et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24, 1005–1014 (2018).
Hochmann, S. et al. Evaluation of modified interferon alpha mRNA constructs for the treatment of non-melanoma skin cancer. Sci. Rep. 8, 12954 (2018).
Holder, P. G. et al. Engineering interferons and interleukins for cancer immunotherapy. Adv. Drug Deliv. Rev. 182, 114112 (2022).
Leonard, J. P. et al. Effects of single-dose interleukin-12 exposure on interleukin-12–associated toxicity and interferon-γ production. Blood 90, 2541–2548 (1997).
Lai, I. et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J. Immunother. Cancer 6, 125 (2018).
Etxeberria, I. et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T cells. Cancer Cell 36, 613–629.e7 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03946800 (2023).
Li, Y. et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893 (2020). This works describes a single intratumoural administration of self-replicating IL12 mRNA lipid nanoparticles that can eradicate large established tumours and induce protective immune memory.
Liu, J. Q. et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 345, 306–313 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03739931 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03871348 (2022).
Sherr, C. J. Principles of tumor suppression. Cell 116, 235–246 (2004).
Soussi, T., Ishioka, C., Claustres, M. & Beroud, C. Locus-specific mutation databases: pitfalls and good practice based on the p53 experience. Nat. Rev. Cancer 6, 83–90 (2006).
Li, T. et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149, 1269–1283 (2012).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Lee, Y. R., Chen, M. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 19, 547–562 (2018).
Ler, L. D. et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl Med. 9, eaai8312 (2017).
Munoz-Fontela, C., Mandinova, A., Aaronson, S. A. & Lee, S. W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 16, 741–750 (2016).
Peng, W. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Lin, Y. X. et al. Reactivation of the tumor suppressor PTEN by mRNA nanoparticles enhances antitumor immunity in preclinical models. Sci. Transl Med. 13, eaba9772 (2021).
Xiao, Y. et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 13, 758 (2022).
Pickar-Oliver, A. & Gersbach, C. A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20, 490–507 (2019).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Price, B. D. & D’Andrea, A. D. Chromatin remodeling at DNA double-strand breaks. Cell 152, 1344–1354 (2013).
Ceccaldi, R., Rondinelli, B. & D’Andrea, A. D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26, 52–64 (2016).
Lieber, M. R., Ma, Y., Pannicke, U. & Schwarz, K. Mechanism and regulation of human non-homologous DNA end-joining. Nat. Rev. Mol. Cell Biol. 4, 712–720 (2003).
Cornu, T. I., Mussolino, C. & Cathomen, T. Refining strategies to translate genome editing to the clinic. Nat. Med. 23, 415–423 (2017).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Zhang, H. X., Zhang, Y. & Yin, H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol. Ther. 27, 735–746 (2019).
Liu, J. et al. Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nat. Protoc. 10, 1842–1859 (2015).
Chew, W. L. Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1408 (2018).
Yin, H. et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 32, 551–553 (2014).
Miller, J. B. et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. 56, 1059–1063 (2017).
Rosenblum, D. et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 6, eabc9450 (2020). This work showcases the potential of using targeted lipid nanoparticles for the delivery of cas9 mRNA and sgRNAs to achieve precise in vivo genome editing, offering promising prospects for future cancer therapies.
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021). The rational design of functional phospholipids presented in this work holds significant potential for advancing gene editing research and its therapeutic applications.
Wagner, D. L. et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 18, 379–393 (2021).
Atsavapranee, E. S., Billingsley, M. M. & Mitchell, M. J. Delivery technologies for T cell gene editing: applications in cancer immunotherapy. EBioMedicine 67, 103354 (2021).
Elsallab, M., Levine, B. L., Wayne, A. S. & Abou-El-Enein, M. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 21, e104–e116 (2020).
Scholler, J. et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl Med. 4, 132ra153 (2012).
MacKay, M. et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 38, 233–244 (2020).
Zhao, Y. et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol. Ther. 13, 151–159 (2006).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Carlsten, M. et al. Efficient mRNA-based genetic engineering of human NK cells with high-affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-associated chemokine CCL19. Front. Immunol. 7, 105 (2016).
Krug, C. et al. A GMP-compliant protocol to expand and transfect cancer patient T cells with mRNA encoding a tumor-specific chimeric antigen receptor. Cancer Immunol. Immunother. 63, 999–1008 (2014).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01355965 (2017).
Maus, M. V. et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 1, 26–31 (2013).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01897415 (2017).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02719782 (2018).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03060356 (2020).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04981691 (2021).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05302037 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02277522 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02624258 (2020).
Svoboda, J. et al. Nonviral RNA chimeric antigen receptor-modified T cells in patients with Hodgkin lymphoma. Blood 132, 1022–1026 (2018).
Tasian, S. K. et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood 129, 2395–2407 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02623582 (2017).
Mangala Prasad, V. et al. Cryo-ET of Env on intact HIV virions reveals structural variation and positioning on the Gag lattice. Cell 185, 641–653.e17 (2022).
Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020).
Zhang, P. et al. Cancer nanomedicine toward clinical translation: obstacles, opportunities, and future prospects. Med 4, 147–167 (2022).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Ferdows, B. E. et al. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 14, 4448–4455 (2022).
Zhao, X. et al. Imidazole-based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem. Int. Ed. 59, 20083–20089 (2020).
Ma, F. et al. Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci. Adv. 6, eabb4429 (2020).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Zou, Y. et al. Blood-brain barrier-penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 8, eabm8011 (2022).
Boehnke, N. et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 377, eabm5551 (2022).
Yang, Z. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 4, 69–83 (2020).
Gurumurthy, C. B., Quadros, R. M. & Ohtsuka, M. Prototype mouse models for researching SEND-based mRNA delivery and gene therapy. Nat. Protoc. 17, 2129–2138 (2022).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021). This research demonstrates that the mammalian retrovirus-like protein PEG10 can bind directly to its own mRNA and package it into extracellular virus-like capsids, potentially serving as an endogenous vector for mRNA-based gene therapy.
Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021). This work reports that the optimized composition, molar ratios and structure of lipid nanoparticles can achieve nebulized delivery of an mRNA encoding a broadly neutralizing antibody for lung disease treatment.
Vogel, A. B. et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol. Ther. 26, 446–455 (2018).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022). This study reports that circRNA vaccines enable effective protection against SARS-CoV-2 in mice and monkeys.
Palmer, C. D. et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat. Med. 28, 1619–1629 (2022). This work reports the use of a self-amplifying mRNA-based neoantigen vaccine in combination with anti-PD1 and anti-CTLA4 in patients with advanced metastatic solid tumours.
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, 1001 (2020).
Ho, T. C. et al. Scaffold-mediated CRISPR-Cas9 delivery system for acute myeloid leukemia therapy. Sci. Adv. 7, eabg3217 (2021).
Hirano, S. et al. Structure of the OMEGA nickase IsrB in complex with omegaRNA and target DNA. Nature 610, 575–581 (2022).
Schuler, G., Hu, C. & Ke, A. Structural basis for RNA-guided DNA cleavage by IscB-omegaRNA and mechanistic comparison with Cas9. Science 376, 1476–1481 (2022).
Altae-Tran, H. et al. The widespread IS200/IS605 transposon family encodes diverse programmable RNA-guided endonucleases. Science 374, 57–65 (2021).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03468244 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03908671 (2019).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT04161755 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03815058 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03948763 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03639714 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05141721 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05359354 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05198752 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05192460 (2023).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05227378 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05202561 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05456165 (2022).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT05533697 (2023).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Fyfe, I. Treatment success in hereditary transthyretin amyloidosis. Nat. Rev. Neurol. 14, 509 (2018).
Hess, P. R., Boczkowski, D., Nair, S. K., Snyder, D. & Gilboa, E. Vaccination with mRNAs encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes CTL responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol. Immunother. 55, 672–683 (2006).
Perche, F. et al. Enhancement of dendritic cells transfection in vivo and of vaccination against B16F10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine 7, 445–453 (2011).
Le Moignic, A. et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release 278, 110–121 (2018).
Lv, H., Zhang, S., Wang, B., Cui, S. & Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 114, 100–109 (2006).
Landesman-Milo, D. & Peer, D. Toxicity profiling of several common RNAi-based nanomedicines: a comparative study. Drug Deliv. Transl. Res. 4, 96–103 (2014).
Kowalski, P. S., Rudra, A., Miao, L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019).
Tateshita, N. et al. Development of a lipoplex-type mRNA carrier composed of an ionizable lipid with a vitamin E scaffold and the KALA peptide for use as an ex vivo dendritic cell-based cancer vaccine. J. Control. Release 310, 36–46 (2019).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019). This study describes the creation of a combinatorial library consisting of ionizable lipid-like materials to identify effective mRNA delivery vehicles capable of facilitating mRNA delivery in vivo and producing potent and specific immune activation.
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Paunovska, K. et al. Analyzing 2000 in vivo drug delivery data points reveals cholesterol structure impacts nanoparticle delivery. ACS Nano 12, 8341–8349 (2018).
Paunovska, K. et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 31, e1807748 (2019).
Li, W. & Szoka, F. C. Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 24, 438–449 (2007).
Eygeris, Y., Gupta, M., Kim, J. & Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 55, 2–12 (2022).
Knop, K., Hoogenboom, R., Fischer, D. & Schubert, U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010).
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Choi, H. Y. et al. Efficient mRNA delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. J. Control. Release 235, 222–235 (2016).
Li, M. et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 228, 9–19 (2016).
Uchida, S. et al. In vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle. PLoS ONE 8, e56220 (2013).
Acknowledgements
This work is supported by Basic Science Research Programs from the National Research Foundation of Korea (no. 2022R1C1C2007637 to S.K.), National Natural Science Foundation of China (no. 82122076 to N.K.), Harvard Medical School/Brigham and Women’s Hospital Department of Anaesthesiology Basic Scientist Grant (no. 2420 BPA075 to W.T.), Nanotechnology Foundation (no. 2022A002721 to W.T.), Gillian Reny Stepping Strong Center for Trauma Innovation Breakthrough Innovator Award (no. 113548 to W.T.), Center for Nanomedicine Research Fund (no. 2019A014810 to W.T.), and Farokhzad Family Distinguished Chair Foundation (no. 018129 to W.T.). W.T. is also a recipient of the Khoury Innovation Award (no. 2020A003219) and the American Heart Association (AHA) Collaborative Science Award (no. 2018A004190).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content, wrote, reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Michael Mitchell, Quanyin Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 5′ and 3′ untranslated regions
-
(UTRs). Non-coding regulatory regions of mRNA that contain multiple regulatory elements.
- 5′ Cap
-
A specially altered nucleotide on the 5′ end of mRNA, which has a substantial role in the initiation of mRNA translation.
- Chimeric antigen receptors
-
(CARs). Receptor proteins that combine antigen binding and T cell activation functions to drive T cells to target specific antigens.
- Cytokines
-
A class of small proteins that modulate autocrine, paracrine and endocrine signalling.
- Electroporation
-
A biological technique in which exogenous substances, such as drugs, DNA or mRNA, are transferred into a cell by applying an electrical field to the cell to increase the permeability of the cell membrane.
- High-performance liquid chromatography
-
(HPLC). An analytical chemistry technique that relies on differences in the chemical interaction of each component of a sample with the sorbent material for the separation, identification and quantification of each component in a mixture.
- Immune tolerant
-
Describes the inability of antigen-specific immune cells to be activated in response to antigen stimulation and to execute a normal immune response.
- Ionizable phospholipid
-
(iPho). A lipid with an ionizable amino group, a phosphate head group and hydrophobic lipid tail; iPhos are positively charged at acidic pH to condense RNA into lipid nanoparticles, but are neutral at physiological pH to minimize toxicity.
- Major histocompatibility complex
-
(MHC). A set of eukaryotic cell surface proteins that can be divided into MHC class I and II molecules.
- Open reading frame
-
(ORF). The most crucial part of mRNA, which contains the coding sequence of the translated protein.
- Peripheral blood mononuclear cells
-
(PBMCs). Blood cells with round nuclei that are generally comprised of lymphocytes (T cells, B cells, natural killer cells), monocytes and a small number of dendritic cells.
- Poly(A) tail
-
A string of adenosine modifications to the 3′ end of mRNA, which plays a crucial part in preventing mRNA degradation and enhancing translation efficiency.
- Regulatory T cells
-
(Treg cells). A subpopulation of T cells that regulate the immune system, maintain tolerance to self antigens and prevent autoimmune diseases.
- RNA-dependent RNA polymerase
-
(RDRP). An enzyme that catalyses the replication of RNA from an RNA template.
- Tumour microenvironment
-
(TME). The non-cancerous environment that surrounds tumour cells, including blood vessels, immune cells, fibroblasts, signalling molecules and extracellular matrix.
- Tumour suppressors
-
Proteins that regulate cells during cell division and replication to prevent cancer.
- Type I interferon
-
(IFN). A cytokine that fulfils an important role in inflammation, immunoregulation, tumour cell recognition and T cell responses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Shi, Q., Huang, X. et al. mRNA-based cancer therapeutics. Nat Rev Cancer 23, 526–543 (2023). https://doi.org/10.1038/s41568-023-00586-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00586-2
This article is cited by
-
Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
Nature Reviews Clinical Oncology (2024)
-
Targeted nanostrategies eliminate pre-metastatic niche of cancer
Nano Research (2024)
-
The breakthrough in vaccination: Nobel Prize in Physiology or Medicine 2023
Structural Chemistry (2024)
-
Advancing personalized medicine in brain cancer: exploring the role of mRNA vaccines
Journal of Translational Medicine (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)