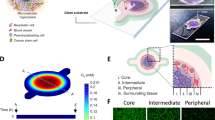
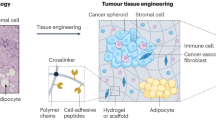
Abstract
Effort invested in the development of new drugs often fails to be translated into meaningful clinical benefits for patients with cancer. The development of more effective anticancer therapeutics and accurate prediction of their clinical merit remain urgent unmet medical needs. As solid cancers have complex and heterogeneous structures composed of different cell types and extracellular matrices, three-dimensional (3D) cancer models hold great potential for advancing our understanding of cancer biology, which has been historically investigated in tumour cell cultures on rigid plastic plates. Advanced 3D bioprinted cancer models have the potential to revolutionize the way we discover therapeutic targets, develop new drugs and personalize anticancer therapies in an accurate, reproducible, clinically translatable and robust manner. These ex vivo cancer models are already replacing existing in vitro systems and could, in the future, diminish or even replace the use of animal models. Therefore, profound understanding of the differences in tumorigenesis between 2D, 3D and animal models of cancer is essential. This Review presents the state of the art of 3D bioprinted cancer modelling, focusing on the biological processes that underlie the molecular mechanisms involved in cancer progression and treatment response as well as on proteomic and genomic signatures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frost & Sullivan. Global Drug Discovery and Early Development Outsourcing Growth Opportunities https://www.reportlinker.com/p06130908/Global-Drug-Discovery-and-Early-Development-Outsourcing-Growth-Opportunities.html?utm_source=GNW (2021).
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 323, 844–853 (2020).
Biotechnology Innovation Organization. Clinical Development Success Rates and Contributing Factors 2011–2020 https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/2021/files/reports/2021-clinical-development-success-rates-2011-2020-v17.pdf (2020).
IQVIA. Global Medicine Spending and Usage Trends: Outlook to 2025. IQVIA Institute Report https://www.iqvia.com/insights/the-iqvia-institute/reports/global-medicine-spending-and-usage-trends-outlook-to-2025 (2021).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Rodrigues, J., Heinrich, M. A., Teixeira, L. M. & Prakash, J. 3D In vitro model (r)evolution: unveiling tumor-stroma interactions. Trends Cancer 7, 249–264 (2021).
Law, A. M. K. et al. Advancements in 3D cell culture systems for personalizing anti-cancer therapies. Front. Oncol. 11, 782766 (2021).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Yoshida, G. J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 13, 4 (2020).
Peres, C. et al. Preclinical models and technologies to advance nanovaccine development. Adv. Drug Deliv. Rev. 172, 148–182 (2021).
Pozzi, S. et al. Meet me halfway: are in vitro 3D cancer models on the way to replace in vivo models for nanomedicine development? Adv. Drug Deliv. Rev. 175, 113760 (2021).
Neufeld, L. et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 7, eabi9119 (2021). This study describes a complex perfusable TME model that includes patient-derived glioblastoma cells, endothelial cells, pericytes, astrocytes and microglia.
Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687 (2018).
Kim, E. et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 588, 664–669 (2020).
Sontheimer-Phelps, A., Hassell, B. A. & Ingber, D. E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 19, 65–81 (2019).
Tang, Y. et al. A biomimetic microfluidic tumor microenvironment platform mimicking the EPR effect for rapid screening of drug delivery systems. Sci. Rep. 7, 9359 (2017).
Ning, L. et al. A 3D bioprinted in vitro model of neuroblastoma recapitulates dynamic tumor-endothelial cell interactions contributing to solid tumor aggressive behavior. Adv. Sci. 9, 2200244 (2022). This study describes a perfusable TME model that includes patient-derived neuroblastoma cells, endothelial cells, adipocyte-derived MSCs and induced pluripotent stem cell-derived MSCs.
Xu, T., Jin, J., Gregory, C., Hickman, J. J. & Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 26, 93–99 (2005).
Xu, F. et al. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 6, 204–212 (2011).
Yi, H.-G. et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 3, 509–519 (2019). This study describes a 3D bioprinted glioblastoma model that reproduces clinically observed patient-derived patterns of treatment resistance to temozolomide or chemoradiation.
Rijal, G. & Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 3, e1700764 (2017).
Utama, R. H. et al. A covalently crosslinked ink for multimaterials drop-on-demand 3D bioprinting of 3D cell cultures. Macromol. Biosci. 21, e2100125 (2021).
Jiang, S. et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Rep. Med. 1, 100161 (2020).
Sbrana, F. V. et al. 3D bioprinting allows the establishment of long-term 3D culture model for chronic lymphocytic leukemia cells. Front. Immunol. 12, 639572 (2021).
Ma, X. et al. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 185, 310–321 (2018).
Mollica, P. A. et al. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 95, 201–213 (2019).
Garreta, E. et al. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 20, 166–178 (2017).
Hakobyan, D. et al. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 12, 035001 (2020). This study describes the fabrication of a rat exocrine pancreatic cell network using laser-assisted bioprinting technology, which enabled the replication of both the initial stages of pancreatic ductal adenocarcinoma and its progression.
Ozturk, M. S. et al. High-resolution tomographic analysis of in vitro 3D glioblastoma tumor model under long-term drug treatment. Sci. Adv. 6, eaay7513 (2020). This study shows that use of a high-resolution tomography platform can markedly improve the imaging of thick 3D bioprinted models.
Hu, M. et al. Integrated genome and tissue engineering enables screening of cancer vulnerabilities in physiologically relevant perfusable ex vivo cultures. Biomaterials 280, 121276 (2022).
Lee, C., Abelseth, E., De La Vega, L. & Willerth, S. Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening. Mater. Today Chem. 12, 78–84 (2019).
Shao, L. et al. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication 12, 035014 (2020).
Hynes, W. et al. Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci. Adv. 6, eabb3308 (2020). This study describes a computational model that can be used to represent fundamental biological phenomena.
Whiteside, T. L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 27, 5904 (2008).
Mishriki, S. et al. Rapid magnetic 3D printing of cellular structures with MCF-7 cell inks. Research 2019, 9854593 (2019).
Schmidt, S. K., Schmid, R., Arkudas, A., Kengelbach-Weigand, A. & Bosserhoff, A. K. Tumor cells develop defined cellular phenotypes after 3D-bioprinting in different bioinks. Cells 8, 1295 (2019).
Diao, J. et al. Role and mechanisms of a three-dimensional bioprinted microtissue model in promoting proliferation and invasion of growth-hormone-secreting pituitary adenoma cells. Biofabrication 11, 025006 (2019).
Duarte Campos, D. F. et al. Exploring cancer cell behavior in vitro in three-dimensional multicellular bioprintable collagen-based hydrogels. Cancers 11, 180 (2019).
Jeong, Y.-M. et al. 3D-printed collagen scaffolds promote maintenance of cryopreserved patients-derived melanoma explants. Cells 10, 589 (2021).
Flores-Torres, S. et al. Alginate-gelatin-Matrigel hydrogels enable the development and multigenerational passaging of patient-derived 3D bioprinted cancer spheroid models. Biofabrication 13, 025001 (2021).
Xie, F. et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 265, 120416 (2021).
Han, J. et al. In vitro breast cancer model with patient-specific morphological features for personalized medicine. Biofabrication 14, 034102 (2022).
Tang, M. et al. Rapid 3D bioprinting of glioblastoma model mimicking native biophysical heterogeneity. Small 17, 2006050 (2021). This study describes the first species-matched 3D bioprinted in vitro models to recapitulate the biophysical heterogeneity of glioblastoma.
Langer, E. M. et al. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell Rep. 26, 608–623.e6 (2019). This study describes multi-cell-type 3D bioprinted tissues that recapitulate aspects of patient-derived tissue and provide insight for drug translational studies.
Zhao, Y. et al. Three-dimensional printing of HeLa cells for cervical tumor model in vitro. Biofabrication 6, 035001 (2014).
Chen, H. et al. 3D printed in vitro tumor tissue model of colorectal cancer. Theranostics 10, 12127–12143 (2020).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30, 668–681 (2016).
Zhou, X. et al. 3D Bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl. Mater. Interf. 8, 30017–30026 (2016).
Back, J. et al. Inflammatory conversion of quiescent osteoblasts by metastatic breast cancer cells through pERK1/2 aggravates cancer-induced bone destruction. Bone Res. 9, 43 (2021).
Kim, J. H. et al. Establishment of three-dimensional bioprinted bladder cancer-on-a-chip with a microfluidic system using bacillus Calmette–Guérin. Int. J. Mol. Sci. 22, 8887 (2021).
Xie, M. et al. 3D biofabrication of microfiber-laden minispheroids: a facile 3D cell co-culturing system. Biomater. Sci. 8, 109–117 (2020).
Ping, Q. et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 28, 984–999 (2021).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Kim, B. S. et al. Construction of tissue-level cancer-vascular model with high-precision position control via in situ 3D cell printing. Small Methods 5, 2100072 (2021).
Meng, F. et al. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 31, e1806899 (2019).
Nothdurfter, D. et al. 3D bioprinted, vascularized neuroblastoma tumor environment in fluidic chip devices for precision medicine drug testing. Biofabrication 14, 035002 (2022).
Dalton, W. The influence of the tumor microenvironment on drug response and drug resistance. Clin. Cancer Res. 14, PL04-03 (2008).
Neophytou, C. M., Panagi, M., Stylianopoulos, T. & Papageorgis, P. The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers 13, 2053 (2021).
Grunewald, L. et al. A reproducible bioprinted 3D tumor model serves as a preselection tool for CAR T cell therapy optimization. Front. Immunol. 12, 689697 (2021). This study describes a highly reproducible 3D bioprinted model for preclinical (in vitro) characterization and optimization of CAR T cells for use in personalized therapy.
Liu, T.-k, Pang, Y., Zhou, Z.-Z., Yao, R. & Sun, W. An integrated cell printing system for the construction of heterogeneous tissue models. Acta Biomater. 95, 245–257 (2019).
Yeini, E. et al. P-selectin axis plays a key role in microglia immunophenotype and glioblastoma progression. Nat. Commun. 12, 1912 (2021).
Tang, M. et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 30, 833–853 (2020).
Heinrich, M. A. et al. 3D-bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 31, e1806590 (2019).
Miranda, M. A. et al. Cytotoxic and chemosensitizing effects of glycoalkaloidic extract on 2D and 3D models using RT4 and patient derived xenografts bladder cancer cells. Mater. Sci. Eng. C. 119, 111460 (2021).
Swaminathan, S., Hamid, Q., Sun, W. & Clyne, A. M. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication 11, 025003–025003 (2019).
Hong, S. & Song, J. M. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 138, 228–239 (2022).
Dai, X., Ma, C., Lan, Q. & Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 8, 045005 (2016).
Mao, S. et al. Bioprinting of patient-derived in vitro intrahepatic cholangiocarcinoma tumor model: establishment, evaluation and anti-cancer drug testing. Biofabrication 12, 045014 (2020).
Singh, M. et al. Controlled three-dimensional tumor microenvironments recapitulate phenotypic features and differential drug response in early vs advanced stage breast cancer. ACS Biomater. Sci. Eng. 4, 421–431 (2018).
Kingsley, D. M. et al. Laser-based 3D bioprinting for spatial and size control of tumor spheroids and embryoid bodies. Acta Biomater. 95, 357–370 (2019).
Qian, Z. M., Li, H., Sun, H. & Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol. Rev. 54, 561–587 (2002).
Charman, W. N. Lymphatic Transport of Drugs (Routledge, 2019).
Cao, X. et al. A tumor-on-a-chip system with bioprinted blood and lymphatic vessel pair. Adv. Funct. Mater. 29, 1807173 (2019). This study describes 3D bioprinted models containing combinations of various blood and lymphatic vessel pairs with different diffusion profiles for biomolecules and anticancer therapies.
Tan, S. K. et al. Drug repositioning in glioblastoma: a pathway perspective. Front. Pharmacol. 9, 218 (2018).
Pushpakom, S. et al. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41–58 (2019).
Scott, S. A. Clinical pharmacogenomics: opportunities and challenges at point of care. Clin. Pharmacol. Ther. 93, 33–35 (2013).
Lierman, E. et al. The ability of sorafenib to inhibit oncogenic PDGFRβ and FLT3 mutants and overcome resistance to other small molecule inhibitors. Haematologica 92, 27–34 (2007).
Tahara, M. et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur. J. Cancer 75, 213–221 (2017).
Li, Y. et al. 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of metuzumab. Biofabrication 11, 034102 (2019).
Hubert, P. & Amigorena, S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. Oncoimmunology 1, 103–105 (2012).
Sharifi, M. et al. 3D bioprinting of engineered breast cancer constructs for personalized and targeted cancer therapy. J. Control. Release 333, 91–106 (2021).
Fatimi, A., Okoro, O. V., Podstawczyk, D., Siminska-Stanny, J. & Shavandi, A. Natural hydrogel-based bio-inks for 3D bioprinting in tissue engineering: a review. Gels 8, 179 (2022).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2016).
Sydney Gladman, A., Matsumoto, E. A., Nuzzo, R. G., Mahadevan, L. & Lewis, J. A. Biomimetic 4D printing. Nat. Mater. 15, 413–418 (2016).
Mordor Intelligence. 3D Bioprinting Market - Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027) https://www.mordorintelligence.com/industry-reports/3d-bioprinting-market (2021).
Acknowledgements
The authors acknowledge partial funding from the European Research Council (ERC) Advanced Grant Agreement no. 835227-3DBrainStorm; ERC Proof of Concept Grant (862580; 3DCanPredict) and the Morris Kahn Foundation (all to R.S.F.). L.N. and E.Y. acknowledge the financial support of their fellowship from the Dan David Prize.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.S.F. declares that she is a Director on the board of Teva Pharmaceutical Industries and receives research funding from Merck for work unrelated to this manuscript. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Teo Xu and Jai Prakash for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Shear thinning
-
A reduction in viscosity caused by shear stress — the physical forces created by liquid flow parallel to the surface of a material.
- Perfusable channels
-
Directional fluid flow can be applied via artificial vessels, in which it is possible to control the speed, rate and composition of the circulating fluid.
- Bioink
-
A composition of materials that can be deposited by a 3D bioprinter to produce a tissue that supports living cells in a 3D manner.
- Crosslinking
-
Formation of permanent or reversible bonds between neighbouring polymer chains creating a network structure.
- Decellularized
-
Natural scaffolds derived from tissues or organs, in which the cellular and nuclear contents are eliminated but the 3D structure and composition of the extracellular matrix are preserved.
- Sacrificial bioink
-
Bioink materials that can be printed and embedded into other materials while forming a solid gel structure and can later be dissolved to create hollow parts, such as microfluidic channels or vascular networks.
- Rheology
-
The science of deformation of materials.
- Epithelial-to-mesenchymal transition
-
(EMT). A process of phenotypic and transcriptomic change in which epithelial-like cells acquire mesenchymal-like properties, such as the ability to detach from their neighbours and migrate to distant sites.
- Support bath
-
A platform that supports low viscosity bioinks extruded within its volume, until they are crosslinked into complex structures.
- Isotropic
-
Mechanical and physical properties that are not affected by the orientation of the atoms when organized in their crystal structure. Isotropic behaviour is observed in pathological remodelling of the extracellular matrix, which facilitates tumour cell invasion.
- Anisotropic
-
Mechanical and physical properties that are affected by the orientation of the atoms when organized in their crystal structure.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neufeld, L., Yeini, E., Pozzi, S. et al. 3D bioprinted cancer models: from basic biology to drug development. Nat Rev Cancer 22, 679–692 (2022). https://doi.org/10.1038/s41568-022-00514-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-022-00514-w
This article is cited by
-
Nanomedicine in cancer therapy
Signal Transduction and Targeted Therapy (2023)
-
Micro-engineering and nano-engineering approaches to investigate tumour ecosystems
Nature Reviews Cancer (2023)
-
How human are our models?
Nature Reviews Bioengineering (2023)
-
Chemiluminescent probes in cancer biology
Nature Reviews Bioengineering (2023)