Abstract
Ductal carcinoma in situ (DCIS) is a non-invasive breast neoplasia that accounts for 25% of all screen-detected breast cancers diagnosed annually. Neoplastic cells in DCIS are confined to the ductal system of the breast, although they can escape and progress to invasive breast cancer in a subset of patients. A key concern of DCIS is overtreatment, as most patients screened for DCIS and in whom DCIS is diagnosed will not go on to exhibit symptoms or die of breast cancer, even if left untreated. However, differentiating low-risk, indolent DCIS from potentially progressive DCIS remains challenging. In this Review, we summarize our current knowledge of DCIS and explore open questions about the basic biology of DCIS, including those regarding how genomic events in neoplastic cells and the surrounding microenvironment contribute to the progression of DCIS to invasive breast cancer. Further, we discuss what information will be needed to prevent overtreatment of indolent DCIS lesions without compromising adequate treatment for high-risk patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
15 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41568-022-00542-6
References
Elshof, L. E. et al. Cause-specific mortality in a population-based cohort of 9799 women treated for ductal carcinoma in situ. Ann. Surg. 267, 952–958 (2018).
Roses, R. E. et al. Ductal carcinoma-in-situ of the breast with subsequent distant metastasis and death. Ann. Surg. Oncol. 18, 2873–2878 (2011).
Ernster, V. L., Barclay, J., Kerlikowske, K., Wilkie, H. & Ballard-Barbash, R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch. Intern. Med. 160, 953–958 (2000).
Bleyer, A. & Welch, H. G. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl. J. Med. 367, 1998–2005 (2012). This analysis of data on the incidence of DCIS, early-stage IBC and late-stage IBC from the Surveillance, Epidemiology, and End Results programme collected from 1976 to 2008 reveals that mammography screening has only marginally reduced the rate at which women present with advanced cancer, suggesting the existence of overdiagnosis.
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018).
American Cancer Society. How common is breast cancer, https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (2019).
Cancer Research UK. In situ breast carcinoma incidence statistics, https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-in-situ (2020).
Netherlands Cancer Registry. National evaluation of breast cancer screening in the Netherlands 2017/2018 https://iknl.nl/getmedia/8b019b63-0eb1-4afa-a824-31c4d10cc86e/Breast_cancer_screening_in_the_Netherlands_2017-2018_en.pdf (2020).
Ward, E. M. et al. Cancer statistics: breast cancer in situ. CA Cancer J. Clin. 65, 481–495 (2015).
Vachon, C. M. et al. Strong evidence of a genetic determinant for mammographic density, a major risk factor for breast cancer. Cancer Res. 67, 8412–8418 (2007).
Alaeikhanehshir, S. et al. The impact of patient characteristics and lifestyle factors on the risk of an ipsilateral event after a primary DCIS: a systematic review. Breast 50, 95–103 (2020).
Flanagan, M. R. et al. Relationship between anthropometric factors and risk of second breast cancer among women with a history of ductal carcinoma in situ. JNCI Cancer Spectr. 2, pky020 (2018).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397 (2017). This comprehensive overview describes the associations of obesity with breast cancer risk and outcome as well as the underlying molecular mechanisms that could explain these associations.
Vachon, C. M. et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol. Biomark. Prev. 16, 43–49 (2007).
Mazzola, E., Cheng, S. C. & Parmigiani, G. The penetrance of ductal carcinoma in situ among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 137, 315–318 (2013).
Bergholtz, H. et al. Comparable cancer-relevant mutation profiles in synchronous ductal carcinoma in situ and invasive breast cancer. Cancer Rep. 3, e1248 (2020).
Hwang, E. S. et al. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin. Cancer Res. 10, 5160–5167 (2004).
van Seijen, M. et al. Variability in grading of ductal carcinoma in situ among an international group of pathologists. J. Pathol. Clin. Res. 7, 233–242 (2021).
Groen, E. J. et al. Prognostic value of histopathological DCIS features in a large-scale international interrater reliability study. Breast Cancer Res. Treat. 183, 759–770 (2020). On the basis of the majority opinion of pathologists, DCIS grade, growth pattern and mitotic activity are reported in this study to be associated with the risk of subsequent IBC in the same breast after treatment with breast-sparing surgery followed by radiotherapy, although inter-rater variability is substantial.
Berg, W. A., Campassi, C., Langenberg, P. & Sexton, M. J. Breast imaging reporting and data system: inter- and intraobserver variability in feature analysis and final assessment. AJR Am. J. Roentgenol. 174, 1769–1777 (2000).
Harrison, B. T., Hwang, E. S., Partridge, A. H., Thompson, A. M. & Schnitt, S. J. Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance trials of ductal carcinoma in situ. Mod. Pathol. 32, 1257–1262 (2019).
Casasent, A. K. et al. Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell 172, 205–217 (2018).
Hernandez, L. et al. Genomic and mutational profiling of ductal carcinomas in situ and matched adjacent invasive breast cancers reveals intra-tumour genetic heterogeneity and clonal selection. J. Pathol. 227, 42–52 (2012).
Doebar, S. C. et al. Gene expression differences between ductal carcinoma in situ with and without progression to invasive breast cancer. Am. J. Pathol. 187, 1648–1655 (2017).
Miron, A. et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 70, 5674–5678 (2010).
Moelans, C. B., de Weger, R. A., Monsuur, H. N., Maes, A. H. & van Diest, P. J. Molecular differences between ductal carcinoma in situ and adjacent invasive breast carcinoma: a multiplex ligation-dependent probe amplification study. Anal. Cell Pathol. 33, 165–173 (2010).
Lesurf, R. et al. Molecular features of subtype-specific progression from ductal carcinoma in situ to invasive breast cancer. Cell Rep. 16, 1166–1179 (2016). This study suggests that the five intrinsic subtypes of breast cancer might help to categorize DCIS and help predict which DCIS have a higher likelihood to progress.
Lips, E. H. et al. Genomic analysis defines clonal relationships of ductal carcinoma in situ and recurrent invasive breast cancer. Nat. Genet. 54, 850–860 (2022). This is an in-depth genomic characterization of initial DCIS and paired invasive recurrences in 95 patients. Seventy-five per cent of the cases clearly showed clonal relatedness, while in 18% of the cases no relationship was seen between the primary DCIS and subsequent invasive recurrence, indicating independent lineages.
Petridis, C. et al. Frequency of pathogenic germline variants in BRCA1, BRCA2, PALB2, CHEK2 and TP53 in ductal carcinoma in situ diagnosed in women under the age of 50 years. Breast Cancer Res. 21, 58 (2019).
Petridis, C. et al. Genetic predisposition to ductal carcinoma in situ of the breast. Breast Cancer Res. 18, 22 (2016).
Grimm, L. J. et al. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann. Surg. Oncol. 24, 3534–3540 (2017).
Ryser, M. D. et al. Cancer outcomes in DCIS patients without locoregional treatment. J. Natl Cancer Inst. 111, 952–960 (2019). This study strongly suggests that patients with DCIS without locoregional treatment have a limited risk of invasive progression, suggesting that there may be overtreatment, especially among patients with increased co-morbidities.
Elshof, L. E. et al. Subsequent risk of ipsilateral and contralateral invasive breast cancer after treatment for ductal carcinoma in situ: incidence and the effect of radiotherapy in a population-based cohort of 10,090 women. Breast Cancer Res. Treat. 159, 553–563 (2016).
Rakovitch, E. et al. Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res. Treat. 138, 581–590 (2013).
Falk, R. S., Hofvind, S., Skaane, P. & Haldorsen, T. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res. Treat. 129, 929–938 (2011).
Maxwell, A. J. et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur. J. Surg. Oncol. 44, 429–435 (2018). This study indicates that there is a need for reproducible grading for DCIS to ensure patients receive accurate treatment if one is looking to use active surveillance for patients with pure low-grade DCIS. In addition, it suggests that low-grade DCIS was much less likely to progress than DCIS of high or intermediate grade.
Maxwell, A. J. et al. Unresected screen detected ductal carcinoma in situ: outcomes of 311 women in the Forget-me–not 2 study. Breast 61, 145–155 (2022).
Worni, M. et al. Trends in treatment patterns and outcomes for ductal carcinoma in situ. J. Natl Cancer Inst. 107, djv263 (2015).
Rakovitch, E. et al. 21-Gene assay and breast cancer mortality in ductal carcinoma in situ. J. Natl Cancer Inst. 113, 572–579 (2021).
van Maaren, M. C. et al. Trends in incidence, treatment, survival and subsequent breast cancer in lobular carcinoma in situ in the Netherlands: a population-based analysis. Breast 59, 376–382 (2021).
Visser, L. L. et al. Predictors of an invasive breast cancer recurrence after DCIS: a systematic review and meta-analyses. Cancer Epidemiol. Biomark. Prev. https://doi.org/10.1158/1055-9965.EPI-18-0976 (2019).
Kerlikowske, K. et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J. Natl Cancer Inst. 102, 627–637 (2010).
Thompson, A. M. et al. Management and 5-year outcomes in 9938 women with screen-detected ductal carcinoma in situ: the UK Sloane Project. Eur. J. Cancer 101, 210–219 (2018).
Shaaban, A. M. et al. Pathological features of 11,337 patients with primary ductal carcinoma in situ (DCIS) and subsequent events: results from the UK Sloane Project. Br. J. Cancer 124, 1009–1017 (2021).
Gierisch, J. M. et al. Prioritization of research addressing management strategies for ductal carcinoma in situ. Ann. Intern. Med. 160, 484–491 (2014). This article outlines a prioritized research agenda for the management of DCIS. PubMed and ClinicalTrials.gov entries were searched and analysed, and the ten largest evidence gaps for DCIS were identified, including patient-reported outcomes in research, better methods to predict risk of invasive cancer, evaluation of active surveillance strategies and testing decision-making tools.
Martelotto, L. G. et al. Whole-genome single-cell copy number profiling from formalin-fixed paraffin-embedded samples. Nat. Med. 23, 376–385 (2017).
Berg, W. A., Arnoldus, C. L., Teferra, E. & Bhargavan, M. Biopsy of amorphous breast calcifications: pathologic outcome and yield at stereotactic biopsy. Radiology 221, 495–503 (2001).
Wilkinson, L., Thomas, V. & Sharma, N. Microcalcification on mammography: approaches to interpretation and biopsy. Br. J. Radiol. 90, 20160594 (2017).
Perry, N. et al. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition-summary document. Ann. Oncol. 19, 614–622 (2008).
Claus, E. B. et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER-2/neu and hormone receptors. Exp. Mol. Pathol. 70, 303–316 (2001).
Leal, C. B., Schmitt, F. C., Bento, M. J., Maia, N. C. & Lopes, C. S. Ductal carcinoma in situ of the breast. Histologic categorization and its relationship to ploidy and immunohistochemical expression of hormone receptors, p53, and c-erbB-2 protein. Cancer 75, 2123–2131 (1995).
Hou, R. et al. Prediction of upstaged ductal carcinoma in situ using forced labeling and domain adaptation. IEEE Trans. Biomed. Eng. 67, 1565–1572 (2020).
Gosling, S. et al. Calcification microstructure reflects breast tissue microenvironment. J. Mammary Gland. Biol. Neoplasia 24, 333–342 (2019).
Kim, H. et al. Clinical outcomes according to molecular subtypes in stage II-III breast cancer patients treated with neoadjuvant chemotherapy followed by surgery and radiotherapy. Asia Pac. J. Clin. Oncol. 13, 329–336 (2017).
Orsaria, P. et al. Clinical outcomes among major breast cancer subtypes after neoadjuvant chemotherapy: impact on breast cancer recurrence and survival. Anticancer. Res. 41, 2697–2709 (2021).
Yu, K. D. et al. Different distribution of breast cancer subtypes in breast ductal carcinoma in situ (DCIS), DCIS with microinvasion, and DCIS with invasion component. Ann. Surg. Oncol. 18, 1342–1348 (2011).
Doebar, S. C. et al. Extent of ductal carcinoma in situ according to breast cancer subtypes: a population-based cohort study. Breast Cancer Res. Treat. 158, 179–187 (2016).
Bergholtz, H. et al. Contrasting DCIS and invasive breast cancer by subtype suggests basal-like DCIS as distinct lesions. NPJ Breast Cancer 6, 26 (2020). This study convincingly shows that RNA-intrinsic centroid-based classifiers do not match between DCIS and IBC owing to the different distribution of subtype frequencies and mutations in DCIS and IBC.
Coates, A. S. et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 26, 1533–1546 (2015).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Solin, L. J. et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J. Natl Cancer Inst. 105, 701–710 (2013).
Rakovitch, E. et al. Multigene expression assay and benefit of radiotherapy after breast conservation in ductal carcinoma in situ. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djw256 (2017).
Nofech-Mozes, S., Hanna, W. & Rakovitch, E. Molecular evaluation of breast ductal carcinoma in situ with Oncotype DX DCIS. Am. J. Pathol. 189, 975–980 (2019).
Raldow, A. C., Sher, D., Chen, A. B. & Punglia, R. S. Cost effectiveness of DCISionRT for guiding treatment of ductal carcinoma in situ. JNCI Cancer Spectr. 4, pkaa004 (2020).
Raldow, A. C., Sher, D., Chen, A. B., Recht, A. & Punglia, R. S. Cost effectiveness of the Oncotype DX DCIS score for guiding treatment of patients with ductal carcinoma in situ. J. Clin. Oncol. 34, 3963–3968 (2016).
Bremer, T. et al. A biological signature for breast ductal carcinoma in situ to predict radiotherapy benefit and assess recurrence risk. Clin. Cancer Res. 24, 5895–5901 (2018).
Weinmann, S. et al. Validation of a ductal carcinoma in situ biomarker profile for risk of recurrence after breast-conserving surgery with and without radiotherapy. Clin. Cancer Res. 26, 4054–4063 (2020).
Mitchell, E. et al. Loss of myoepithelial calponin-1 characterizes high-risk ductal carcinoma in situ cases, which are further stratified by T cell composition. Mol. Carcinog. 59, 701–712 (2020).
Nagasawa, S. et al. Genomic profiling reveals heterogeneous populations of ductal carcinoma in situ of the breast. Commun. Biol. 4, 438 (2021).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Yates, L. R. et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 21, 751–759 (2015).
Pareja, F. et al. Whole-exome sequencing analysis of the progression from non-low-grade ductal carcinoma in situ to invasive ductal carcinoma. Clin. Cancer Res. 26, 3682–3693 (2020).
Agahozo, M. C. et al. PIK3CA mutations in ductal carcinoma in situ and adjacent invasive breast cancer. Endocr. Relat. Cancer 26, 471–482 (2019).
Roylance, R. et al. Comparative genomic hybridization of breast tumors stratified by histological grade reveals new insights into the biological progression of breast cancer. Cancer Res. 59, 1433–1436 (1999).
Kitamura, M. et al. Progression potential of ductal carcinoma in situ assessed by genomic copy number profiling. Pathobiology 86, 92–101 (2019).
Buerger, H. et al. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J. Pathol. 189, 521–526 (1999).
Abba, M. C. et al. A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res. 75, 3980–3990 (2015).
Afghahi, A. et al. Chromosomal copy number alterations for associations of ductal carcinoma in situ with invasive breast cancer. Breast Cancer Res. 17, 108 (2015).
Pang, J. B. et al. Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod. Pathol. 30, 952–963 (2017).
Latta, E. K., Tjan, S., Parkes, R. K. & O’Malley, F. P. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod. Pathol. 15, 1318–1325 (2002).
Van Bockstal, M. et al. Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch. 465, 275–289 (2014).
Lambein, K. et al. Comparison of HER2 amplification status among breast cancer subgroups offers new insights in pathways of breast cancer progression. Virchows Arch. 471, 575–587 (2017).
Miligy, I. M. et al. The clinical and biological significance of HER2 over-expression in breast ductal carcinoma in situ: a large study from a single institution. Br. J. Cancer 120, 1075–1082 (2019).
Park, K., Han, S., Kim, H. J., Kim, J. & Shin, E. HER2 status in pure ductal carcinoma in situ and in the intraductal and invasive components of invasive ductal carcinoma determined by fluorescence in situ hybridization and immunohistochemistry. Histopathology 48, 702–707 (2006).
Vincent-Salomon, A. et al. Integrated genomic and transcriptomic analysis of ductal carcinoma in situ of the breast. Clin. Cancer Res. 14, 1956–1965 (2008).
Begon, D. Y., Delacroix, L., Vernimmen, D., Jackers, P. & Winkler, R. Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J. Biol. Chem. 280, 24428–24434 (2005).
Powe, D. G. et al. Investigating AP-2 and YY1 protein expression as a cause of high HER2 gene transcription in breast cancers with discordant HER2 gene amplification. Breast Cancer Res. 11, R90 (2009).
Lin, C. Y. et al. Genomic landscape of ductal carcinoma in situ and association with progression. Breast Cancer Res. Treat. 178, 307–316 (2019).
Li, H. et al. PIK3CA mutations mostly begin to develop in ductal carcinoma of the breast. Exp. Mol. Pathol. 88, 150–155 (2010).
Martinez-Saez, O. et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 22, 45 (2020).
Kumar, A. & Carrera, A. C. New functions for PI3K in the control of cell division. Cell Cycle 6, 1696–1698 (2007).
Rodgers, S. J., Ferguson, D. T., Mitchell, C. A. & Ooms, L. M. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci. Rep. https://doi.org/10.1042/BSR20160432 (2017).
Eeles, R. A., Bartkova, J., Lane, D. P. & Bartek, J. The role of TP53 in breast cancer development. Cancer Surv. 18, 57–75 (1993).
Meijnen, P., Peterse, J. L., Antonini, N., Rutgers, E. J. & van de Vijver, M. J. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br. J. Cancer 98, 137–142 (2008).
Klajic, J. et al. Quantitative DNA methylation analyses reveal stage dependent DNA methylation and association to clinico-pathological factors in breast tumors. BMC Cancer 13, 456 (2013).
Muggerud, A. A. et al. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. 12, R3 (2010).
Pang, J. M. et al. Methylation profiling of ductal carcinoma in situ and its relationship to histopathological features. Breast Cancer Res. 16, 423 (2014).
Verschuur-Maes, A. H., de Bruin, P. C. & van Diest, P. J. Epigenetic progression of columnar cell lesions of the breast to invasive breast cancer. Breast Cancer Res. Treat. 136, 705–715 (2012).
Lee, J. S. et al. Quantitative promoter hypermethylation profiles of ductal carcinoma in situ in North American and Korean women: potential applications for diagnosis. Cancer Biol. Ther. 7, 1398–1406 (2008).
Moelans, C. B., Verschuur-Maes, A. H. & van Diest, P. J. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J. Pathol. 225, 222–231 (2011).
Kim, H., Kim, C. Y., Park, K. H. & Kim, A. Clonality analysis of multifocal ipsilateral breast carcinomas using X-chromosome inactivation patterns. Hum. Pathol. 78, 106–114 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ying, Z. & Beronja, S. Embryonic barcoding of equipotent mammary progenitors functionally identifies breast cancer drivers. Cell Stem Cell 26, 403–419 (2020).
Giraddi, R. R. et al. Stem and progenitor cell division kinetics during postnatal mouse mammary gland development. Nat. Commun. 6, 8487 (2015).
Scheele, C. L. et al. Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542, 313–317 (2017). This study shows that the majority of terminal end bud cells function as highly proliferative, lineage-committed MaSCs, heterogeneous in their expression profile. Through cell rearrangements during terminal end bud bifurcation, each MaSC is able to contribute actively to long-term growth, not directly linked to a single expression profile.
Zhou, J. et al. Stem cells and cellular origins of breast cancer: updates in the rationale, controversies, and therapeutic implications. Front. Oncol. 9, 820 (2019).
Watson, C. J. & Khaled, W. T. Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment. Development https://doi.org/10.1242/dev.169862 (2020).
Williams, J. M. & Daniel, C. W. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 97, 274–290 (1983).
Silberstein, G. B. & Daniel, C. W. Glycosaminoglycans in the basal lamina and extracellular matrix of serially aged mouse mammary ducts. Mech. Ageing Dev. 24, 151–162 (1984).
van Amerongen, R., Bowman, A. N. & Nusse, R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11, 387–400 (2012).
Van Keymeulen, A. et al. Lineage-restricted mammary stem cells sustain the development, homeostasis, and regeneration of the estrogen receptor positive lineage. Cell Rep. 20, 1525–1532 (2017).
Keymeulen, A. V. et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525, 119–123 (2015).
Davis, F. M. et al. Single-cell lineage tracing in the mammary gland reveals stochastic clonal dispersion of stem/progenitor cell progeny. Nat. Commun. 7, 13053 (2016).
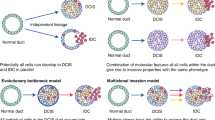
Casasent, A. K., Edgerton, M. & Navin, N. E. Genome evolution in ductal carcinoma in situ: invasion of the clones. J. Pathol. 241, 208–218 (2017). In this review, the role of intratumour heterogeneity in the progression of DCIS to IDC in the context of independent lineages, evolutionary bottlenecks and multiclonal invasion is discussed, along with their relevance to the diagnosis of DCIS and the treatment of patients with DCIS.
Trinh, A. et al. Genomic alterations during the in situ to invasive ductal breast carcinoma transition shaped by the immune system. Mol. Cancer Res. 19, 623–635 (2021).
Kroigard, A. B. et al. Clonal expansion and linear genome evolution through breast cancer progression from pre-invasive stages to asynchronous metastasis. Oncotarget 6, 5634–5649 (2015).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Poste, G. & Fidler, I. J. The pathogenesis of cancer metastasis. Nature 283, 139–146 (1980).
Walens, A. et al. Adaptation and selection shape clonal evolution of tumors during residual disease and recurrence. Nat. Commun. 11, 5017 (2020).
Welter, L. et al. Treatment response and tumor evolution: lessons from an extended series of multianalyte liquid biopsies in a metastatic breast cancer patient. Cold Spring Harb. Mol. Case Stud. https://doi.org/10.1101/mcs.a005819 (2020).
Maggrah, A. et al. Paired ductal carcinoma in situ and invasive breast cancer lesions in the D-loop of the mitochondrial genome indicate a cancerization field effect. Biomed. Res. Int. 2013, 379438 (2013).
Desmedt, C. et al. Uncovering the genomic heterogeneity of multifocal breast cancer. J. Pathol. 236, 457–466 (2015).
McCrorie, A. D. et al. Multifocal breast cancers are more prevalent in BRCA2 versus BRCA1 mutation carriers. J. Pathol. Clin. Res. 6, 146–153 (2020).
Visser, L. L. et al. Discordant marker expression between invasive breast carcinoma and corresponding synchronous and preceding DCIS. Am. J. Surg. Pathol. 43, 1574–1582 (2019).
Foschini, M. P. et al. Genetic clonal mapping of in situ and invasive ductal carcinoma indicates the field cancerization phenomenon in the breast. Hum. Pathol. 44, 1310–1319 (2013).
Sakr, R. A. et al. PI3K pathway activation in high-grade ductal carcinoma in situ-implications for progression to invasive breast carcinoma. Clin. Cancer Res. 20, 2326–2337 (2014).
Ottesen, G. L., Christensen, I. J., Larsen, J. K., Hansen, B. & Andersen, A. J. Flow cytometric DNA analysis of breast cancers with predominance of carcinoma in situ: a comparison of the premalignant and malignant components. Clin. Cancer Res. 1, 881–888 (1995).
Sontag, L. & Axelrod, D. E. Evaluation of pathways for progression of heterogeneous breast tumors. J. Theor. Biol. 232, 179–189 (2005).
Mai, K. T. Morphological evidence for field effect as a mechanism for tumour spread in mammary Paget’s disease. Histopathology 35, 567–576 (1999).
Asioli, S., Morandi, L., Cavatorta, C., Cucchi, M. C. & Foschini, M. P. The impact of field cancerization on the extent of duct carcinoma in situ (DCIS) in breast tissue after conservative excision. Eur. J. Surg. Oncol. 42, 1806–1813 (2016).
Tan, M. P. Integration of ‘sick lobe hypothesis’ with concept of field cancerisation for a personalised surgical margin for breast conserving surgery. J. Surg. Oncol. 116, 954–955 (2017).
Going, J. J. & Mohun, T. J. Human breast duct anatomy, the ‘sick lobe’ hypothesis and intraductal approaches to breast cancer. Breast Cancer Res. Treat. 97, 285–291 (2006). This study reveals highly detailed central and peripheral duct anatomy in the human breast. Such knowledge is required for understanding normal breast development, for understanding the growth of cancer precursors and for developing the intraductal approach to breast cancer.
Petrova, S. C. et al. Regulation of breast cancer oncogenesis by the cell of origin’s differentiation state. Oncotarget 11, 3832–3848 (2020).
Tan, M. P. & Tot, T. The sick lobe hypothesis, field cancerisation and the new era of precision breast surgery. Gland. Surg. 7, 611–618 (2018).
Dooley, W., Bong, J. & Parker, J. Redefining lumpectomy using a modification of the “sick lobe” hypothesis and ductal anatomy. Int. J. Breast Cancer 2011, 726384 (2011).
Tot, T. The theory of the sick breast lobe and the possible consequences. Int. J. Surg. Pathol. 15, 369–375 (2007).
Park, S., Supek, F. & Lehner, B. Systematic discovery of germline cancer predisposition genes through the identification of somatic second hits. Nat. Commun. 9, 2601 (2018).
Knudson, A. G. Jr Heredity and human cancer. Am. J. Pathol. 77, 77–84 (1974).
Konishi, H. et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc. Natl Acad. Sci. USA 108, 17773–17778 (2011).
Gao, Y. et al. Single-cell sequencing deciphers a convergent evolution of copy number alterations from primary to circulating tumor cells. Genome Res. 27, 1312–1322 (2017).
Wang, F. et al. MEDALT: single-cell copy number lineage tracing enabling gene discovery. Genome Biol. 22, 70 (2021).
Tegze, B. et al. Parallel evolution under chemotherapy pressure in 29 breast cancer cell lines results in dissimilar mechanisms of resistance. PLoS ONE 7, e30804 (2012).
Brommesson, S. et al. Tiling array-CGH for the assessment of genomic similarities among synchronous unilateral and bilateral invasive breast cancer tumor pairs. BMC Clin. Pathol. 8, 6 (2008).
Regitnig, P., Ploner, F., Maderbacher, M. & Lax, S. F. Bilateral carcinomas of the breast with local recurrence: analysis of genetic relationship of the tumors. Mod. Pathol. 17, 597–602 (2004).
Lim, B., Lin, Y. & Navin, N. Advancing cancer research and medicine with single-cell genomics. Cancer Cell 37, 456–470 (2020).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019).
Badve, S. S. et al. Multi-protein spatial signatures in ductal carcinoma in situ (DCIS) of breast. Br. J. Cancer 124, 1150–1159 (2021).
Ma, X. J. et al. Gene expression profiles of human breast cancer progression. Proc. Natl Acad. Sci. USA 100, 5974–5979 (2003).
Porter, D. et al. Molecular markers in ductal carcinoma in situ of the breast. Mol. Cancer Res. 1, 362–375 (2003).
Castro, N. P. et al. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 10, R87 (2008).
Song, G. et al. Identification of aberrant gene expression during breast ductal carcinoma in situ progression to invasive ductal carcinoma. J. Int. Med. Res. 48, 0300060518815364 (2020).
Dettogni, R. S. et al. Potential biomarkers of ductal carcinoma in situ progression. BMC Cancer 20, 119 (2020).
Lee, S. et al. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 72, 4574–4586 (2012).
Schuetz, C. S. et al. Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res. 66, 5278–5286 (2006).
Abba, M. C. et al. Transcriptomic changes in human breast cancer progression as determined by serial analysis of gene expression. Breast Cancer Res. 6, R499–R513 (2004).
Knudsen, E. S. et al. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res. Treat. 133, 1009–1024 (2012).
Krstic, M. et al. TBX3 promotes progression of pre-invasive breast cancer cells by inducing EMT and directly up-regulating SLUG. J. Pathol. 248, 191–203 (2019).
Coradini, D., Boracchi, P., Ambrogi, F., Biganzoli, E. & Oriana, S. Cell polarity, epithelial-mesenchymal transition, and cell-fate decision gene expression in ductal carcinoma in situ. Int. J. Surg. Oncol. 2012, 984346 (2012).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Coradini, D., Boracchi, P., Oriana, S., Biganzoli, E. & Ambrogi, F. Cell identity disruption in breast cancer precursors. Anticancer. Res. 34, 1307–1319 (2014).
Deshmukh, A. P. et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl Acad. Sci. USA 118, e2102050118 (2021).
Sorlie, T. et al. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: gene expression analyses across three different platforms. BMC Genomics 7, 127 (2006).
Oliemuller, E. et al. SOX11 promotes epithelial/mesenchymal hybrid state and alters tropism of invasive breast cancer cells. Elife https://doi.org/10.7554/eLife.58374 (2020).
Oliemuller, E. et al. SOX11 promotes invasive growth and ductal carcinoma in situ progression. J. Pathol. 243, 193–207 (2017).
Ma, X. J., Dahiya, S., Richardson, E., Erlander, M. & Sgroi, D. C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 11, R7 (2009).
Allinen, M. et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6, 17–32 (2004).
Yoosuf, N., Navarro, J. F., Salmen, F., Stahl, P. L. & Daub, C. O. Identification and transfer of spatial transcriptomics signatures for cancer diagnosis. Breast Cancer Res. 22, 6 (2020).
Li, Y. H. et al. Visualization and analysis of gene expression in Stanford type A aortic dissection tissue section by spatial transcriptomics. Front. Genet 12, 698124 (2021).
Wu, S. Z. et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 53, 1334–1347 (2021).
Hu, M. et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell 13, 394–406 (2008).
Gil Del Alcazar, C. R. et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 7, 1098–1115 (2017). This study reported fewer activated GZMB+CD8+ T cells in IBC than in DCIS and a decrease in CD8+ signatures in IBC.
Acerbi, I. et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7, 1120–1134 (2015).
Allen, M. D. et al. Altered microenvironment promotes progression of preinvasive breast cancer: myoepithelial expression of alphavbeta6 integrin in DCIS identifies high-risk patients and predicts recurrence. Clin. Cancer Res. 20, 344–357 (2014).
Chanson, L. et al. Self-organization is a dynamic and lineage-intrinsic property of mammary epithelial cells. Proc. Natl Acad. Sci. USA 108, 3264–3269 (2011).
Duivenvoorden, H. M. et al. Myoepithelial cell-specific expression of stefin A as a suppressor of early breast cancer invasion. J. Pathol. 243, 496–509 (2017).
Sarper, M. et al. Loss of MMP-8 in ductal carcinoma in situ (DCIS)-associated myoepithelial cells contributes to tumour promotion through altered adhesive and proteolytic function. Breast Cancer Res. 19, 33 (2017).
Wang, L. et al. TGF-beta1 stimulates epithelial-mesenchymal transition and cancer-associated myoepithelial cell during the progression from in situ to invasive breast cancer. Cancer Cell Int. 19, 343 (2019).
Risom, T. et al. Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma. Cell 185, 299–310 (2022). This Human Tumor Atlas network Breast PreCancer Atlas study suggests that myoepithelial disruption is more advanced in patients with DCIS who do not develop IBC.
Conklin, M. W. et al. Collagen alignment as a predictor of recurrence after ductal carcinoma in situ. Cancer Epidemiol. Biomark. Prev. 27, 138–145 (2018).
Toss, M. S. et al. Collagen (XI) alpha-1 chain is an independent prognostic factor in breast ductal carcinoma in situ. Mod. Pathol. https://doi.org/10.1038/s41379-019-0286-9 (2019).
Toss, M. S. et al. Geometric characteristics of collagen have independent prognostic significance in breast ductal carcinoma in situ: an image analysis study. Mod. Pathol. https://doi.org/10.1038/s41379-019-0296-7 (2019).
Toss, M. S. et al. Prolyl-4-hydroxylase alpha subunit 2 (P4HA2) expression is a predictor of poor outcome in breast ductal carcinoma in situ (DCIS). Br. J. Cancer 119, 1518–1526 (2018).
Habel, L. A. et al. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol. Biomark. Prev. 19, 2488–2495 (2010).
Huo, C. W. et al. High mammographic density in women is associated with protumor inflammation. Breast Cancer Res. 20, 92 (2018).
Strand, S. H. et al. DCIS genomic signatures define biology and correlate with clinical outcome: a Human Tumor Atlas Network (HTAN) analysis of TBCRC 038 and RAHBT cohorts. Preprint at bioRxiv https://doi.org/10.1101/2021.06.16.448585.
Morita, M. et al. CD8+ tumor-infiltrating lymphocytes contribute to spontaneous “healing” in HER2-positive ductal carcinoma in situ. Cancer Med. 5, 1607–1618 (2016).
Agahozo, M. C. et al. Immune response and stromal changes in ductal carcinoma in situ of the breast are subtype dependent. Mod. Pathol. 33, 1773–1782 (2020).
Pruneri, G. et al. The prevalence and clinical relevance of tumor-infiltrating lymphocytes (TILs) in ductal carcinoma in situ of the breast. Ann. Oncol. 28, 321–328 (2017). This study assesses TILs by the criteria of the International Immuno-Oncology Biomarker Working Group in a large cohort of patients with pure DCIS (1,488 patients) with known outcome.
Almekinders, M. M. et al. Comprehensive multiplexed immune profiling of the ductal carcinoma in situ immune microenvironment regarding subsequent ipsilateral invasive breast cancer risk. Br. J. Cancer https://doi.org/10.1038/s41416-022-01888-2 (2022). In this comprehensive analysis, multiplexed profiling of the DCIS immune microenvironment in a large, well-annotated case–control series of pure DCIS does not reveal an association between the investigated immune factors and subsequent ipsilateral IBC risk.
Toss, M. S. et al. The prognostic significance of immune microenvironment in breast ductal carcinoma in situ. Br. J. Cancer https://doi.org/10.1038/s41416-020-0797-7 (2020).
Campbell, M. J. et al. Characterizing the immune microenvironment in high-risk ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 161, 17–28 (2017).
Thike, A. A. et al. Higher densities of tumour-infiltrating lymphocytes and CD4+ T cells predict recurrence and progression of ductal carcinoma in situ of the breast. Histopathology 76, 852–864 (2020).
Chen, X. Y. et al. Higher density of stromal M2 macrophages in breast ductal carcinoma in situ predicts recurrence. Virchows Arch. https://doi.org/10.1007/s00428-019-02735-1 (2020).
Agahozo, M. C. et al. Ductal carcinoma in situ of the breast: immune cell composition according to subtype. Mod. Pathol. https://doi.org/10.1038/s41379-019-0331-8 (2019).
Chen, X. Y. et al. Breast ductal carcinoma in situ associated with microinvasion induces immunological response and predicts ipsilateral invasive recurrence. Virchows Arch: https://doi.org/10.1007/s00428-020-02959-6 (2020).
Darvishian, F. et al. Tumor-infiltrating lymphocytes in a contemporary cohort of women with ductal carcinoma in situ (DCIS). Ann. Surg. Oncol. 26, 3337–3343 (2019).
Farolfi, A. et al. Tumor-infiltrating lymphocytes (TILs) and risk of a second breast event after a ductal carcinoma in situ. Front. Oncol. 10, 1486 (2020).
Hendry, S. et al. Relationship of the breast ductal carcinoma in situ immune microenvironment with clinicopathological and genetic features. Clin. Cancer Res. 23, 5210–5217 (2017).
Kim, M. et al. Immune microenvironment in ductal carcinoma in situ: a comparison with invasive carcinoma of the breast. Breast Cancer Res. https://doi.org/10.1186/s13058-020-01267-w (2020).
Ramachandra, S., Machin, L., Ashley, S., Monaghan, P. & Gusterson, B. A. Immunohistochemical distribution of c-erbB-2 in in situ breast carcinoma-a detailed morphological analysis. J. Pathol. 161, 7–14 (1990).
Toss, M. S. et al. Prognostic significance of tumor-infiltrating lymphocytes in ductal carcinoma in situ of the breast. Mod. Pathol. 31, 1226–1236 (2018).
Narayanan, P. L. et al. Unmasking the immune microecology of ductal carcinoma in situ with deep learning. NPJ Breast Cancer 7, 19 (2021).
Almekinders, M. M. M. et al. Breast adipocyte size associates with ipsilateral invasive breast cancer risk after ductal carcinoma in situ. NPJ Breast Cancer 7, 31 (2021).
Morris, P. G. et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 4, 1021–1029 (2011).
Lee, J. Y., Sohn, K. H., Rhee, S. H. & Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276, 16683–16689 (2001).
Meyer, D. S. et al. Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res. 71, 4344–4351 (2011).
Liu, X. et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl Acad. Sci. USA 104, 12111–12116 (2007).
Cardiff, R. D., Moghanaki, D. & Jensen, R. A. Genetically engineered mouse models of mammary intraepithelial neoplasia. J. Mammary Gland. Biol. Neoplasia 5, 421–437 (2000).
Crist, K. A., Chaudhuri, B., Shivaram, S. & Chaudhuri, P. K. Ductal carcinoma in situ in rat mammary gland. J. Surg. Res. 52, 205–208 (1992).
Thompson, H. J. & Singh, M. Rat models of premalignant breast disease. J. Mammary Gland. Biol. Neoplasia 5, 409–420 (2000).
Hong, Y. et al. Mouse-INtraDuctal (MIND): an in vivo model for studying the underlying mechanisms of DCIS malignancy. J. Pathol. https://doi.org/10.1002/path.5820 (2021). This hallmark publication describes the development of the mouse intraductal model, in which patient-derived DCIS epithelial cells are injected intraductally and allowed to progress naturally in mice to study the dynamics of DCIS initiation and progression.
Annunziato, S., Barazas, M., Rottenberg, S. & Jonkers, J. Genetic dissection of cancer development, therapy response, and resistance in mouse models of breast cancer. Cold Spring Harb. Symp. Quant. Biol. 81, 141–150 (2016).
Bu, W. & Li, Y. Intraductal injection of lentivirus vectors for stably introducing genes into rat mammary epithelial cells in vivo. J. Mammary Gland. Biol. Neoplasia 25, 389–396 (2020).
Behbod, F., Gomes, A. M. & Machado, H. L. Modeling human ductal carcinoma in situ in the mouse. J. Mammary Gland. Biol. Neoplasia 23, 269–278 (2018).
Valdez, K. E. et al. Human primary ductal carcinoma in situ (DCIS) subtype-specific pathology is preserved in a mouse intraductal (MIND) xenograft model. J. Pathol. 225, 565–573 (2011).
Koren, S. et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature 525, 114–118 (2015).
Acknowledgements
This work was supported by Cancer Research UK and by KWF Kankerbestrijding (reference C38317/A24043). A.K.C. acknowledges support from the CPRIT Single Cell Genomics Center (RP180684) for funding. N.E.N. acknowledges support from the US National Cancer Institute (RO1CA240526) and the CPRIT Single Cell Genomics Center (RP180684). E.S.H. is funded by RFA-CA-17-035 (NIH), 1505-30497 (PCORI), BCRF 19-074 (BCRF), DOD BC132057 and R01 CA185138-01. S.N.-Z. is funded by a Cancer Research UK Advanced Clinician Scientist Fellowship (C60100/A23916) and an NIHR Research Professorship (NIHR 301607) and is supported by the NIHR Cambridge Biomedical Research Centre (BRC-125-20014). D.C. is funded by the Cancer Research UK PRECISION award. E.S.H, A.M.T and D.C. are supported by the PCORI-funded COMET study through the Alliance for Clinical Trials in Oncology Foundation. J.J and J.v.R. acknowledge funding by the Oncode Institute. C.M. is supported by the AVL Donation Investment Fund of the AVL Foundation. The authors thank the Grand Challenge PRECISION Consortium Steering Group. They also thank T. Kumar and M. Edgerton from MD Anderson Cancer Center and E. J. Sawyer from King’s College London for their feedback and support in the preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
A.K.C., M.M.A. and C.M. contributed equally to this work. J.W., A.K.C., M.A., C.M., A.M.T., J.v.R. and N.E.N researched data for the article. J.W., A.K.C., M.M.A., C.M., P.B., A.T., J.J., E.L., J.v.R., E.S.H., S.N.-Z. and N.E.N. made substantial contributions to discussion of the content. J.W., A.K.C., M.M.A., C.M., P.B., J.v.R., S.N.-Z. and N.E.N. wrote the manuscript. All authors were involved in the review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.N.Z. has filed the following patents on the use of mutational signatures for clinical applications: PCT/EP2017/06029, PCT/EP2017/060289, PCT/EP2017/060279, PCT/EP2017/060298 and PCT/EP2017/084409. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
COMET: https://clinicaltrials.gov/ct2/show/NCT02926911?term=comet&cond=DCIS&draw=2&rank=1
LORD: https://clinicaltrials.gov/ct2/show/NCT02492607
LORETTA: https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000028298
LORIS: https://www.birmingham.ac.uk/research/crctu/trials/loris/index.aspx
Glossary
- Synchronous DCIS–IBC
-
Cases of ductal carcinoma in situ (DCIS) and invasive breast cancer (IBC) that were diagnosed at the same time.
- Breast-conserving surgery
-
(BCS). Surgery to remove only part of the breast tissue.
- Active surveillance
-
Also called ‘active monitoring’, closely watching a patient’s condition but not giving any treatment unless the results of regularly scheduled tests show that the condition is getting worse.
- Lobular carcinoma in situ
-
A non-invasive neoplastic proliferation of discohesive cells, originating in the terminal duct lobular units.
- Sequential DCIS and/or IBC
-
General term for when ductal carcinoma in situ (DCIS) has recurred as DCIS or invasive breast cancer (IBC).
- Contralateral DCIS and/or IBC
-
General term for when ductal carcinoma in situ (DCIS) and/or invasive breast cancer (IBC) events occur on the opposite sides of the body.
- Ipsilateral DCIS and/or IBC
-
General term for when ductal carcinoma in situ (DCIS) and/or invasive breast cancer (IBC) events occur on the same side, often the same region, of the body as the initial DCIS.
- Pure DCIS
-
Ductal carcinoma in situ (DCIS) without invasive growth.
- Rudimental ductal tree
-
The small ductal network formed during embryonic stages, from which the adult network is formed during puberty.
- Sick lobes
-
A ductal tree with a normal phenotype that carries a large number of mutant cells, thereby priming it for tumour initiation.
- Mitochondrial D-loop
-
The non-coding displacement (D) loop of mitochondrial DNA, which lacks histone protection.
- Sequential DCIS–IBC
-
Ductal carcinoma in situ (DCIS) that has recurred as invasive breast cancer (IBC).
- Immunoediting
-
The process in which the immune system can constrain or promote tumour development through the stages of elimination, equilibrium and escape.
- Neoantigen load
-
The amount of mutations in neoplastic cells that translate into new (non-self) peptides that are presented by human leukocyte antigen at the cell surface.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Casasent, A.K., Almekinders, M.M., Mulder, C. et al. Learning to distinguish progressive and non-progressive ductal carcinoma in situ. Nat Rev Cancer 22, 663–678 (2022). https://doi.org/10.1038/s41568-022-00512-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-022-00512-y
This article is cited by
-
Progression from ductal carcinoma in situ to invasive breast cancer: molecular features and clinical significance
Signal Transduction and Targeted Therapy (2024)
-
Non-progressive breast carcinomas detected at mammography screening: a population study
Breast Cancer Research (2023)
-
Researchers dig into cancer niches
Nature Methods (2023)