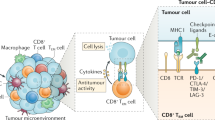
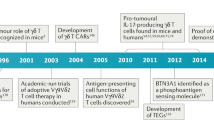
Abstract
Although immunotherapy research to date has focused largely on T cells, there is mounting evidence that tumour-infiltrating B cells and plasma cells (collectively referred to as tumour-infiltrating B lymphocytes (TIL-Bs)) have a crucial, synergistic role in tumour control. In many cancers, TIL-Bs have demonstrated strong predictive and prognostic significance in the context of both standard treatments and immune checkpoint blockade, offering the prospect of new therapeutic opportunities that leverage their unique immunological properties. Drawing insights from autoimmunity, we review the molecular phenotypes, architectural contexts, antigen specificities, effector mechanisms and regulatory pathways relevant to TIL-Bs in human cancer. Although the field is young, the emerging picture is that TIL-Bs promote antitumour immunity through their unique mode of antigen presentation to T cells; their role in assembling and perpetuating immunologically ‘hot’ tumour microenvironments involving T cells, myeloid cells and natural killer cells; and their potential to combat immune editing and tumour heterogeneity through the easing of self-tolerance mechanisms. We end by discussing the most promising approaches to enhance TIL-B responses in concert with other immune cell subsets to extend the reach, potency and durability of cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thommen, D. S. et al. A transcriptionally and functionally distinct PD-1+CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 24, 994–1004 (2018).
Workel, H. H. et al. A transcriptionally distinct CXCL13+CD103+CD8+ T-cell population is associated with B-cell recruitment and neoantigen load in human cancer. Cancer Immunol. Res. 7, 784–796 (2019).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789 (2019).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Reuschenbach, M., von Knebel Doeberitz, M. & Wentzensen, N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol. Immunother. 58, 1535–1544 (2009).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Sng, J. et al. AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol. 4, eaav6778 (2019). This study demonstrates that defects in T cell central tolerance can markedly affect the B cell compartment, leading to autoantibody production.
Finney, J., Watanabe, A., Kelsoe, G. & Kuraoka, M. Minding the gap: the impact of B-cell tolerance on the microbial antibody repertoire. Immunol. Rev. 292, 24–36 (2019).
Burnett, D. L., Reed, J. H., Christ, D. & Goodnow, C. C. Clonal redemption and clonal anergy as mechanisms to balance B cell tolerance and immunity. Immunol. Rev. 292, 61–75 (2019). This Review describes the concept of clonal redemption, in which anergic B cells can mutate away from self-reactivity during somatic hypermutation to produce antibodies against foreign antigens.
Brink, R. & Phan, T. G. Self-reactive B cells in the germinal center reaction. Annu. Rev. Immunol. 36, 339–360 (2018).
Meffre, E. & O’Connor, K. C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 292, 90–101 (2019).
Wang, E. Y. et al. Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021).
Qiu, C. C., Caricchio, R. & Gallucci, S. Triggers of autoimmunity: the role of bacterial infections in the extracellular exposure of lupus nuclear autoantigens. Front. Immunol. 10, 1–15 (2019).
Sanz, I. et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol. 10, 1–17 (2019). This Review describes the core markers required to reproducibly distinguish the different phenotypic subsets of B cells.
Stewart, A. et al. Single-cell transcriptomic analyses define distinct peripheral B cell subsets and discrete development pathways. Front. Immunol. 12, 602539 (2021).
Glass, D. R. et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity 53, 217–232 (2020). This work presents an atlas of peripheral B cell subsets isolated from normal lymphoid tissues from human participants.
Holmes, A. B. et al. Single-cell analysis of germinal-center B cells informs on lymphoma cell of origin and outcome. J. Exp. Med. 217, e20200483 (2020).
He, S. et al. Single-cell transcriptome profiling an adult human cell atlas of 15 major organs. Genome Biol. 21, 294 (2020).
Allie, S. R. & Randall, T. D. Resident memory B cells. Viral Immunol. 33, 282–293 (2020).
Weisel, N. M., Weisel, F. J., Farber, D. L. & Borghesi, L. A. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood 136, 2774–2785 (2021).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019).
Jenks, S. A. et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739 (2018). This study identifies DN2 B cells as predominant producers of autoantibodies in lupus.
Li, Y., Li, Z. & Hu, F. Double-negative (DN) B cells: an under-recognized effector memory B cell subset in autoimmunity. Clin. Exp. Immunol. 205, 119–127 (2021).
Chang, H. D. et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 61, 86–91 (2019).
Oleinika, K., Mauri, C. & Salama, A. D. Effector and regulatory B cells in immune-mediated kidney disease. Nat. Rev. Nephrol. 15, 11–26 (2019).
Matsumoto, M. et al. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 34, 703–714 (2011).
Miles, K. et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc. Natl Acad. Sci. USA 109, 887–892 (2012).
Yoshizaki, A. et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268 (2012).
Rosser, E. C. et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat. Med. 20, 1334–1339 (2014).
Menon, M., Blair, P. A., Isenberg, D. A. & Mauri, C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 44, 683–697 (2016).
Saulep-Easton, D. et al. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia 30, 163–172 (2016).
Fehres, C. M. et al. APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front. Immunol. 10, 1368 (2019).
Wang, R. X. et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641 (2014).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Kaltenmeier, C. et al. CD4+ T cell–derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J. Immunol. 194, 3768–3777 (2015).
Fridman, W. H. et al. B cells and cancer: to B or not to B? J. Exp. Med. 218, e20200851 (2021).
Nielsen, J. S. et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27– memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 18, 3281–3292 (2012). This study is the first to describe the co-localization and combined prognostic effects of tumour-infiltrating CD20+ B cells and CD8+ T cells in human cancer, highlighting the importance of T cell–B cell interactions in effective antitumour immunity.
Centuori, S. M. et al. Double-negative (CD27–IgD–) B cells are expanded in NSCLC and inversely correlate with affinity-matured B cell populations. J. Transl. Med. 16, 1–8 (2018).
Montfort, A. et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin. Cancer Res. 23, 250–262 (2017).
Gong, L. et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun. 12, 1540 (2021).
Bruno, T. C. et al. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non–small cell lung cancer patients. Cancer Immunol. Res. 5, 898–907 (2017). This work is the first report of exhausted B cells and their association with Treg cells in human cancer.
Hu, Q. et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 12, 2186 (2021). This study provides a comprehensive atlas of TIL-Bs in patients with breast cancer, and reports B cell signatures (identified by single-cell sequencing) that significantly associate with improved survival.
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308 (2018).
Qian, J. et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res. 30, 745–762 (2020).
Ruffin, A. T. et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 12, 3349 (2021).
Chen, J. et al. Single-cell transcriptome and antigen-immunoglobin analysis reveals the diversity of B cells in non-small cell lung cancer. Genome Biol. 21, 152 (2020).
Mei, Y. et al. Single-cell analyses reveal suppressive tumor microenvironment of human colorectal cancer. Clin. Transl. Med. 11, e422 (2021).
Olalekan, S., Xie, B., Back, R., Eckart, H. & Basu, A. Characterizing the tumor microenvironment of metastatic ovarian cancer by single-cell transcriptomics. Cell Rep. 35, 109165 (2021).
Wang, W. et al. Multiregion single-cell sequencing reveals the transcriptional landscape of the immune microenvironment of colorectal cancer. Clin. Transl. Med. 11, e253 (2021).
Zhang, S., Liu, Z., Wu, D., Chen, L. & Xie, L. Single-cell RNA-seq analysis reveals microenvironmental infiltration of plasma cells and hepatocytic prognostic markers in HCC with cirrhosis. Front. Oncol. 10, 596318 (2020).
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2020). This paper shows that in patients with HPV+ tumours, TIL-Bs produce HPV-specific IgG antibodies, providing strong evidence that TIL-B can recognize tumour-specific antigens.
Lu, Y. et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell 180, 1081–1097 (2020). This study shows that neoadjuvant chemotherapy of breast cancer induces ICOSL+ B cells via complement signalling, which in turn boosts T cell-mediated antitumour responses.
Cillo, A. R. et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 52, 183–199 (2020).
Lambrechts, D. et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 24, 1277–1289 (2018).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Murakami, Y. et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci. Rep. 9, 13083 (2019).
Cai, C. et al. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumor Biol. 37, 8209–8218 (2016).
Shalapour, S. et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345 (2017). This work shows that chronic liver inflammation is accompanied by IgA+PD-L1+IL-10+ plasmocytes that suppress CD8+ T cells and accelerate the development of hepatocellular carcinoma.
Shalapour, S. et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015).
Spear, S. et al. Discrepancies in the tumor microenvironment of spontaneous and orthotopic murine models of pancreatic cancer uncover a new immunostimulatory phenotype for B cells. Front. Immunol. 10, 542 (2019).
Hollern, D. P. et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell 179, 1191–1206 (2019). This paper shows that in mouse breast cancer models, responses to immune checkpoint inhibitors depend on B cell activation by TFH cells, highlighting that B cell/T cell signatures could be used as a biomarker to predict such responses.
Shaul, M. E. et al. Tumor-associated neutrophils drive B-cell recruitment and their differentiation to plasma cells. Cancer Immunol. Res. 9, 811–824 (2021).
Ouyang, F. Z. et al. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat. Commun. 7, 13453 (2016).
Zhou, M. et al. Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88−NF-κB signal pathway. Oncoimmunology 5, e1180485 (2016).
Wejksza, K. et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor α. J. Immunol. 190, 2575–2584 (2013).
Lindner, S. et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 73, 2468–2479 (2013).
Domblides, C. et al. Tumor-associated tertiary lymphoid structures: from basic and clinical knowledge to therapeutic manipulation. Front. Immunol. 12, 698604 (2021).
Noël, G. et al. Functional TH1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Invest. 131, e139905 (2021). This study reveals that T follicular regulatory cells (CD25+CXCR5+GARP+FOXP3+) can inhibit TFH cells in TLSs, thereby dampening TLS-driven humoral responses.
Kinker, G. S. et al. B cell orchestration of anti-tumor immune responses: a matter of cell localization and communication. Front. Cell Dev. Biol. 9, 678127 (2021).
Jenks, S. A., Cashman, K. S., Woodruff, M. C., Lee, F. E. H. & Sanz, I. Extrafollicular responses in humans and SLE. Immunol. Rev. 288, 136–148 (2019). This Review describes the three main B cell activation pathways and further highlights how the extrafollicular pathway might help to produce pathogenic autoantibodies in lupus.
Ruddle, N. H. Basics of inducible lymphoid organs. Curr. Top. Microbiol. Immunol. 426, 1–19 (2020).
Kranich, J. & Krautler, N. J. How follicular dendritic cells shape the B-cell antigenome. Front. Immunol. 7, 225 (2016).
Cyster, J. G. & Allen, C. D. C. B cell responses: cell interaction dynamics and decisions. Cell 177, 524–540 (2019).
Graus, F. & Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 16, 535–548 (2019).
Small, M. et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 135, 569–579 (2018).
Kroeger, D. R., Milne, K. & Nelson, B. H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res. 22, 3005–3015 (2016).
Elsner, R. A. & Shlomchik, M. J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53, 1136–1150 (2020).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017). This study shows that the synovium of rheumatoid arthritis contains expanded TPH cells that, similar to TFH cells, express a wide array of factors to support B cell activation and function.
Yoshitomi, H. & Ueno, H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell. Mol. Immunol. 18, 523–527 (2021).
Eisenbarth, S. C. et al. CD4+ T cells that help B cells — a proposal for uniform nomenclature. Trends Immunol. 42, 658–669 (2021).
Wang, S. et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11chiT-bet+ B cells in SLE. Nat. Commun. 9, 1758 (2018).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Gu-Trantien, C. et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2, e91487 (2017). This study highlights the importance of tumour-infiltrating CXCL13-producing TFH cells in promoting memory B cell differentiation and humoral antitumour immunity in human breast cancer.
Li, J. P. et al. PD-1+CXCR5–CD4+Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer 9, e002101 (2021).
Webb, J. R., Milne, K., Kroeger, D. R. & Nelson, B. H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 141, 293–302 (2016).
Hansen, M. H., Nielsen, H. & Ditzel, H. J. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc. Natl Acad. Sci. USA 98, 12659–12664 (2001).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Lin, Z. et al. Pan-cancer analysis of genomic properties and clinical outcome associated with tumor tertiary lymphoid structure. Sci. Rep. 10, 21530 (2020).
Maxime, M. F. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541 (2022).
Zaenker, P. & Ziman, M. R. Serologic autoantibodies as diagnostic cancer biomarkers — a review. Cancer Epidemiol. Biomark. Prev. 22, 2161–2181 (2013).
Sharonov, G. V., Serebrovskaya, E. O., Yuzhakova, D. V., Britanova, O. V. & Chudakov, D. M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 20, 294–307 (2020).
Macdonald, I. K., Parsy-Kowalska, C. B. & Chapman, C. J. Autoantibodies: opportunities for early cancer detection. Trends Cancer 3, 198–213 (2017).
Shimada, H., Ochiai, T. & Nomura, F. Titration of serum p53 antibodies in 1085 patients with various types of malignant tumors a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer 97, 682–689 (2003).
Oshima, Y. et al. NY-ESO-1 autoantibody as a tumor-specific biomarker for esophageal cancer: screening in 1969 patients with various cancers. J. Gastroenterol. 51, 30–34 (2016).
Raza, A. et al. Unleashing the immune response to NY-ESO-1 cancer testis antigen as a potential target for cancer immunotherapy. J. Transl. Med. 18, 140 (2020).
Fischer, E. et al. Cryptic epitopes induce high-titer humoral immune response in patients with cancer. J. Immunol. 185, 3095–3102 (2010).
Ishikawa, T. et al. Tumor-specific immunological recognition of frameshift-mutated peptides in colon cancer with microsatellite instability. Cancer Res. 63, 5564–5572 (2003).
Reis, B. S. et al. Prostate cancer progression correlates with increased humoral immune response to a human endogenous retrovirus GAG protein. Clin. Cancer Res. 19, 6112–6125 (2013).
Blixt, O. et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 13, R25 (2011).
Wang, X. et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med. 353, 1224–1235 (2005).
Soussi, T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 60, 1777–1788 (2000).
Mazor, R. D. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell https://doi.org/10.1016/j.cell.2022.02.012 (2022).
Garaud, S. et al. Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor-infiltrating B cells in breast cancer. Front. Immunol. 9, 2660 (2018).
Shen, P. & Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15, 441–451 (2015).
Garaud, S. et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight 4, e129641 (2019).
Rodriguez, A. B. et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep. 36, 109422 (2021). This work shows that in a mouse melanoma model, TLS development is organized by cancer-associated fibroblasts via the TNF receptor pathway, and their presence correlates with the response to immunotherapy.
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Hua, Z. & Hou, B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol. Rev. 296, 24–35 (2020).
Lanzavecchia, A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol. 8, 773–793 (1990).
Tolar, P. Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 17, 621–634 (2017).
Rivera, A., Chen, C. C., Ron, N., Dougherty, J. P. & Ron, Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13, 1583–1593 (2001).
Lin, R. H., Mamula, M. J., Hardin, J. A. & Janeway, C. A. Induction of autoreactive B cells allows priming of autoreactive T cells. J. Exp. Med. 173, 1433–1439 (1991).
Smith, M. J., Simmons, K. M. & Cambier, J. C. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat. Rev. Nephrol. 13, 712–720 (2017).
Giles, J. R., Kashgarian, M., Koni, P. A. & Shlomchik, M. J. B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J. Immunol. 195, 2571–2579 (2015).
Molnarfi, N. et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 210, 2921–2937 (2013).
O’Neill, S. K. et al. Expression of CD80/86 on B cells is essential for autoreactive T cell activation and the development of arthritis. J. Immunol. 179, 5109–5116 (2007).
Iversen, R. et al. Efficient T cell–B cell collaboration guides autoantibody epitope bias and onset of celiac disease. Proc. Natl Acad. Sci. USA 116, 15134–15139 (2019).
Jelcic, I. et al. Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 175, 85–100 (2018).
Wennhold, K. et al. CD86+ antigen-presenting B cells are increased in cancer, localize in tertiary lymphoid structures, and induce specific T-cell responses. Cancer Immunol. Res. 9, 1098–1108 (2021).
Cui, C. et al. Neoantigen driven B cell and CD4+ T follicular helper cell collaboration promotes robust anti-tumor CD8+ T cell responses. Cell 184, 1–18 (2021).
Lo, J. A. et al. Epitope spreading toward wild type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci. Transl. Med. 13, eabd8636 (2021).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014). This study shows that in patients with both scleroderma and cancer, somatic mutations in the POLR3A gene are associated with autoantibodies against non-mutated regions of the corresponding protein (RPC1), suggesting that immune responses to tumour-associated neoantigens may trigger humoral responses against self-epitopes via antigen spreading.
Zitvogel, L., Perreault, C., Finn, O. J. & Kroemer, G. Beneficial autoimmunity improves cancer prognosis. Nat. Rev. Clin. Oncol. 18, 591–602 (2021).
Michaud, D., Steward, C. R., Mirlekar, B. & Pylayeva-Gupta, Y. Regulatory B cells in cancer. Immunol. Rev. 299, 74–92 (2021).
Zhou, X., Su, Y.-X., Lao, X.-M., Liang, Y.-J. & Liao, G.-Q. CD19+IL-10+ regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4+ T cells to CD4+Foxp3+ regulatory T cells. Oral. Oncol. 53, 27–35 (2016).
Wang, W. W. et al. CD19+CD24hiCD38hi Bregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6, 33486–33499 (2015).
Ishigami, E. et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 26, 180–189 (2019).
Xiao, X. et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 6, 546–559 (2016).
Mirlekar, B. et al. B cell-derived IL35 drives STAT3-dependent CD8+ T-cell exclusion in pancreatic cancer. Cancer Immunol. Res. 8, 292–308 (2020).
Hagn, M. et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol. Cell Biol. 90, 457–467 (2012).
Lundy, S. K. Killer B lymphocytes: the evidence and the potential. Inflamm. Res. 58, 345–357 (2009).
Tao, H. et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10. Eur. J. Immunol. 45, 999–1009 (2015).
Jeske, S. S. et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother. 69, 1205–1216 (2020).
Zhang, B. et al. B cell-derived GABA elicits IL-10+ macrophages to limit anti-tumour immunity. Nature 599, 471–476 (2021).
Logtenberg, M. E. W., Scheeren, F. A. & Schumacher, T. N. The CD47–SIRPα immune checkpoint. Immunity 52, 742–752 (2020).
Biswas, S. et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 591, 464–470 (2021).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype–immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017).
Ferris, R. L., Jaffee, E. M. & Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 28, 4390–4399 (2010).
Hu, X. et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat. Genet. 51, 560–567 (2019). This paper presents a bioinformatic survey of B cell responses across cancers using data from TCGA.
Doubrovina, E. S. et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol. 171, 6891–6899 (2003).
Roumenina, L. T., Daugan, M. V., Petitprez, F., Sautès-Fridman, C. & Fridman, W. H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019).
Roumenina, L. T. et al. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol. Res. 7, 1091–1105 (2019).
Kouser, L. et al. Emerging and novel functions of complement protein C1q. Front. Immunol. 6, 317 (2015).
Kwak, J. W. et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 78, 143–156 (2018).
Foss, S. et al. TRIM21 — from intracellular immunity to therapy. Front. Immunol. 10, 2049 (2019).
Rhodes, D. A. & Isenberg, D. A. TRIM21 and the function of antibodies inside cells. Trends Immunol. 38, 916–926 (2017).
Gu, Y. et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med. 25, 312–322 (2019). This work shows that in a mouse breast cancer model, anti-HSPA4 IgG produced by TIL-Bs activates the CXCR4/SDF1a pathway in tumour cells, thereby promoting lymph node metastasis.
Lo, C. S. et al. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin. Cancer Res. 23, 925–934 (2017).
Zirakzadeh, A. A. et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clin. Immunol. 176, 63–70 (2017).
Morcrette, G. et al. APC germline hepatoblastomas demonstrate cisplatin-induced intratumor tertiary lymphoid structures. Oncoimmunology 8, e1584547 (2019).
Silina, K. et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 78, 1308–1320 (2018).
Brase, J. C. et al. Role of tumor-infiltrating B cells in clinical outcome of patients with melanoma treated with dabrafenib plus trametinib. Clin. Cancer Res. 27, 4500–4510 (2021).
Chelvanambi, M., Fecek, R. J., Taylor, J. L. & Storkus, W. J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 9, e001906 (2021).
Vito, A. et al. Immune checkpoint blockade in triple negative breast cancer influenced by B cells through myeloid-derived suppressor cells. Commun. Biol. 4, 859 (2021).
Wang, X. et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol. Immunol. 109, 20–26 (2019).
Das, R. et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J. Clin. Invest. 128, 715–720 (2018).
Guan, H. et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19+ B lymphocytes and T cells in invasive breast cancer. Oncoimmunology 5, e1075112 (2016).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Budczies, J. et al. A gene expression signature associated with B cells predicts benefit from immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology 10, 1860586 (2021).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Cottrell, T. R. et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 29, 1853–1860 (2018).
van Dijk, N. et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med. 26, 1839–1844 (2020).
Kim, S. S. et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res. 26, 3345–3359 (2020).
Sánchez-Alonso, S. et al. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother. Cancer 8, e001187 (2020).
Vonderheide, R. H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Jackaman, C., Cornwall, S., Graham, P. T. & Nelson, D. J. CD40-activated B cells contribute to mesothelioma tumor regression. Immunol. Cell Biol. 89, 255–267 (2011).
Irenaeus, S. M. M. et al. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int. J. Cancer 145, 1189–1199 (2019).
Vonderheide, R. H. et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
van Hooren, L. et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat. Commun. 12, 4127 (2021).
Leonard, W. J., Lin, J. X. & O’Shea, J. J. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity 50, 832–850 (2019).
Papillion, A. & Ballesteros-Tato, A. The potential of harnessing IL-2-mediated immunosuppression to prevent pathogenic B cell responses. Front. Immunol. 12, 667342 (2021).
Yarchoan, M. et al. Effects of B cell-activating factor on tumor immunity. JCI Insight 5, e136417 (2020).
Rubio, A. J., Porter, T. & Zhong, X. Duality of B cell–CXCL13 axis in tumor immunology. Front. Immunol. 11, 521110 (2020).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Maldonado, L. et al. Vaccination: intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci. Transl. Med. 6, 221ra13 (2014).
Lutz, E. R. et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2, 616–631 (2014).
Wennhold, K. et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget 8, 27740–27753 (2017).
Wennhold, K. et al. Using antigen-specific B cells to combine antibody and T cell–based cancer immunotherapy. Cancer Immunol. Res. 5, 730–743 (2017).
Lee-Chang, C. et al. Activation of 4-1BBL+ B cells with CD40 agonism and IFNγ elicits potent immunity against glioblastoma. J. Exp. Med. 218, e20200913 (2021).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Joshi, N. S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Ziebart, A. et al. The influence of chemotherapy on adenosine-producing B cells in patients with head and neck squamous cell carcinoma. Oncotarget 9, 5834–5847 (2018).
Lee-Chang, C. et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 191, 4141–4151 (2013).
Yarchoan, M. et al. MEK inhibition suppresses B regulatory cells and augments anti-tumor immunity. PLoS ONE 14, e0224600 (2019).
Das, S. & Bar-Sagi, D. BTK signaling drives CD1dhiCD5+ regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene 38, 3316–3324 (2019).
Lu, R. M. et al. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1–30 (2020).
Heemskerk, N. et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J. Clin. Invest. 131, e134680 (2021).
Zhang, W. et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front. Immunol. 11, 18 (2020).
Geller, A. & Yan, J. The role of membrane bound complement regulatory proteins in tumor development and cancer immunotherapy. Front. Immunol. 10, 1074 (2019).
Germain, C. et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 189, 832–844 (2014). This work shows that in lung cancer, B cells display features of an ongoing humoral response, including recognition of tumour-associated antigens, and predict patient outcome when present with mature dendritic cells.
Yasuda, M. et al. Antigens recognized by IgG derived from tumor-infiltrating B lymphocytes in human lung cancer. Anticancer. Res. 26, 3607–3611 (2006).
Yasuda, M. et al. Tumor-infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res. 62, 1751–1756 (2002).
Pavoni, E. et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 7, 1–17 (2007).
Kotlan, B. et al. Novel ganglioside antigen identified by B cells in human medullary breast carcinomas: the proof of principle concerning the tumor-infiltrating B lymphocytes. J. Immunol. 175, 2278–2285 (2005).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Wouters, M. C. A. & Nelson, B. H. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin. Cancer Res. 24, 6125–6135 (2018).
Castino, G. F. et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 5, e1085147 (2016).
Posch, F. et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 7, e1378844 (2018).
Gunderson, A. J. et al. Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 10, e1900635 (2021).
Lynch, K. T. et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J. Immunother. Cancer 9, e002273 (2021).
Vanhersecke, L. et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2, 794–802 (2021). This large study involving three independent cohorts shows that the presence of mature TLSs is associated with response to checkpoint blockade of the PD-1 pathway.
Gago da Graça, C., van Baarsen, L. G. M. & Mebius, R. E. Tertiary lymphoid structures: diversity in their development, composition, and role. J. Immunol. 206, 273–281 (2021).
Acknowledgements
The authors thank K. Singh, T. Rastogi and K. Milne for technical assistance with multicolour immunofluorescence staining and image processing for Fig. 3. C.M.L. is supported by postdoctoral fellowships from Canadian Institutes of Health Research (CIHR) (Banting postdoctoral fellowships programme, 429161) and Michael Smith Foundation for Health Research (MSFHR) (RT-2020-0630). A.C.B. is supported by a Doctoral Award from CIHR (Frederick Banting and Charles Best Canada Graduate Scholarship, FBD — 177882). M.G. and D.P.H. were supported by funds from the National Institutes of Health (NIH P30 CA014195), 2021 Metavivor Early Career Investigator Award, and the San Diego Padres Pedal the Cause C3 Collaborative Translational Cancer Pilot Project Award.
Author information
Authors and Affiliations
Contributions
All authors researched literature, contributed to discussions of the content of the article and edited or reviewed the manuscript before submission. C.M.L. and B.H.N. wrote the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Claudia Jakubzick, who co-reviewed with Kavita Rawat, Shiv Pillai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Human protein atlas: https://www.proteinatlas.org/
Supplementary information
Glossary
- Exhausted or dysfunctional CD8+ and CD4+ TILs
-
(Exhausted or dysfunctional CD8+ and CD4+ tumour-infiltrating lymphocytes). Adysfunctional T cell state triggered by chronic antigen exposure and characterized by poor effector functions (for example, cytokine production), sustained expression of inhibitory receptors (for example, PD-1, cytotoxic T lymphocyte-associated protein 4 (CTLA4)) and widespread transcriptomic changes. Exhausted T cells can be placed on a dysfunction continuum (pre-dysfunctional, early dysfunctional and late dysfunctional) based on their clonality (intermediate to high) and their expression of C–X–C motif chemokine ligand 13 (CXCL13) and inhibitory receptors (intermediate to high).
- Clonal redemption
-
A process by which moderately self-reactive anergic B cells are recruited to the germinal centre, where somatic hypermutation gives them the opportunity to mutate away from self-reactivity.
- CD40
-
A molecule central to B cell activation; upon binding to its ligand expressed by activated T cells, CD40 promotes B cell survival and proliferation, germinal centre formation and development of memory B cells and long-lived plasma cells (PCs).
- Somatic hypermutation
-
A process by which antigen-activated B cells acquire somatic mutations in their B cell receptor (BCR) sequence, thereby leading to receptor diversification.
- Affinity maturation
-
A process by which the diverse repertoire of B cell receptors (BCRs) generated by somatic hypermutation is screened to select clones with the highest affinity for their cognate antigen.
- Secondary lymphoid organs
-
Peripheral organs, such as the spleen and lymph nodes, that provide the optimal environment to generate and quench adaptive immune responses.
- Antibody-dependent cellular cytotoxicity
-
(ADCC). A process by which Fc receptor-expressing effector cells, such as natural killer cells and macrophages, recognize antibody-coated cells and orchestrate their destruction through the release of cytotoxic granules.
- Antibody-dependent cellular phagocytosis
-
(ADCP). A process by which Fc receptor-expressing phagocytes, typically macrophages, recognize antibody-coated cells and destroy them by engulfment.
Rights and permissions
About this article
Cite this article
Laumont, C.M., Banville, A.C., Gilardi, M. et al. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer 22, 414–430 (2022). https://doi.org/10.1038/s41568-022-00466-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-022-00466-1
This article is cited by
-
Single-cell sequencing reveals the heterogeneity of B cells and tertiary lymphoid structures in muscle-invasive bladder cancer
Journal of Translational Medicine (2024)
-
Roles of exosomes in immunotherapy for solid cancers
Cell Death & Disease (2024)
-
Cell Senescence-Related Genes as Biomarkers for Prognosis and Immunotherapeutic Response in Colon Cancer
Biochemical Genetics (2024)
-
Characterization of genomic instability-related genes predicts survival and therapeutic response in lung adenocarcinoma
BMC Cancer (2023)
-
CD79A work as a potential target for the prognosis of patients with OSCC: analysis of immune cell infiltration in oral squamous cell carcinoma based on the CIBERSORTx deconvolution algorithm
BMC Oral Health (2023)