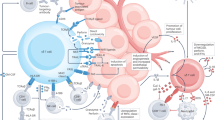
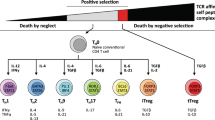
Abstract
The potential of cancer immunotherapy relies on the mobilization of immune cells capable of producing antitumour cytokines and effectively killing tumour cells. These are major attributes of γδ T cells, a lymphoid lineage that is often underestimated despite its major role in tumour immune surveillance, which has been established in a variety of preclinical cancer models. This situation notwithstanding, in particular instances the tumour microenvironment seemingly mobilizes γδ T cells with immunosuppressive or tumour-promoting functions, thus emphasizing the importance of regulating γδ T cell responses in order to realize their translation into effective cancer immunotherapies. In this Review we outline both seminal work and recent advances in our understanding of how γδ T cells participate in tumour immunity and how their functions are regulated in experimental models of cancer. We also discuss the current strategies aimed at maximizing the therapeutic potential of human γδ T cells, on the eve of their exploration in cancer clinical trials that may position them as key players in cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Silva-Santos, B., Serre, K. & Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 15, 683–691 (2015).
Willcox, B. E. & Willcox, C. R. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat. Immunol. 20, 121–128 (2019).
Vermijlen, D., Gatti, D., Kouzeli, A., Rus, T. & Eberl, M. γδ T cell responses: how many ligands will it take till we know? Semin. Cell Dev. Biol. 84, 75–86 (2018).
Adams, E. J., Gu, S. & Luoma, A. M. Human γ δ T cells: evolution and ligand recognition. Cell. Immunol. 296, 31–40 (2015).
Melandri, D. et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19, 1352–1365 (2018).
Girardi, M. et al. Regulation of cutaneous malignancy by γδ T cells. Science 294, 605–609 (2001). Seminal work using TCR δ chain −/− mice to demonstrate a protective role for mouse γδ T cells in chemically induced skin cancer.
Gao, Y. et al. γδ T cells provide an early source of interferon γ in tumor immunity. J. Exp. Med. 198, 433–442 (2003).
Street, S. E. et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. J. Exp. Med. 199, 879–884 (2004).
Liu, Z. et al. Protective immunosurveillance and therapeutic antitumor activity of γδ T cells demonstrated in a mouse model of prostate cancer. J. Immunol. 180, 6044–6053 (2008).
Lanca, T. et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J. Immunol. 190, 6673–6680 (2013).
He, W. et al. Naturally activated V γ 4 γ δ T cells play a protective role in tumor immunity through expression of eomesodermin. J. Immunol. 185, 126–133 (2010).
Simoes, A. E., Di Lorenzo, B. & Silva-Santos, B. Molecular determinants of target cell recognition by human γδ T cells. Front. Immunol. 9, 929 (2018).
Strid, J. et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 9, 146–154 (2008).
Strid, J., Sobolev, O., Zafirova, B., Polic, B. & Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 334, 1293–1297 (2011).
Dalessandri, T., Crawford, G., Hayes, M., Castro Seoane, R. & Strid, J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat. Commun. 7, 12080 (2016). A study showing a crucial effect of IL-13-producing γδ T cells in maintaining skin integrity and therefore protecting from carcinogenesis.
Cao, G. et al. mTOR inhibition potentiates cytotoxicity of Vγ4 γδ T cells via up-regulating NKG2D and TNF-α. J. Leukoc. Biol. 100, 1181–1189 (2016).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Groh, V. et al. Broad tumor-associated expression and recognition by tumor-derived γ δ T cells of MICA and MICB. Proc. Natl Acad. Sci. USA 96, 6879–6884 (1999).
Kong, Y. et al. The NKG2D ligand ULBP4 binds to TCRγ9/δ2 and induces cytotoxicity to tumor cells through both TCRγδ and NKG2D. Blood 114, 310–317 (2009).
Lanca, T. et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T cell cytotoxicity. Blood 115, 2407–2411 (2010).
Correia, D. V., Lopes, A. & Silva-Santos, B. Tumor cell recognition by γδ T lymphocytes: T cell receptor versus NK-cell receptors. Oncoimmunology 2, e22892 (2013).
Viey, E. et al. Phosphostim-activated γ δ T cells kill autologous metastatic renal cell carcinoma. J. Immunol. 174, 1338–1347 (2005).
Alexander, A. A. et al. Isopentenyl pyrophosphate-activated CD56+ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 14, 4232–4240 (2008).
Todaro, M. et al. Efficient killing of human colon cancer stem cells by γδ T lymphocytes. J. Immunol. 182, 7287–7296 (2009).
D’Asaro, M. et al. Vγ9Vδ2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J. Immunol. 184, 3260–3268 (2010).
Dokouhaki, P. et al. NKG2D regulates production of soluble TRAIL by ex vivo expanded human γδ T cells. Eur. J. Immunol. 43, 3175–3182 (2013).
Li, Z. et al. IFN-γ enhances HOS and U2OS cell lines susceptibility to γδ T cell-mediated killing through the Fas/Fas ligand pathway. Int. Immunopharmacol. 11, 496–503 (2011).
Couzi, L. et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 119, 1418–1427 (2012).
Tokuyama, H. et al. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs—rituximab and trastuzumab. Int. J. Cancer 122, 2526–2534 (2008).
Capietto, A. H., Martinet, L. & Fournie, J. J. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J. Immunol. 187, 1031–1038 (2011).
Gertner-Dardenne, J. et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 113, 4875–4884 (2009).
Oberg, H. H. et al. Novel bispecific antibodies increase γδ T cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74, 1349–1360 (2014).
Schiller, C. B. et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget 7, 83392–83408 (2016).
Fisher, J. P. et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 20, 5720–5732 (2014).
Riond, J., Rodriguez, S., Nicolau, M. L., al Saati, T. & Gairin, J. E. In vivo major histocompatibility complex class I (MHCI) expression on MHCIlow tumor cells is regulated by γδ T and NK cells during the early steps of tumor growth. Cancer Immun. 9, 10 (2009).
Brandes, M., Willimann, K. & Moser, B. Professional antigen-presentation function by human γδ T Cells. Science 309, 264–268 (2005).
Altvater, B. et al. Activated human γδ T cells induce peptide-specific CD8+ T cell responses to tumor-associated self-antigens. Cancer Immunol. Immunother. 61, 385–396 (2012).
Mao, C. et al. Tumor-activated TCRγδ(+) T cells from gastric cancer patients induce the antitumor immune response of TCRαβ(+) T cells via their antigen-presenting cell-like effects. J. Immunol. Res. 2014, 593562 (2014).
Himoudi, N. et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J. Immunol. 188, 1708–1716 (2012).
Muto, M., Baghdadi, M., Maekawa, R., Wada, H. & Seino, K. Myeloid molecular characteristics of human γδ T cells support their acquisition of tumor antigen-presenting capacity. Cancer Immunol. Immunother. 64, 941–949 (2015).
Maniar, A. et al. Human γδ T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood 116, 1726–1733 (2010).
Nussbaumer, O., Gruenbacher, G., Gander, H. & Thurnher, M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood 118, 2743–2751 (2011).
Wen, L. et al. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. J. Exp. Med. 183, 2271–2282 (1996).
Huang, Y. et al. γδ T cells affect IL-4 production and B cell tolerance. Proc. Natl Acad. Sci. USA 112, E39–E48 (2015).
Rezende, R. M. et al. γδ T cells control humoral immune response by inducing T follicular helper cell differentiation. Nat. Commun. 9, 3151 (2018).
Bansal, R. R., Mackay, C. R., Moser, B. & Eberl, M. IL-21 enhances the potential of human γδ T cells to provide B cell help. Eur. J. Immunol. 42, 110–119 (2012).
Shimura, E. et al. Epidermal γδ T cells sense precancerous cellular dysregulation and initiate immune responses. Int. Immunol. 22, 329–340 (2010).
Crawford, G. et al. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat. Immunol. 19, 859–870 (2018). A work demonstrating that γδ T cells provide help for B cells to class-switch to IgE production, resulting in a protective response against tumour development.
Mattarollo, S. R. et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 71, 4809–4820 (2011).
Ma, Y. et al. Contribution of IL-17-producing γ δ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 208, 491–503 (2011).
Medina, B. D. et al. Oncogenic kinase inhibition limits Batf3-dependent dendritic cell development and antitumor immunity. J. Exp. Med. https://doi.org/10.1084/jem.20180660 (2019).
Wakita, D. et al. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 40, 1927–1937 (2010).
Carmi, Y. et al. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J. Immunol. 186, 3462–3471 (2011).
Benevides, L. et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 75, 3788–3799 (2015).
Kimura, Y. et al. IL-17A-producing CD30(+) Vδ1 T cells drive inflammation-induced cancer progression. Cancer Sci. 107, 1206–1214 (2016).
Kulig, P. et al. IL17A-mediated endothelial breach promotes metastasis formation. Cancer Immunol. Res. 4, 26–32 (2016).
Ma, S. et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 74, 1969–1982 (2014).
Rei, M. et al. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl Acad. Sci. USA 111, E3562–E3570 (2014). The description of a pro-tumour axis between mouse IL-17-producing γδ T cells and pro-inflammatory and pro-angiogenic macrophages in an ovarian cancer mouse model.
Patin, E. C. et al. Type I IFN receptor signaling controls IL7-dependent accumulation and activity of protumoral IL17A-producing γδT cells in breast cancer. Cancer Res. 78, 195–204 (2018).
McAllister, F. et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25, 621–637 (2014). A study that shows a pathogenic role for IL-17, provided in the TME by γδ T cells and T H 17 cells, in pancreatic tumour development and progression.
Housseau, F. et al. Redundant innate and adaptive sources of IL17 production drive colon tumorigenesis. Cancer Res. 76, 2115–2124 (2016).
Wu, S. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022 (2009).
Coffelt, S. B. et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015). The study that reveals for the first time a pro-metastatic role for mouse IL-17-producing γδ T cells in a breast cancer mouse model.
Busch, S. E. et al. Lung cancer subtypes generate unique immune responses. J. Immunol. 197, 4493–4503 (2016).
Jin, C. et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell 176, 998–1013 (2019).
Van Hede, D. et al. Human papillomavirus oncoproteins induce a reorganization of epithelial-associated γδ T cells promoting tumor formation. Proc. Natl Acad. Sci. USA 114, E9056–E9065 (2017).
Gosmann, C., Mattarollo, S. R., Bridge, J. A., Frazer, I. H. & Blumenthal, A. IL-17 suppresses immune effector functions in human papillomavirus-associated epithelial hyperplasia. J. Immunol. 193, 2248–2257 (2014).
Ness-Schwickerath, K. J., Jin, C. & Morita, C. T. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ2 T cells. J. Immunol. 184, 7268–7280 (2010).
Caccamo, N. et al. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood 118, 129–138 (2011).
Patil, R. S. et al. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 139, 869–881 (2016).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
Wu, P. et al. γδ T 17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40, 785–800 (2014). γδT The first report of a pro-tumour role for human IL-17-producing γδ T cells in colorectal cancer.
Meraviglia, S. et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 6, e1347742 (2017).
Kargl, J. et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 8, 14381 (2017).
Lo Presti, E. et al. Squamous cell tumors recruit γδ T cells producing either IL17 or IFNγ depending on the tumor stage. Cancer Immunol. Res. 5, 397–407 (2017).
Wesch, D., Glatzel, A. & Kabelitz, D. Differentiation of resting human peripheral blood γ δ T cells toward Th1− or Th2-phenotype. Cell. Immunol. 212, 110–117 (2001).
Hao, J. et al. Regulatory role of Vγ1 γδ T cells in tumor immunity through IL-4 production. J. Immunol. 187, 4979–4986 (2011).
Mao, Y. et al. A new effect of IL-4 on human γδ T cells: promoting regulatory Vδ1 T cells via IL-10 production and inhibiting function of Vδ2 T cells. Cell. Mol. Immunol. 13, 217–228 (2016).
Sundblad, V., Morosi, L. G., Geffner, J. R. & Rabinovich, G. A. Galectin-1: a jack-of-all-trades in the resolution of acute and chronic inflammation. J. Immunol. 199, 3721–3730 (2017).
Daley, D. et al. γδ T cells support pancreatic oncogenesis by restraining αβ T cell activation. Cell 166, 1485–1499 (2016).
Gunderson, A. J. et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 6, 270–285 (2016).
Khosravi, N. et al. IL22 promotes Kras-mutant lung cancer by induction of a protumor immune response and protection of stemness properties. Cancer Immunol. Res. 6, 788–797 (2018).
McKenzie, D. R. et al. IL-17-producing γδ T cells switch migratory patterns between resting and activated states. Nat. Commun. 8, 15632 (2017).
Kersten, K. et al. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. Oncoimmunology 6, e1334744 (2017).
Glatzel, A. et al. Patterns of chemokine receptor expression on peripheral blood γ δ T lymphocytes: strong expression of CCR5 is a selective feature of V δ 2/V γ 9 γ δ T cells. J. Immunol. 168, 4920–4929 (2002).
Ye, J. et al. Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer Res. 73, 6137–6148 (2013).
Ribot, J. C., Ribeiro, S. T., Correia, D. V., Sousa, A. E. & Silva-Santos, B. Human γδ thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J. Immunol. 192, 2237–2243 (2014).
Correia, D. V. et al. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 118, 992–1001 (2011).
Yamaguchi, T. et al. Interleukin-15 effectively potentiates the in vitro tumor-specific activity and proliferation of peripheral blood γδT cells isolated from glioblastoma patients. Cancer Immunol. Immunother. 47, 97–103 (1998).
Van Acker, H. H. et al. Interleukin-15-cultured dendritic cells enhance anti-tumor γ δ T cell functions through IL-15 secretion. Front. Immunol. 9, 658 (2018).
Haas, J. D. et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur. J. Immunol. 39, 3488–3497 (2009).
Li, W. et al. Effect of IL-18 on expansion of γδ T cells stimulated by zoledronate and IL-2. J. Immunother. 33, 287–296 (2010).
Thedrez, A. et al. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V γ 9V δ 2 T cells for adoptive immunotherapy. J. Immunol. 182, 3423–3431 (2009).
Wang, X. et al. IL-36γ transforms the tumor microenvironment and promotes type 1 lymphocyte-mediated antitumor immune responses. Cancer Cell 28, 296–306 (2015).
Yi, Y. et al. The functional impairment of HCC-infiltrating γδ T cells, partially mediated by regulatory T cells in a TGFβ- and IL-10-dependent manner. J. Hepatol. 58, 977–983 (2013).
Sacchi, A. et al. Myeloid-derived suppressor cells specifically suppress IFN-γ production and antitumor cytotoxic activity of Vδ2 T cells. Front. Immunol. 9, 1271 (2018).
Sabbione, F. et al. Neutrophils suppress γδ T cell function. Eur. J. Immunol. 44, 819–830 (2014).
Li, L. et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene 38, 2830–2843 (2018).
Rossi, C. et al. Boosting γδ T cell-mediated antibody-dependent cellular cytotoxicity by PD-1 blockade in follicular lymphoma. Oncoimmunology 8, 1554175 (2019).
Iwasaki, M. et al. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur. J. Immunol. 41, 345–355 (2011).
Hoeres, T., Holzmann, E., Smetak, M., Birkmann, J. & Wilhelm, M. PD-1 signaling modulates interferon-γ production by Gamma Delta (γδ) T-Cells in response to leukemia. Oncoimmunology 8, 1550618 (2019).
Siegers, G. M., Dutta, I., Lai, R. & Postovit, L. M. Functional plasticity of γ δ T cells and breast tumor targets in hypoxia. Front. Immunol. 9, 1367 (2018).
Rodrigues, N. V. et al. Low-density lipoprotein uptake inhibits the activation and antitumor functions of human Vγ9Vδ2 T cells. Cancer. Immunol. Res. 6, 448–457 (2018).
Mattarollo, S. R., Kenna, T., Nieda, M. & Nicol, A. J. Chemotherapy and zoledronate sensitize solid tumour cells to Vγ9Vδ2 T cell cytotoxicity. Cancer Immunol. Immunother. 56, 1285–1297 (2007).
Todaro, M., Meraviglia, S., Caccamo, N., Stassi, G. & Dieli, F. Combining conventional chemotherapy and γδ T cell-based immunotherapy to target cancer-initiating cells. Oncoimmunology 2, e25821 (2013).
Wang, Z. et al. Decitabine enhances Vγ9Vδ2 T cell-mediated cytotoxic effects on osteosarcoma cells via the NKG2DL-NKG2D axis. Front. Immunol. 9, 1239 (2018).
Niu, C. et al. Decitabine inhibits γ δ T cell cytotoxicity by promoting KIR2DL2/3 expression. Front. Immunol. 9, 617 (2018).
Bhat, S. A., Vedpathak, D. M. & Chiplunkar, S. V. Checkpoint blockade rescues the repressive effect of histone deacetylases inhibitors on γδ T cell function. Front. Immunol. 9, 1615 (2018).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Michel, M. L. et al. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proc. Natl Acad. Sci. USA 109, 17549–17554 (2012).
Tang, Q. et al. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediators Inflamm. 2013, 713859 (2013).
Douguet, L. et al. Inflammation drives nitric oxide synthase 2 expression by γδ T cells and affects the balance between melanoma and vitiligo associated melanoma. Oncoimmunology 7, e1484979 (2018).
Douguet, L. et al. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of γδ17 T cells in melanoma. Oncoimmunology 5, e1208878 (2016).
Baek, A. E. et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 8, 864 (2017).
Kathania, M. et al. Itch inhibits IL-17-mediated colon inflammation and tumorigenesis by ROR-γt ubiquitination. Nat. Immunol. 17, 997–1004 (2016).
Mensurado, S. et al. Tumor-associated neutrophils suppress pro-tumoral IL-17+ γδ T cells through induction of oxidative stress. PLOS Biol. 16, e2004990 (2018).
Liu, Y. et al. CD11b+Ly6G+ cells inhibit tumor growth by suppressing IL-17 production at early stages of tumorigenesis. Oncoimmunology 5, e1061175 (2016).
Bialasiewicz, A. A., Ma, J. X. & Richard, G. Alpha/β- and γ/δ TCR(+) lymphocyte infiltration in necrotising choroidal melanomas. Br. J. Ophthalmol. 83, 1069–1073 (1999).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Tosolini, M. et al. Assessment of tumor-infiltrating TCRVγ9Vδ2 γδ lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology 6, e1284723 (2017).
Gibbons, D. L. et al. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur. J. Immunol. 39, 1794–1806 (2009).
Peng, G. et al. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27, 334–348 (2007).
Ma, C. et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 189, 5029–5036 (2012).
Ye, J. et al. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J. Immunol. 190, 2403–2414 (2013).
Wilhelm, M. et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 102, 200–206 (2003). A pioneering clinical trial showing the safety and efficacy of in vivo Vγ9Vδ2 T cell activation in patients with lymphoma.
Kobayashi, H. et al. Safety profile and anti-tumor effects of adoptive immunotherapy using γ-δ T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol. Immunother. 56, 469–476 (2007).
Nicol, A. J. et al. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 105, 778–786 (2011).
Fournie, J. J. et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 10, 35–41 (2013). A review providing an insightful discussion of the limited success of cancer clinical trials based on Vγ9Vδ2 T cells.
Wistuba-Hamprecht, K. et al. Proportions of blood-borne Vδ1+ and Vδ2+ T cells are associated with overall survival of melanoma patients treated with ipilimumab. Eur. J. Cancer 64, 116–126 (2016).
Gubin, M. M. et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint cancer therapy. Cell 175, 1443 (2018).
de Weerdt, I. et al. Improving CLL Vγ9Vδ2-T cell fitness for cellular therapy by ex vivo activation and ibrutinib. Blood 132, 2260–2272 (2018).
de Bruin, R. C. G. et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology 7, e1375641 (2017).
Oberg, H. H. et al. Tribody [(HER2)2xCD16] is more effective than trastuzumab in enhancing γδ T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells. Front. Immunol. 9, 814 (2018).
Grunder, C. et al. γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs. Blood 120, 5153–5162 (2012).
Marcu-Malina, V. et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood 118, 50–59 (2011). A work that establishes a proof of principle for transferring Vγ9Vδ2 TCRs into αβ T cells to improve their antitumour efficacy and safety.
Straetemans, T. et al. GMP-grade manufacturing of T cells engineered to express a defined γδTCR. Front. Immunol. 9, 1062 (2018).
Netherlands Trial Register. Trial NL6357 (NTR6541). TrialRegister.nl https://www.trialregister.nl/trial/6357 (2017).
Di Marco Barros, R. et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell 167, 203–218 (2016).
Almeida, A. R. et al. δ1 T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof of concept. Clin. Cancer Res. 22, 5795–5804 (2016). A study providing preclinical proof of principle for an application of Vδ1 + cells in adoptive immunotherapy of CLL.
Di Lorenzo, B. et al. Broad cytotoxic targeting of acute myeloid leukemia by polyclonal δ one T cells. Cancer Immunol. Res. 7, 552–558 (2019).
Benveniste, P. M. et al. Generation and molecular recognition of melanoma-associated antigen-specific human γδ T cells. Sci. Immunol. 3, eaav4036 (2018).
Mirzaei, H. R., Mirzaei, H., Lee, S. Y., Hadjati, J. & Till, B. G. Prospects for chimeric antigen receptor (CAR) γδ T cells: a potential game changer for adoptive T cell cancer immunotherapy. Cancer Lett. 380, 413–423 (2016).
Rischer, M. et al. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 126, 583–592 (2004).
Deniger, D. C. et al. Bispecific T cells expressing polyclonal repertoire of endogenous γδ T cell receptors and introduced CD19-specific chimeric antigen receptor. Mol. Ther. 21, 638–647 (2013).
Salter, A. I., Pont, M. J. & Riddell, S. R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 131, 2621–2629 (2018).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 24, 1504–1506 (2018).
Ruella, M. et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499–1503 (2018).
Zumwalde, N. A. et al. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight 2, 93179 (2017).
Jarry, U. et al. Stereotaxic administrations of allogeneic human Vγ9Vδ2 T cells efficiently control the development of human glioblastoma brain tumors. Oncoimmunology 5, e1168554 (2016).
Deniger, D. C. et al. Activating and propagating polyclonal γδ T cells with broad specificity for malignancies. Clin. Cancer Res. 20, 5708–5719 (2014).
Qaqish, A. et al. Adoptive transfer of phosphoantigen-specific γδ T cell subset attenuates Mycobacterium tuberculosis infection in nonhuman primates. J. Immunol. 198, 4753–4763 (2017).
Sicard, H. et al. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J. Immunol. 175, 5471–5480 (2005).
Godder, K. T. et al. Long term disease-free survival in acute leukemia patients recovering with increased γδ T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 39, 751–757 (2007). An important paper showing that upon stem cell transplantation, efficient Vδ1 + T cell reconstitution is associated with improved clinical outcomes in patients with leukaemia.
Airoldi, I. et al. γδ T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes. Blood 125, 2349–2358 (2015).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Cheng, M. et al. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res. 74, 4030–4041 (2014).
Munoz-Ruiz, M., Sumaria, N., Pennington, D. J. & Silva-Santos, B. Thymic determinants of γδ T cell differentiation. Trends Immunol. 38, 336–344 (2017).
Tripodo, C. et al. Gamma-δ T cell lymphomas. Nat. Rev. Clin. Oncol. 6, 707–717 (2009).
Foppoli, M. & Ferreri, A. J. Gamma-δ t-cell lymphomas. Eur. J. Haematol. 94, 206–218 (2015).
Matos, D. M., Rizzatti, E. G., Fernandes, M., Buccheri, V. & Falcao, R. P. γδ and αβ T cell acute lymphoblastic leukemia: comparison of their clinical and immunophenotypic features. Haematologica 90, 264–266 (2005).
Ribeiro, S. T. et al. Casein kinase 2 controls the survival of normal thymic and leukemic γδ T cells via promotion of AKT signaling. Leukemia 31, 1603–1610 (2017).
Saito, H. et al. Complete primary structure of a heterodimeric T cell receptor deduced from cDNA sequences. Nature 309, 757–762 (1984).
Brenner, M. B. et al. Identification of a putative second T cell receptor. Nature 322, 145–149 (1986).
Bank, I. et al. A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature 322, 179–181 (1986).
Borst, J. et al. A T cell receptor γ/CD3 complex found on cloned functional lymphocytes. Nature 325, 683–688 (1987).
Fournie, J. J. & Bonneville, M. Stimulation of γδ T cells by phosphoantigens. Res. Immunol. 147, 338–347 (1996).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T cell subset. Blood 120, 2269–2279 (2012). A seminal paper on butyrophilin subfamily 3 member A1 and its role in inducing cellular stress-sensing by human Vγ9Vδ2 T cells.
Acknowledgements
We thank D. Correia, A. Simões, H. Norell and K. Serre (iMM JLA) for insightful discussions on this topic. We acknowledge funding from the European Research Council (CoG_646701 to B.S.-S.), Cancer Research UK Glasgow Centre (A25142 to S.B.C.), Breast Cancer Now (2018JulPR1101 to S.B.C.), Wellcome Trust (208990/Z/17/Z to S.B.C.), Tenovus Scotland (Project S17-17 to S.B.C.) and Fundação para a Ciência e a Tecnologia / Ministério da Ciência, Tecnologia e Ensino Superior through Fundos do Orçamento de Estado (refs. UID/BIM/50005/2019 and PD/BD/114099/2015).
Reviewer information
Nature Reviews Cancer thanks W. Havran, B. Willcox and F. Dieli for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
B.S.-S., S.M. and S.B.C. researched the data for the article and contributed equally to the writing as well as to the review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
B.S.-S. is a co-founder and shareholder of Lymphact, the company that developed DOT cells, which was acquired in 2018 by GammaDelta Therapeutics (London, UK). S.M. and S.B.C. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Triplebodies
-
Immunoligands consisting of three tandem single-chain variable fragments with three distinct specificities.
- Aminobisphosphonate
-
A drug type that derives from bisphosphonates and is commonly used in bone-related disorders to avoid excessive bone resorption.
- Immunoglobulin class switching
-
Mechanism by which B cells change the isotype of immunoglobulin produced, altering its effector function.
- Germinal centre
-
A site within the spleen and lymph nodes where B cells proliferate, differentiate and perform immunoglobulin class switching.
- V(D)J recombination
-
Also known as somatic recombination. The somatic rearrangement of variable (V), diversity (D) and joining (J) regions of the genes that encode antigen receptors, leading to repertoire diversity of both T cell and B cell receptors.
- Angiogenic switch
-
Time point during tumour progression when the pro-angiogenic factors outcompete the antiangiogenic ones, leading to transition between a dormant avascularized hyperplasia and an outgrowing vascularized tumour.
- Thymocytes
-
Haematopoietic progenitor cells present in the thymus gland.
- Oxygen tension
-
Partial pressure of oxygen molecules dissolved in a liquid (such as blood plasma).
- Mevalonate pathway
-
Also known as the isoprenoid pathway. An essential metabolic pathway that gives rise to two five-carbon building blocks, called isopentenyl pyrophosphate and dimethylallyl purophosphate, that are converted into isoprenoids. Metabolites of this pathway accumulate in metabolically distressed cells.
- Nanobody
-
An antibody with a single monomeric domain.
- Stereotaxic administration
-
Delivery of a compound into the brain using an external, three-dimensional frame of reference, usually based on the Cartesian coordinate system.
- Haploidentical stem cell transplantation
-
Treatment of blood disorders involving the replacement of the patient’s haematopoietic cells by healthy partially (50%) human leukocyte antigen-matched haematopoietic progenitors.
Rights and permissions
About this article
Cite this article
Silva-Santos, B., Mensurado, S. & Coffelt, S.B. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer 19, 392–404 (2019). https://doi.org/10.1038/s41568-019-0153-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0153-5
This article is cited by
-
Tumor reactive γδ T cells contribute to a complete response to PD-1 blockade in a Merkel cell carcinoma patient
Nature Communications (2024)
-
Anti-Anisakis antibodies in colon cancer patients and their relationship with γδ T-cells
Parasitology Research (2024)
-
γδ T cells in oral diseases
Inflammation Research (2024)
-
DNT cells mediate resistance to CAR-T cells therapy in a pediatric patient with relapsed and refractory B-ALL
Annals of Hematology (2024)
-
Extracellular vesicles remodel tumor environment for cancer immunotherapy
Molecular Cancer (2023)