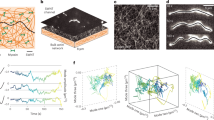
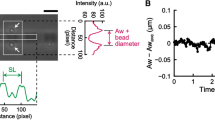
Abstract
Actomyosin networks play essential roles in many cellular processes, including intracellular transport, cell division and cell motility, and exhibit many spatiotemporal patterns. Despite extensive research, how the interplay between network mechanics, turnover and geometry leads to these different patterns is not well understood. We focus on the size-dependent behaviour of contracting actomyosin networks in the presence of turnover, using a reconstituted system based on cell extracts encapsulated in water-in-oil droplets. We show that the system can self-organize into different global contraction patterns, exhibiting persistent contractile flows in smaller droplets and periodic contractions in the form of waves or spirals in larger droplets. The transition between continuous and periodic contraction occurs at a characteristic length scale that is inversely dependent on the network contraction rate. These dynamics are captured by a theoretical model that considers the coexistence of different local density-dependent mechanical states with distinct rheological properties. The model shows how large-scale contractile behaviours emerge from the interplay between network percolation, which is essential for long-range force transmission, and rearrangements due to advection and turnover. Our findings thus demonstrate how varied contraction patterns can arise from the same microscopic constituents, without invoking specific biochemical regulation, merely by changing the system geometry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data used to support the findings of this paper are included in the plots and Supplementary Information. Any further details are available from K.K. upon request.
Code availability
The codes used for data analysis are publicly available as follows: Particle image velocimetry (PIV): https://github.com/nivieru/dropletsRhoV; Optical flow: https://ars.els-cdn.com/content/image/1-s2.0-S0006349516300339-mmc7.txt; Tracer bead tracking: https://site.physics.georgetown.edu/matlab/code.html; Spectral time-lapse (STL): https://zenodo.org/record/7663. The Matlab codes used for solving the 1D and 2D partial differential model equations as well as the the 1D discrete stochastic code are available at: https://github.com/mariyasavinov/SizeDependentWaves.git.
References
Field, C. M. & Lenart, P. Bulk cytoplasmic actin and its functions in meiosis and mitosis. Curr. Biol. 21, R825–R830 (2011).
Salbreux, G., Charras, G. & Paluch, E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22, 536–545 (2012).
Munjal, A. & Lecuit, T. Actomyosin networks and tissue morphogenesis. Development 141, 1789–1793 (2014).
Koenderink, G. H. & Paluch, E. K. Architecture shapes contractility in actomyosin networks. Curr. Opin. Cell Biol. 50, 79–85 (2018).
Murrell, M., Oakes, P. W., Lenz, M. & Gardel, M. L. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486–498 (2015).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Lenart, P. et al. A contractile nuclear actin network drives chromosome congression in oocytes. Nature 436, 812–818 (2005).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499 (2009).
Jodoin, J. N. et al. Stable force balance between epithelial cells arises from F-actin turnover. Dev. Cell 35, 685–697 (2015).
Nishikawa, M., Naganathan, S. R., Julicher, F. & Grill, S. W. Controlling contractile instabilities in the actomyosin cortex. eLife 6, e19595 (2017).
Agarwal, P. & Zaidel-Bar, R. Principles of actomyosin regulation in vivo. Trends Cell Biol. 29, 150–163 (2019).
Backouche, F., Haviv, L., Groswasser, D. & Bernheim-Groswasser, A. Active gels: dynamics of patterning and self-organization. Phys. Biol. 3, 264 (2006).
Bendix, P. M. et al. A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys. J. 94, 3126–3136 (2008).
Reymann, A.-C. et al. Actin network architecture can determine myosin motor activity. Science 336, 1310–1314 (2012).
Kohler, S. & Bausch, A. R. Contraction mechanisms in composite active actin networks. PLoS ONE 7, e39869 (2012).
Alvarado, J., Sheinman, M., Sharma, A., MacKintosh, F. C. & Koenderink, G. H. Molecular motors robustly drive active gels to a critically connected state. Nat. Phys. 9, 591–597 (2013).
Ennomani, H. et al. Architecture and connectivity govern actin network contractility. Curr. Biol. 26, 616–626 (2016).
Alvarado, J., Sheinman, M., Sharma, A., MacKintosh, F. C. & Koenderink, G. H. Force percolation of contractile active gels. Soft Matter 13, 5624–5644 (2017).
Belmonte, J. M., Leptin, M. & Nedelec, F. A theory that predicts behaviors of disordered cytoskeletal networks. Mol. Syst. Biol. 13, 941 (2017).
McFadden, W. M., McCall, P. M., Gardel, M. L. & Munro, E. M. Filament turnover tunes both force generation and dissipation to control long-range flows in a model actomyosin cortex. PLoS Comput. Biol. 13, e1005811 (2017).
Hiraiwa, T. & Salbreux, G. Role of turnover in active stress generation in a filament network. Phys. Rev. Lett. 116, 188101 (2016).
Brieher, W. Mechanisms of actin disassembly. Mol. Biol. Cell 24, 2299–2302 (2013).
Malik-Garbi, M. et al. Scaling behaviour in steady-state contracting actomyosin networks. Nat. Phys. 15, 509–516 (2019).
Pinot, M. et al. Confinement induces actin flow in a meiotic cytoplasm. Proc. Natl Acad. Sci. USA 109, 11705–11710 (2012).
Abu-Shah, E. & Keren, K. Symmetry breaking in reconstituted actin cortices. eLife 3, e01433 (2014).
Tan, T. H. et al. Self-organization of stress patterns drives state transitions in actin cortices. Sci. Adv. 4, eaar2847 (2018).
Ezzell, R. M., Brothers, A. J. & Cande, W. Z. Phosphorylation-dependent contraction of actomyosin gels from amphibian eggs. Nature 306, 620–622 (1983).
Field, C. M. et al. Actin behavior in bulk cytoplasm is cell cycle regulated in early vertebrate embryos. J. Cell Sci. 124, 2086–2095 (2011).
Sakamoto, R. et al. Tug-of-war between actomyosin-driven antagonistic forces determines the positioning symmetry in cell-sized confinement. Nat. Commun. 11, 1–13 (2020).
Sakamoto, R., Miyazaki, M. & Maeda, Y. T. State transitions of a confined actomyosin system controlled through contractility and polymerization rate. Phys. Rev. Res. 5, 013208 (2023).
Pohl, T. in Lecture Notes in Biomathematics: Biological Motion, Vol. 89 (eds Alt, W. & Hoffmann, G.) 85–94 (Springer, 1990).
Alt, W. & Dembo, M. Cytoplasm dynamics and cell motion: two-phase flow models. Math. Biosci. 156, 207–228 (1999).
Ierushalmi, N. et al. Centering and symmetry breaking in confined contracting actomyosin networks. eLife 9, e55368 (2020).
Wuhr, M. et al. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr. Biol. 24, 1467–1475 (2014).
Zicha, D. et al. Rapid actin transport during cell protrusion. Science 300, 142–145 (2003).
Keren, K., Yam, P. T., Kinkhabwala, A., Mogilner, A. & Theriot, J. A. Intracellular fluid flow in rapidly moving cells. Nat. Cell Biol. 11, 1219–1224 (2009).
Ideses, Y. et al. Spontaneous buckling of contractile poroelastic actomyosin sheets. Nat. Commun. 9, 2461 (2018).
Sadhukhan, P., Schumann, O. & Heussinger, C. Elasto-plastic response of reversibly crosslinked biopolymer bundles. Eur. Phys. J. E 37, 1–9 (2014).
Wollrab, V. et al. Polarity sorting drives remodeling of actin-myosin networks. J. Cell Sci. 132, jcs219717 (2019).
Bueno, C., Liman, J., Schafer, N. P., Cheung, M. S. & Wolynes, P. G. A generalized Flory-Stockmayer kinetic theory of connectivity percolation and rigidity percolation of cytoskeletal networks. PLoS Comput. Biol. 18, e1010105 (2022).
Freedman, S. L., Hocky, G. M., Banerjee, S. & Dinner, A. R. Nonequilibrium phase diagrams for actomyosin networks. Soft Matter 14, 7740–7747 (2018).
Banerjee, S., Gardel, M. L. & Schwarz, U. S. The actin cytoskeleton as an active adaptive material. Annu. Rev. Condens. Matter Phys. 11, 421–439 (2020).
García-Arcos, J. M. et al. Advected percolation in the actomyosin cortex drives amoeboid cell motility. Preprint at https://www.biorxiv.org/content/10.1101/2022.07.14.500109v1.abstract (2022).
Bement, W. M. et al. Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17, 1471–1483 (2015).
Allard, J. & Mogilner, A. Traveling waves in actin dynamics and cell motility. Curr. Opin. Cell Biol. 25, 107–115 (2013).
Staddon, M. F., Munro, E. M. & Banerjee, S. Pulsatile contractions and pattern formation in excitable actomyosin cortex. PLoS Comput. Biol. 18, e1009981 (2022).
Mak, M., Zaman, M. H., Kamm, R. D. & Kim, T. Interplay of active processes modulates tension and drives phase transition in self-renewing, motor-driven cytoskeletal networks. Nat. Commun. 7, 1–12 (2016).
Yu, Q., Li, J., Murrell, M. P. & Kim, T. Balance between force generation and relaxation leads to pulsed contraction of actomyosin networks. Biophys. J. 115, 2003–2013 (2018).
Banerjee, D. S., Munjal, A., Lecuit, T. & Rao, M. Actomyosin pulsation and flows in an active elastomer with turnover and network remodeling. Nat. Commun. 8, 1121 (2017).
Dierkes, K., Sumi, A., Solon, J. E. O. & Salbreux, G. Spontaneous oscillations of elastic contractile materials with turnover. Phys. Rev. Lett. 113, 148102 (2014).
Hannezo, E., Dong, B., Recho, P., Joanny, J.-F. C. & Hayashi, S. Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes. Proc. Natl Acad. Sci. USA 112, 8620–8625 (2015).
Naganathan, S. R., Fürthauer, S., Nishikawa, M., Julicher, F. & Grill, S. W. Active torque generation by the actomyosin cell cortex drives left-right symmetry breaking. eLife 3, e04165 (2014).
Tee, Y. H. et al. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat. Cell Biol. 17, 445–457 (2015).
Bressloff, P. C. Mean-field theory of globally coupled integrate-and-fire neural oscillators with dynamic synapses. Phys. Rev. E 60, 2160 (1999).
Bailles, A. I., Gehrels, E. W. & Lecuit, T. Mechanochemical principles of spatial and temporal patterns in cells and tissues. Annu. Rev. Cell Dev. Biol. 38, 321–347 (2022).
Field, C. M., Nguyen, P. A., Ishihara, K., Groen, A. C. & Mitchison, T. J. in Methods in Enzymology, Vol. 540 (ed. Vale, R. D.) 399–415 (Elsevier, 2014).
Abu-Shah, E., Malik-Garbi, M. & Keren, K. Building a Cell from Its Component Parts (eds Ross, J. & Marshall, W.) Ch. 15 (Elsevier, 2014).
Vig, D. K., Hamby, A. E. & Wolgemuth, C. W. On the quantification of cellular velocity fields. Biophys. J. 110, 1469–1475 (2016).
Madan, C. R. & Spetch, M. L. Visualizing and quantifying movement from pre-recorded videos: the spectral time-lapse (STL) algorithm. F1000Research 3, 19 (2014).
Acknowledgements
We thank L. Garion for the help with extract preparation. We thank M. Piel, R. Voituriez and Juan Manuel García-Arcos for stimulating discussions and sharing unpublished results. We thank J.-F.C. Joanny for the helpful advice on the modelling. We thank P. Lenart, G. Bunin, A. Tayar, A. Frishman and S. Ro for comments on the paper. This work was supported by a grant from the United States–Israel Binational Science Foundation to K.K. and B. Goode (grant no. 2017158). M.S. and A.M. are supported by National Science Foundation grants DMS 1953430 and DMS 2052515.
Author information
Authors and Affiliations
Contributions
A.K., N.I. and K.K. designed the experiments. A.K. and N.I. performed the experiments. A.K., N.I. and K.K. analysed the data. A.M., M.S., K.K. and A.K. developed the model. A.K., M.S., A.M. and K.K. wrote the paper. All co-authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Anne Bernheim-Groswasser, Patrick McCall and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Wave front propagation in a droplet with periodic contraction.
Extended Data Fig. 2
Periodic contraction in different geometries.
Extended Data Fig. 3
The movement of tracer beads in a droplet exhibiting periodic contraction.
Extended Data Fig. 4
Periodic contraction in the form of spirals.
Extended Data Fig. 5
Contraction rate depends on droplet size.
Extended Data Fig. 6
Numerical solution of the 1D model.
Extended Data Fig. 7
Modeling the dynamics of droplets with changing actin turnover or contraction rates.
Extended Data Fig. 8
Predicted wave period as a function of system size.
Extended Data Fig. 9
2D contractile ring model.
Extended Data Fig. 10
Different contractile behaviors obtained from simulations of a discrete agent-based model for different parameter values.
Supplementary information
Supplementary Information
Supplementary text, extended data fig. legends for Figs. 1–10 and supplementary video legends for Videos 1–16.
Supplementary Video 1
Size-dependent contraction patterns.
Supplementary Video 2
Continuous actomyosin network contraction.
Supplementary Video 3
Periodic actomyosin network contraction as concentric waves.
Supplementary Video 4
Periodic actomyosin network contraction in an elongated capillary.
Supplementary Video 5
Periodic actomyosin network contraction in a spherical droplet.
Supplementary Video 6
Global actomyosin network contraction in a very large droplet.
Supplementary Video 7
The movement of tracer beads in a droplet exhibiting periodic contraction.
Supplementary Video 8
Periodic actomyosin network contraction as a clockwise spiral.
Supplementary Video 9
Mixed contraction pattern in droplet with an off-centre contraction centre.
Supplementary Video 10
Periodic contraction with different actin-associated proteins.
Supplementary Video 11
Contraction with limited turnover.
Supplementary Video 12
Transition from continuous to periodic contraction.
Supplementary Video 13
Model simulations of the size-dependent contractile behaviour.
Supplementary Video 14
Model simulations of the transition from continuous to periodic contraction in a system characterized by a contraction rate that increases over time.
Supplementary Video 15
Local contractions in droplets with enhanced myosin activity.
Supplementary Video 16
Local contractions in droplets with enhanced filament capping.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krishna, A., Savinov, M., Ierushalmi, N. et al. Size-dependent transition from steady contraction to waves in actomyosin networks with turnover. Nat. Phys. 20, 123–134 (2024). https://doi.org/10.1038/s41567-023-02271-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-023-02271-5
This article is cited by
-
Molecular motors make waves and sculpt patterns
Nature Physics (2024)