Abstract
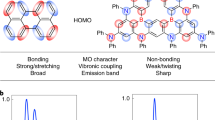
Organic light-emitting diode (OLED) technology is promising for ultrahigh-definition displays and other applications, but further improvements in efficiency and colour purity are desired. Here, we designed and synthesized an ultrapure green emitter called DBTN-2, which is organoboron based and features a highly distorted fused π-conjugated molecular design. This design concept substantially reduces the relaxation energy between the geometries of the excited and ground states, leading to a full-width at half-maximum emission of only 20 nm. Furthermore, the different excitation characters of the singlet and triplet states enhance the spin–orbit couplings leading to highly efficient operation. The introduction of the multiple carbazole moieties gives rise to a charge-resonance-type excitation feature of the triplet states, thus resulting in a high density of the triplet states and a rate of reverse intersystem crossing (kRISC) as fast as 1.7 × 105 s−1. An ultrapure green OLED exploiting DBTN-2 as an emitter without optimized cavity effects and colour filters operated with Commission Internationale de l’Eclairage coordinates of (0.19, 0.74), satisfying the requirement for a commercial green OLED display. Moreover, in combination with a photoluminescence quantum yield of near 100% and a strong horizontal dipole orientation in the doped film, an excellent external quantum efficiency of 35.2% with suppressed efficiency roll-off is simultaneously obtained.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that all relevant data are included in the paper and its Supplementary Information.
References
Chen, H. W. et al. Going beyond the limit of an LCD’s color gamut. Light Sci. Appl. 6, 17043 (2017).
Steckel, J. S., Ho, J. & Coe-Sullivan, S. QDs generate light for next-generation displays features. Photon. Spectra 48, 55–61 (2014).
Zhu, R., Luo, Z., Chen, H., Dong, Y. & Wu, S. T. Realizing Rec. 2020 color gamut with quantum dot displays. Opt. Express 23, 23680–23693 (2015).
Liu, Y. C., Li, C. S., Ren, Z. J., Yan, S. K. & Bryce, M. R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 3, 18020 (2018).
Xiao, L. et al. Recent progresses on materials for electrophosphorescent organic light-emitting devices. Adv. Mater. 23, 926–952 (2011).
Xu, Y., Xu, P., Hu, D. & Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 50, 1030–1069 (2021).
Ha, J. M., Hur, S. H., Pathak, A., Jeong, J. E. & Woo, H. Y. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 13, 53 (2021).
Sun, Y. & Forrest, S. R. Enhanced light out-coupling of organic light-emitting devices using embedded low-index grids. Nat. Photon. 2, 483–487 (2008).
Reineke, S. et al. White organic light-emitting diodes with fluorescent tube efficiency. Nature 459, 234–238 (2009).
Helander, M. G. et al. Chlorinated indium tin oxide electrodes with high work function for organic device compatibility. Science 332, 944–947 (2011).
Hatakeyama, T. et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 28, 2777–2781 (2016).
Kondo, Y. et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photon. 13, 678–683 (2019).
Wei, J. et al. Indolo[3,2,1-jk]carbazole embedded multiple-resonance fluorophors for narrowband deep-blue electroluminescence with EQE ≈ 34.7% and CIEy ≈ 0.085. Angew. Chem. Int. Ed. 60, 12269–12273 (2021).
Yang, M., Park, I. S. & Yasuda, T. Full-color, narrowband, and high-efficiency electroluminescence from boron and carbazole embedded polycyclic heteroaromatics. J. Am. Chem. Soc. 142, 19468–19472 (2020).
Zhang, Y. et al. Multi-resonance deep-red emitters with shallow potential-energy surfaces to surpass energy-gap law. Angew. Chem. Int. Ed. 60, 20498–20503 (2021).
Chan, C.-Y. et al. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photon. 15, 203–207 (2021).
Jeon, S. O. et al. High-efficiency, long-lifetime deep-blue organic light-emitting diodes. Nat. Photon. 15, 208–215 (2021).
Yang, M. et al. Wide-range color tuning of narrowband emission in multi-resonance organoboron delayed fluorescence materials through rational imine/amine functionalization. Angew. Chem. Int. Ed. 60, 23142–23147 (2021).
Zhang, Y. et al. Achieving pure green electroluminescence with CIEy of 0.69 and EQE of 28.2% from an aza-fused multi-resonance emitter. Angew. Chem. Int. Ed. 59, 17499–17503 (2020).
Ikeda, N. et al. Solution-processable pure green thermally activated delayed fluorescence emitter based on the multiple resonance effect. Adv. Mater. 32, 2004072 (2020).
Cai, X. et al. Achieving 37.1% green electroluminescent efficiency and 0.09 eV full width at half maximum based on a ternary boron–oxygen–nitrogen embedded polycyclic aromatic system. Angew. Chem. Int. Ed. 61, e202200337 (2022).
Pershin, A. et al. Highly emissive excitons with reduced exchange energy in thermally activated delayed fluorescent molecules. Nat. Commun. 10, 597 (2019).
Baronas, P. et al. Helical molecular orbitals to induce spin–orbit coupling in oligoyne-bridged bifluorenes. J. Phys. Chem. Lett. 12, 6827–6833 (2021).
Saltiel, J. & Kumar, V. K. Photophysics of diphenylacetylene: light from the “dark state”. J. Phys. Chem. A 116, 10548–10558 (2012).
Bulashevich, K. A., Kulik, A. V. & Karpov, S. Y. Optimal ways of colour mixing for high-quality white-light LED sources. Phys. Status Solidi A 212, 914–919 (2015).
Cui, L.-S. et al. Fast spin-flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photon. 14, 636–642 (2020).
Noda, H. et al. Critical role of intermediate electronic states for spin-flip processes in charge-transfer-type organic molecules with multiple donors and acceptors. Nat. Mater. 18, 1084–1090 (2019).
Chen, X. K., Coropceanu, V. & Bredas, J. L. Assessing the nature of the charge-transfer electronic states in organic solar cells. Nat. Commun. 9, 5295 (2018).
Huang, Y., Hsiang, E. L., Deng, M. Y. & Wu, S. T. Mini-LED, micro-LED and OLED displays: present status and future perspectives. Light Sci. Appl. 9, 105 (2020).
Hsiang, E. L., Yang, Z., Yang, Q., Lan, Y. F. & Wu, S. T. Prospects and challenges of mini‐LED, OLED, and micro‐LED displays. J. Soc. Inf. Disp. 29, 446–465 (2021).
Frisch, M. J. et al. Gaussian 16, revision C.01 (Gaussian, Inc., 2016).
Neese, F., Wennmohs, F., Becker, U. & Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 152, 224108 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 52130304 (X.-H.Z.), 51821002 (X.-H.Z.), 52003185 (K.W.) and 52003186 (Y.-Z.S.)), the National Key Research and Development Program of China (grant numbers 2020YFA0714601 (K.W.) and 2020YFA0714604 (K.W.)), and Suzhou Key Laboratory of Functional Nano and Soft Materials, Collaborative Innovation Center of Suzhou Nano Science and Technology, the 111 Project (X.-H.Z.). X.-K.C. acknowledges the New Faculty Start-up Grant of the City University of Hong Kong (7200709 and 9610547), and the financial support from the Kyulux Inc.
Author information
Authors and Affiliations
Contributions
K.W., X.-K.C., C.A. and X.-H.Z. conceived the project. X.-K.C. carried out all of the theoretical simulations, and proposed the molecular-design concept. K.W. and X.-C.F. designed the compound and devices. X.-C.F. synthesized and characterized the organic compound with the help of Y.-Z.S. and Y.-C.C.; X.-C.F. performed the photophysical measurements and fabricated the OLED devices. Y.-T.L. measured the molecular orientation in the thin films. X.-C.F., K.W., X.-K.C., C.A. and X.-H.Z. wrote the manuscript. X.-H.Z. supervised the project. J.Y. commented on the manuscript. All authors discussed the results and commented on the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Photonics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, XC., Wang, K., Shi, YZ. et al. Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design. Nat. Photon. 17, 280–285 (2023). https://doi.org/10.1038/s41566-022-01106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-022-01106-8
This article is cited by
-
Bright, efficient, and stable pure-green hyperfluorescent organic light-emitting diodes by judicious molecular design
Nature Communications (2024)
-
A figure of merit for efficiency roll-off in TADF-based organic LEDs
Nature (2024)
-
Highly efficient multi-resonance thermally activated delayed fluorescence material toward a BT.2020 deep-blue emitter
Nature Communications (2024)
-
Efficient, narrow-band, and stable electroluminescence from organoboron-nitrogen-carbonyl emitter
Nature Communications (2024)
-
Valley-centre tandem perovskite light-emitting diodes
Nature Nanotechnology (2024)