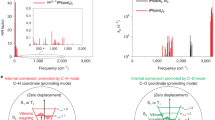
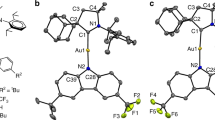
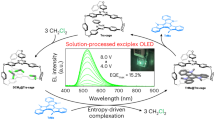
Abstract
Gold(iii) complexes are attractive candidates as phosphorescent dopants in organic light-emitting devices for high-luminance full-colour displays. However, no data on the stability of such devices have been reported to date. Through rational molecular design and synthesis, we have successfully generated a new class of cyclometalated gold(iii) C^C^N complexes with tunable emission colours spanning from sky-blue to red. These complexes exhibit high photoluminescence quantum yields of up to 80% in solid-state thin films, excellent solubility and high thermal stability. Solution-processable and vacuum-deposited organic light-emitting devices based on these complexes operate with external quantum efficiencies of up to 11.9% and 21.6%, respectively, and operational half-lifetimes of up to 83,000 h at 100 cd m−2.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Tang, C. W. & VanSlyke, S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987).
Burroughes, J. H. et al. Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990).
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 90, 5048–5051 (2001).
Ikai, M., Tokito, S., Sakamoto, Y., Suzuki, T. & Taga, Y. Highly efficient phosphorescence from organic light-emitting devices with an exciton-block layer. Appl. Phys. Lett. 79, 156–158 (2001).
D’Andrade, B. W. et al. High-efficiency yellow double-doped organic light-emitting devices based on phosphor-sensitized fluorescence. Appl. Phys. Lett. 79, 1045–1047 (2001).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photon. 8, 326–332 (2014).
Hofbeck, T., Monkowius, U. & Yersin, H. Highly efficient luminescence of Cu(I) compounds: thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 137, 399–404 (2015).
Seino, Y., Inomata, S., Sasabe, H., Pu, Y. J. & Kido, J. High-performance green OLEDs using thermally activated delayed fluorescence with a power efficiency of over 100 lm W−1. Adv. Mater. 28, 2638–2643 (2016).
Kim, D.-H. et al. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nat. Photon. 12, 98–104 (2018).
Wu, T.-L. et al. Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off. Nat. Photon. 12, 235–240 (2018).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Adachi, C., Baldo, M. A., Forrest, S. R. & Thompson, M. E. High-efficiency organic electrophosphorescent devices with tris(2-phenylpyridine)iridium doped into electron-transporting materials. Appl. Phys. Lett. 77, 904–906 (2000).
Zhang, B. et al. High-efficiency single emissive layer white organic light-emitting diodes based on solution-processed dendritic host and new orange-emitting iridium complex. Adv. Mater. 24, 1873–1877 (2012).
Udagawa, K., Sasabe, H., Cai, C. & Kido, J. Low-driving-voltage blue phosphorescent organic light-emitting devices with external quantum efficiency of 30%. Adv. Mater. 26, 5062–5066 (2014).
Ly, T. K. et al. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photon. 11, 63–68 (2017).
Zhu, Z.-Q., Klimes, K., Holloway, S. & Li, J. Efficient cyclometalated platinum(II) complex with superior operational stability. Adv. Mater. 29, 1605002 (2017).
Wong, K. M.-C. et al. A novel class of phosphorescent gold(III) alkynyl-based organic light-emitting devices with tunable colour. Chem. Commun. 2, 2906–2908 (2005).
Au, V. K.-M. et al. High-efficiency green organic light-emitting devices utilizing phosphorescent bis-cyclometalated alkynylgold(III) complexes. J. Am. Chem. Soc. 132, 14273–14278 (2010).
Tang, M.-C., Tsang, D. P.-K., Chan, M. M.-Y., Wong, K. M.-C. & Yam, V. W.-W. Dendritic luminescent gold(III) complexes for highly efficient solution-processable organic light-emitting devices. Angew. Chem. Int. Ed. 52, 446–449 (2013).
Tang, M.-C. et al. Bipolar gold(III) complexes for solution-processable organic light-emitting devices with a small efficiency roll-off. J. Am. Chem. Soc. 136, 17861–17868 (2014).
Wong, B. Y.-W., Wong, H.-L., Wong, Y.-C., Chan, M.-Y. & Yam, V. W.-W. Versatile synthesis of luminescent tetradentate cyclometalated alkynylgold(III) complexes and their application in solution-processable organic light-emitting devices. Angew. Chem. Int. Ed. 56, 302–305 (2017).
Tang, M.-C. et al. Versatile design strategy for highly luminescent vacuum-evaporable and solution-processable tridentate gold(III) complexes with monoaryl auxiliary ligands and their applications for phosphorescent organic light emitting devices. J. Am. Chem. Soc. 139, 9341–9349 (2017).
Cheng, G., Chan, K. T., To, W.-P. & Che, C.-M. Color tunable organic light-emitting devices with external quantum efficiency over 20% based on strongly luminescent gold(III) complexes having long-lived emissive excited states. Adv. Mater. 26, 2540–2546 (2014).
To, W.-P. et al. Highly luminescent pincer gold(III) aryl emitters: thermally activated delayed fluorescence and solution-processed OLEDs. Angew. Chem. Int. Ed. 56, 14036–14041 (2017).
Zhang, F. et al. Iridium(III) complexes bearing oxadiazol-substituted amide ligands: color tuning and application in highly efficient phosphorescent organic light-emitting diodes. J. Mater. Chem. C 5, 9146–9156 (2017).
Lam, E. S.-H., Lam, W. H. & Yam, V. W.-W. A study on the effect of dianionic tridentate ligands on the radiative and nonradiative processes for gold(III) alkynyl systems by a computational approach. Inorg. Chem. 54, 3624–3630 (2015).
Kumar, R., Linden, A. & Nevado, C. Luminescent (N^C^C) gold(III) complexes: stabilized gold(III) fluorides. Angew. Chem. Int. Ed. 54, 14287–14290 (2015).
Yam, V. W.-W., Wong, K. M.-C., Hung, L.-L. & Zhu, N. Luminescent gold(III) alkynyl complexes: synthesis, structural characterization, and luminescence properties. Angew. Chem. Int. Ed. 44, 3107–3110 (2005).
Wong, K. M.-C., Hung, L.-L., Lam, W. H., Zhu, N. & Yam, V. W.-W. A class of luminescent cyclometalated alkynylgold(III) complexes: synthesis, characterization, and electrochemical, photophysical, and computational studies of [Au(C^N^C)(C≡C−R)] (C^N^C = κ3C,N,C bis-cyclometalated 2,6-diphenylpyridyl). J. Am. Chem. Soc. 129, 4350–4365 (2007).
To, W.-P. et al. Strongly luminescent gold(III) complexes with long-lived excited states: high emission quantum yields, energy up-conversion, and nonlinear optical properties. Angew. Chem. Int. Ed. 52, 6648–6652 (2013).
Crosby, G. A. & Demas, J. N. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 75, 991–1024 (1971).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 71, 3–8 (2015).
Spek, A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 71, 9–18 (2015).
Stratmann, R. E., Scuseria, G. E. & Frisch, M. J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 109, 8218–8224 (1998).
Bauernschmitt, R. & Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 256, 454–464 (1996).
Casida, M. E., Jamorski, C., Casida, K. C. & Salahub, D. R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 108, 4439–4449 (1998).
Gaussian 09 (Revision D.01) (Gaussian, Inc., Wallingford CT, 2013).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396 (1997).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Cossi, M., Rega, N., Scalmani, G. & Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 24, 669–681 (2003).
Lu, T. in sobMECP Program; http://sobereva.com/286/.
Harvey, J. N. & Aschi, M. Spin-forbidden dehydrogenation of methoxy cation: a statistical view. Phys. Chem. Chem. Phys. 1, 5555–5563 (1999).
Harvey, J. N., Aschi, M., Schwarz, H. & Koch, W. The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor. Chem. Acc. 99, 95–99 (1998).
Andrae, D., Haussermann, U., Dolg, M., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 77, 123–141 (1990).
Ehlers, A. W. et al. A set of f-polarization functions for pseudo-potential basis-sets of the transition-metals Sc-Cu, Y-Ag and La-Au. Chem. Phys. Lett. 208, 111–114 (1993).
Hehre, W. J., Ditchfie., R. & Pople, J. A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Hariharan, P. C. & Pople, J. A. The influence of polarization functions on molecular-orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973).
Francl, M. M. et al. Self-consistent molecular-orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 77, 3654–3665 (1982).
Acknowledgements
V.W.-W.Y. acknowledges UGC funding administrated by The University of Hong Kong (HKU) for supporting the Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry Facilities under the Support for Interdisciplinary Research in Chemical Science, the HKU Development Fund and the Dr. Hui Wai Haan Fund for funding the X-Ray Diffractometer Facilities, and the support from HKU University Research Committee (URC) Strategically Oriented Research Theme on Functional Materials for Molecular Electronics Towards Materials and Energy Applications. The work described in this paper was fully supported by a grant from the University Grants Committee Areas of Excellence (AoE) Scheme from the Hong Kong Special Administrative Region, China (project no. AoE/P-03/08). L.-K.L. acknowledges the receipt of postgraduate studentships from HKU. The computations were performed using the HKU ITS research computing facilities. W.-L. Cheung is acknowledged for his assistance in the preparation of solution-processable OLED fabrication. K.-H. Low is acknowledged for his assistance in X-ray crystal structure data collection, and L.-Y. Yao and C.-H. Lee are acknowledged for their assistance in X-ray crystal structure determination.
Author information
Authors and Affiliations
Contributions
V.W.-W.Y. initiated and designed the research. V.W.-W.Y., M.-C.T. and L.-K.L. designed the gold(iii) complexes. L.-K.L., M.-C.T. and W.-K.K. conducted the synthesis, characterization, electrochemical and photophysical measurements of the gold(iii) complexes. S.-L.L. and M.-Y.C. carried out the OLED fabrication and characterizations. M.N. performed and analysed the computational calculations. V.W.-W.Y. supervised the work. All authors discussed the results and contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
V.W.-W.Y., M.-C.T., M.-Y.C and L.-K.L. have filed the following patent applications: The University of Hong Kong, Hong Kong; V.W.-W.Y., M.-C.T., M.-Y.C. and C.-H. Lee, Molecular design and OLED application. US provisional patent application no. 62/403,799 (pending); The University of Hong Kong, Hong Kong; V.W.-W.Y., M.-C.T., M.-Y.C., C.-H. Lee and L.-K.L. Molecular design and OLED application. PCT patent application no. PCT/CN2017/105241 (pending).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Further chemical analysis of the complexes
Rights and permissions
About this article
Cite this article
Li, LK., Tang, MC., Lai, SL. et al. Strategies towards rational design of gold(iii) complexes for high-performance organic light-emitting devices. Nature Photon 13, 185–191 (2019). https://doi.org/10.1038/s41566-018-0332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41566-018-0332-z
This article is cited by
-
Enhancing operational stability of OLEDs based on subatomic modified thermally activated delayed fluorescence compounds
Nature Communications (2023)
-
Carbene-stabilized enantiopure heterometallic clusters featuring EQE of 20.8% in circularly-polarized OLED
Nature Communications (2023)
-
Luminescent gold(III) exciplexes enable efficient multicolor electroluminescence
Science China Chemistry (2023)
-
Efficient and stable organic light-emitting devices employing phosphorescent molecular aggregates
Nature Photonics (2021)
-
Chiral lanthanide lumino-glass for a circularly polarized light security device
Communications Chemistry (2020)