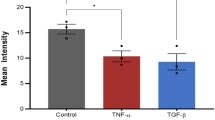
Abstract
The therapeutic potential of liposomes to deliver drugs into inflamed tissue is well documented. Liposomes are believed to largely transport drugs into inflamed joints by selective extravasation through endothelial gaps at the inflammatory sites, known as the enhanced permeation and retention effect. However, the potential of blood-circulating myeloid cells for the uptake and delivery of liposomes has been largely overlooked. Here we show that myeloid cells can transport liposomes to inflammatory sites in a collagen-induced arthritis model. It is shown that the selective depletion of the circulating myeloid cells reduces the accumulation of liposomes up to 50–60%, suggesting that myeloid-cell-mediated transport accounts for more than half of liposomal accumulation in inflamed regions. Although it is widely believed that PEGylation inhibits premature liposome clearance by the mononuclear phagocytic system, our data show that the long blood circulation times of PEGylated liposomes rather favours uptake by myeloid cells. This challenges the prevailing theory that synovial liposomal accumulation is primarily due to the enhanced permeation and retention effect and highlights the potential for other pathways of delivery in inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Other relevant data are available for research purposes from the corresponding authors upon request. Source data are provided with this paper.
References
Sercombe, L. et al. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 6, 286 (2015).
Giulimondi, F. et al. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat. Commun. 10, 3686 (2019).
Suk, J. S., Xu, Q., Kim, N., Hanes, J. & Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99, 28–51 (2016).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105, 14265–14270 (2008).
Ren, H. et al. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces 11, 20304–20315 (2019).
Yang, M., Feng, X., Ding, J., Chang, F. & Chen, X. Nanotherapeutics relieve rheumatoid arthritis. J. Control. Release 252, 108–124 (2017).
Gawne, P. J. et al. PET imaging of liposomal glucocorticoids using 89 Zr-oxine: theranostic applications in inflammatory arthritis. Theranostics 10, 3867–3879 (2020).
Metselaar, J. M. et al. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 63, 348–353 (2004).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 46, 6387–6392 (1986).
Danhier, F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 244, 108–121 (2016).
Davignon, J. L. et al. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology 52, 590–598 (2013).
Kaplan, M. J. Role of neutrophils in systemic autoimmune diseases. Arthritis Res. Ther. 15, 219 (2013).
Izar, M. C. O. et al. Monocyte subtypes and the CCR2 chemokine. Clin. Sci. (Lond.) 131, 1215–1224 (2017).
McInnes, I. B. & Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 389, 2328–2337 (2017).
Dammes, N. et al. Conformation-sensitive targeting of lipid nanoparticles for RNA therapeutics. Nat. Nanotechnol. 16, 1030–1038 (2021).
Sofias, A. M., Andreassen, T. & Hak, S. Nanoparticle ligand-decoration procedures affect in vivo interactions with immune cells. Mol. Pharm. 15, 5754–5761 (2018).
Chu, D., Gao, J. & Wang, Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano 9, 11800–11811 (2015).
Karathanasis, E. et al. Selective targeting of nanocarriers to neutrophils and monocytes. Ann. Biomed. Eng. 37, 1984–1992 (2009).
Veiga, N. et al. Leukocyte-specific siRNA delivery revealing IRF8 as a potential anti-inflammatory target. J. Control. Release 313, 33–41 (2019).
Vargason, A. M., Anselmo, A. C. & Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5, 951–967 (2021).
El Kebir, D. E. & Filep, J. G. Modulation of neutrophil apoptosis and the resolution of inflammation through β2 integrins. Front. Immunol. 4, 60 (2013).
Braeckmans, K. et al. Sizing nanomatter in biological fluids by fluorescence single particle tracking. Nano Lett. 10, 4435–4442 (2010).
Chen, D., Ganesh, S., Wang, W. & Amiji, M. Plasma protein adsorption and biological identity of systemically administered nanoparticles. Nanomedicine 12, 2113–2135 (2017).
De Chermont, Q. L. M. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl Acad. Sci. USA 104, 9266–9271 (2007).
Smith, W. J. et al. Lipophilic indocarbocyanine conjugates for efficient incorporation of enzymes, antibodies and small molecules into biological membranes. Biomaterials 161, 57 (2018).
Hofkens, W., Storm, G., Van Den Berg, W. B. & Van Lent, P. L. Liposomal targeting of glucocorticoids to the inflamed synovium inhibits cartilage matrix destruction during murine antigen-induced arthritis. Int. J. Pharm. 416, 486–492 (2011).
Kratofil, R. M., Kubes, P. & Deniset, J. F. Monocyte conversion during inflammation and injury. Arterioscler. Thromb. Vasc. Biol. 37, 35–42 (2017).
Gschwandtner, M., Derler, R. & Midwood, K. S. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 10, 2759 (2019).
Seeuws, S. et al. A multiparameter approach to monitor disease activity in collagen-induced arthritis. Arthritis Res. Ther. 12, R160 (2010).
Tu, J. et al. Ontogeny of synovial macrophages and the roles of synovial macrophages from different origins in arthritis. Front. Immunol. 10, 1146 (2019).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Inglis, J. J. et al. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res. Ther. 9, R113 (2007).
Asquith, D. L., Miller, A. M., McInnes, I. B. & Liew, F. Y. Animal models of rheumatoid arthritis. Eur. J. Immunol. 39, 2040–2044 (2009).
Wipke, B. T. & Allen, P. M. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J. Immunol. 167, 1601–1608 (2001).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Kulkarni, J. A., Witzigmann, D., Chen, S., Cullis, P. R. & Van Der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 52, 2435–2444 (2019).
Zhu, X. et al. Surface de-PEGylation controls nanoparticle-mediated siRNA delivery in vitro and in vivo. Theranostics 7, 1990–2002 (2017).
Cambré, I. et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat. Commun. 9, 4613 (2018).
Meghraoui-Kheddar, A., Barthelemy, S., Boissonnas, A. & Combadière, C. Revising CX3CR1 expression on murine classical and non-classical monocytes. Front. Immunol. 11, 1117 (2020).
Kinne, R. W. Macrophages in rheumatoid arthritis. Arthritis Res. Ther. 2, 189 (2000).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Wyatt Shields, C. et al. Cellular backpacks for macrophage immunotherapy. Sci. Adv. 6, eaaz6579 (2020).
Kumar, R. A., Li, Y., Dang, Q. & Yang, F. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 65, 348–359 (2018).
Kim, J. & Sahay, G. Nanomedicine hitchhikes on neutrophils to the inflamed lung. Nat. Nanotechnol. 17, 1–2 (2021).
Palchetti, S. et al. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta Biomembr. 1858, 189–196 (2016).
Schöttler, S. et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 11, 372–377 (2016).
Francia, V., Schiffelers, R. M., Cullis, P. R. & Witzigmann, D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjugate Chem. 31, 2046–2059 (2020).
Dale, D. C., Boxer, L., & Liles, W. C. The phagocytes: neutrophils and monocytes. Blood 112, 935–945 (2008).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Novobrantseva, T. I. et al. Systemic RNAi-mediated gene silencing in nonhuman primate and rodent myeloid cells. Mol. Ther. Nucleic Acids 1, e4 (2012).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Lenart, K. et al. A third dose of the unmodified COVID-19 mRNA vaccine CVnCoV enhances quality and quantity of immune responses. Mol. Ther. Methods Clin. Dev. 27, 309–323 (2022).
Jafarzadeh, A., Chauhan, P., Saha, B., Jafarzadeh, S. & Nemati, M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 257, 118102 (2020).
Martinez, F. O., Combes, T. W., Orsenigo, F. & Gordon, S. Monocyte activation in systemic Covid-19 infection: assay and rationale. eBioMedicine 59, 102964 (2020).
Zhang, D. et al. COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 109, 13–22 (2020).
Pence, B. D. Severe COVID-19 and aging: are monocytes the key? GeroScience 42, 1051–1061 (2020).
Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R. & Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 11, 1446 (2020).
Yoshimura, T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine 98, 71–78 (2017).
Parihar, A., Eubank, T. D. & Doseff, A. I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2, 204–215 (2010).
Yang, J., Zhang, L., Yu, C., Yang, X. F. & Wang, H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2, 1 (2014).
Lammers, T. et al. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 15, 622–624 (2020).
Benchimol, M. J., Bourne, D., Moghimi, S. M. & Simberg, D. Pharmacokinetic analysis reveals limitations and opportunities for nanomedicine targeting of endothelial and extravascular compartments of tumors. J. Drug Target. 27, 690–698 (2019).
Fang, J., Nakamura, H. & Maeda, H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151 (2011).
Brocato, T. A. et al. Understanding the connection between nanoparticle uptake and cancer treatment efficacy using mathematical modeling. Sci. Rep. 8, 7538 (2018).
Avnir, Y. et al. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: a novel approach to treating autoimmune arthritis. Arthritis Rheum. 58, 119–129 (2008).
Avnir, Y. et al. Fabrication principles and their contribution to the superior in vivo therapeutic efficacy of nano-liposomes remote loaded with glucocorticoids. PLoS ONE 6, e25721 (2011).
Verbeke, R. et al. Broadening the message: a nanovaccine co-loaded with messenger RNA and α-GalCer induces antitumor immunity through conventional and natural killer T cells. ACS Nano 13, 1655–1669 (2019).
Kulkarni, J. A. et al. Fusion-dependent formation of lipid nanoparticles containing macromolecular payloads. Nanoscale 11, 9023–9031 (2019).
Kulkarni, J. A. et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano 12, 4787–4795 (2018).
Hirota, S., De Ilarduya, C. T., Barron, L. G. & Szoka, F. C. Simple mixing device to reproducibly prepare cationic lipid-DNA complexes (lipoplexes). Biotechniques 27, 286–290 (1999).
Kulkarni, J. A. et al. Rapid synthesis of lipid nanoparticles containing hydrophobic inorganic nanoparticles. Nanoscale 9, 13600–13609 (2017).
Kannan, K., Ortmann, R. A. & Kimpel, D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12, 167–181 (2005).
Seemann, S., Zohles, F. & Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 24, 60 (2017).
Acknowledgements
J.D. acknowledges funding from the Special Research Fund (BOF) from Ghent University (grant no. 01D30517). R.V., S.M. and I.A. acknowledge funding from the Research Foundation-Flanders (FWO-V) (grant nos. 1275023N, 1S73120N and 1S40923N, respectively). I.L. and S.C.D.S acknowledge the FWO-V (grant agreement no. G016221N). I.L. acknowledges financial support from the Ghent University Special Research Fund (UGent-BOF) for Concerted Research Actions (GOA). The work of R.v.d.M. is supported by the Netherlands Research Council (NWO: ZonMW Vici grant no. 016.176.622 to W. J. M. Mulder). D.E. acknowledges funding from the FWO-V (grant nos. 3G0C7719, 3G067219 and G082023N) and Stichting tegen Kanker (STI) (grant no. STI.STK.2023.0005.01). We thank B.D and C.V. from the pre-clinical core imaging facility Infinity Lab (UGent).
Author information
Authors and Affiliations
Contributions
I.L., D.E. and S.C.D.S. designed the project. J.D. was responsible for the protocols and project management. J.D., S.M. and I.A. carried out the liposome fabrication. J.D., R.V., S.M., I.A., H.D., T.D., J.C. and I.L. carried out the mice experiments. T.D. and J.C. were responsible for scoring, caretaking and harvesting the mice. J.D., R.V., S.M. and I.A. performed the IVIS imaging of the mice. J.V.D. and G.V.I. conducted the ImageStream experiments via the VIB Flow Core. J.D., I.L. and D.E. conceptualized the autologous transfusion experiment. J.D., D.P., S.M. and I.L. contributed to the conceptualization of the depletion experiment. J.D. analysed the data. R.v.d.M. helped with the fabrication and guidance of the mRNA LNPs. J.D., R.V. and I.L. wrote the manuscript with input from S.C.D.S, D.E., P.J. and R.v.d.M. All the authors contributed to the discussions and the final work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Katharina Maisel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–22.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deprez, J., Verbeke, R., Meulewaeter, S. et al. Transport by circulating myeloid cells drives liposomal accumulation in inflamed synovium. Nat. Nanotechnol. 18, 1341–1350 (2023). https://doi.org/10.1038/s41565-023-01444-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01444-w