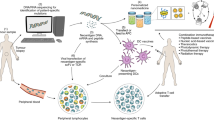
Abstract
Solid tumours display a limited response to immunotherapies. By contrast, haematological malignancies exhibit significantly higher response rates to immunotherapies as compared with solid tumours. Among several microenvironmental and biological disparities, the differential expression of unique immune regulatory molecules contributes significantly to the interaction of blood cancer cells with immune cells. The self-ligand receptor of the signalling lymphocytic activation molecule family member 7 (SLAMF7), a molecule that is critical in promoting the body’s innate immune cells to detect and engulf cancer cells, is expressed nearly exclusively on the cell surface of haematologic tumours, but not on solid ones. Here we show that a bispecific nanobioconjugate that enables the decoration of SLAMF7 on the surface of solid tumours induces robust phagocytosis and activates the phagocyte cyclic guanosine monophosphate–adenosine monophosphate synthase–stimulator of interferon genes (cGAS–STING) pathway, sensitizing the tumours to immune checkpoint blockade. Our findings support an immunological conversion strategy that uses nano-adjuvants to improve the effectiveness of immunotherapies for solid tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. The datasets generated and analysed during the study are publicly available at https://osf.io/jxy9z. Source data are provided with this paper.
References
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Vonderheide, R. H. CD47 blockade as another immune checkpoint therapy for cancer. Nat. Med. 21, 1122–1123 (2015).
Weiskopf, K. et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341, 88–91 (2013).
Logtenberg, M. E. W., Scheeren, F. A. & Schumacher, T. N. The CD47-SIRPα immune checkpoint. Immunity 52, 742–752 (2020).
Jalil, A. R., Andrechak, J. C. & Discher, D. E. Macrophage checkpoint blockade: results from initial clinical trials, binding analyses, and CD47-SIRPα structure–function. Antib. Ther. 3, 80–94 (2020).
Zhang, W. et al. Advances in anti-tumor treatments targeting the CD47/SIRPα axis. Front. Immunol. 11, 18 (2020).
Sikic, B. I. et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 37, 946–953 (2019).
Ansell, S. M. et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 27, 2190–2199 (2021).
Eladl, E. et al. Role of CD47 in hematological malignancies. J. Hematol. Oncol. 13, 96 (2020).
Chen, J. et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature 544, 493–497 (2017).
Feng, M. et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019).
Uger, R. & Johnson, L. Blockade of the CD47-SIRPα axis: a promising approach for cancer immunotherapy. Expert Opin. Biol. Ther. 20, 5–8 (2020).
Zhong, C. et al. Poly(I:C) enhances the efficacy of phagocytosis checkpoint blockade immunotherapy by inducing IL-6 production. J. Leukoc. Biol. 110, 1197–1208 (2021).
Cao, X. et al. Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer. J. Immunother. Cancer 9, e002022 (2021).
Zhang, A. L. et al. Dual targeting of CTLA-4 and CD47 on T-reg cells promotes immunity against solid tumors. Sci. Transl. Med. 13, eabg8693 (2021).
Shi, Y. & Lammers, T. Combining nanomedicine and immunotherapy. Acc. Chem. Res. 52, 1543–1554 (2019).
Yuan, H. et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Nanotechnol. 12, 763–769 (2017).
Weissleder, R., Kelly, K., Sun, E. Y., Shtatland, T. & Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 23, 1418–1423 (2005).
Gordon, S. R. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545, 495–499 (2017).
Pazina, T. et al. Enhanced SLAMF7 homotypic interactions by elotuzumab improves NK cell killing of multiple myeloma. Cancer Immunol. Res. 7, 1633–1646 (2019).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Li, X. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 7, 891–899 (2022).
Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25 (2013).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Alizadeh, D. et al. IFNγ is critical for CAR T cell-mediated myeloid activation and induction of endogenous immunity. Cancer Discov. 11, 2248–2265 (2021).
Pitter, M. R. & Zou, W. Uncovering the immunoregulatory function and therapeutic potential of the PD-1/PD-L1 axis in cancer. Cancer Res. 81, 5141–5143 (2021).
Jiang, X. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 18, 10 (2019).
Su, S. et al. Immune checkpoint inhibition overcomes ADCP-induced immunosuppression by macrophages.Cell 175, 442–457.e23 (2018).
von Roemeling, C. A. et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 11, 1508 (2020).
Kosaka, A. et al. CD47 blockade enhances the efficacy of intratumoral STING-targeting therapy by activating phagocytes. J. Exp. Med. 218, e20200792 (2021).
Hopfner, K. P. & Hornung, V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol. 21, 501–521 (2020).
Martin, G. R., Blomquist, C. M., Henare, K. L. & Jirik, F. R. Stimulator of interferon genes (STING) activation exacerbates experimental colitis in mice. Sci. Rep. 9, 14281 (2019).
Abdullah, A. et al. STING-mediated type-I interferons contribute to the neuroinflammatory process and detrimental effects following traumatic brain injury. J. Neuroinflammation 15, 323 (2018).
Mathur, V. et al. Activation of the STING-dependent type I interferon response reduces microglial reactivity and neuroinflammation. Neuron 96, 1290–1302.e6 (2017).
Li, Z. et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv. Sci. (Weinh.) 9, e2201734 (2022).
Zitvogel, L., Galluzzi, L., Kepp, O., Smyth, M. J. & Kroemer, G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 15, 405–414 (2015).
Salvagno, C. et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat. Cell Biol. 21, 511–521 (2019).
Zhang, Z. et al. Folate receptor α associated with triple-negative breast cancer and poor prognosis. Arch. Pathol. Lab. Med. 138, 890–895 (2014).
Song, D. G. et al. Effective adoptive immunotherapy of triple-negative breast cancer by folate receptor-alpha redirected CAR T cells is influenced by surface antigen expression level. J. Hematol. Oncol. 9, 56 (2016).
Aldea, M. et al. Overcoming resistance to tumor-targeted and immune-targeted therapies. Cancer Discov. 11, 874–899 (2021).
Han, C. et al. Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat. Immunol. 21, 546–554 (2020).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Liao, J. B. et al. Preservation of tumor–host immune interactions with luciferase-tagged imaging in a murine model of ovarian cancer. J. Immunother. Cancer 3, 16 (2015).
Qie, Y. et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci. Rep. 6, 26269 (2016).
Mosser, D. M. & Zhang, X. Activation of murine macrophages. Curr. Protoc. Immunol. 83, 14.2.1–14.2.8 (2008).
Weiskopf, K. et al. Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341, 88–91 (2013).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Evans, B. C. et al. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 73, 50166 (2013).
Acknowledgements
This study was supported in part by a Susan G. Komen Foundation Career Catalyst Research Grant (CCR19605871) and a National Cancer Institute Grant (1K08 CA241070) to W.J., a Department of Defense Grant (W81XWH-19-1-0325) to B.Y.S.K. and a Cancer Center Support (Core) Grant (P30 CA016672) from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center (PI: P. W. Pisters). The authors thank C. Wogan of the Division of Radiation Oncology, MD Anderson Cancer Center, for editorial assistance.
Author information
Authors and Affiliations
Contributions
Y.L., W.J. and B.Y.S.K. conceived the study and designed the experiments. Y.L., K.H., D.L. and Y.Q. performed the experiments and generated the data. Y.L., K.H., W.J. and B.Y.S.K. analysed the data and interpreted the results. All authors helped to write the paper.
Corresponding authors
Ethics declarations
Competing interests
The University of Texas MD Anderson Cancer Center has filed a patent application on the technology and intellectual property reported here for which Y.L., K.H., W.J. and B.Y.S.K are inventors. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Jeffrey Hubbell, Gabriele Multhoff and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–30.
Source data
Source Data Fig. 1
Unprocessed gel.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Y., Huntoon, K., Lee, D. et al. Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy. Nat. Nanotechnol. 17, 1332–1341 (2022). https://doi.org/10.1038/s41565-022-01245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-022-01245-7
This article is cited by
-
Synthetic cationic helical polypeptides for the stimulation of antitumour innate immune pathways in antigen-presenting cells
Nature Biomedical Engineering (2024)
-
CD47 masks pro-phagocytic ligands in cis on tumor cells to suppress antitumor immunity
Nature Immunology (2023)
-
Combining a first-in-class SLAMF7 antibody with SIRPα blockade for anti-tumor immunity
Nature Immunology (2023)
-
The ligation between ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis and antitumor immunity
Nature Immunology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)