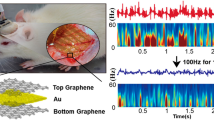
Abstract
Mapping the entire frequency bandwidth of brain electrophysiological signals is of paramount importance for understanding physiological and pathological states. The ability to record simultaneously DC-shifts, infraslow oscillations (<0.1 Hz), typical local field potentials (0.1–80 Hz) and higher frequencies (80–600 Hz) using the same recording site would particularly benefit preclinical epilepsy research and could provide clinical biomarkers for improved seizure onset zone delineation. However, commonly used metal microelectrode technology suffers from instabilities that hamper the high fidelity of DC-coupled recordings, which are needed to access signals of very low frequency. In this study we used flexible graphene depth neural probes (gDNPs), consisting of a linear array of graphene microtransistors, to concurrently record DC-shifts and high-frequency neuronal activity in awake rodents. We show here that gDNPs can reliably record and map with high spatial resolution seizures, pre-ictal DC-shifts and seizure-associated spreading depolarizations together with higher frequencies through the cortical laminae to the hippocampus in a mouse model of chemically induced seizures. Moreover, we demonstrate the functionality of chronically implanted devices over 10 weeks by recording with high fidelity spontaneous spike-wave discharges and associated infraslow oscillations in a rat model of absence epilepsy. Altogether, our work highlights the suitability of this technology for in vivo electrophysiology research, and in particular epilepsy research, by allowing stable and chronic DC-coupled recordings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data obtained to evaluate the main findings of the paper are openly available in Zenodo at https://doi.org/10.5281/zenodo.5655535. All other raw data are available from the corresponding author upon reasonable request.
Code availability
Python v.3.7 packages (Matplotlib v.3.2.0 and Numpy v.1.17.4) and the following Python library were used for electric characterization of the gSGFET arrays: https://github.com/aguimera/PyGFET. A custom Simulink model was used for graphene microtransistor electrophysiological data acquisition; contact g.tec medical engineering for code access. Electrophysiological data were analysed using Python v.3.7 packages (Matplotlib v.3.2.0, Numpy v.1.17.4, Pandas v.0.25.3, seaborn v.0.9.0, Neo v.0.8.0 and Elephant) and the custom library PhyREC (https://github.com/aguimera/PhyREC/tree/PhyREC4). Custom scripts can be found at Zenodo (https://doi.org/10.5281/zenodo.5655535). Immunohistochemical data analysis was performed using Python v.3.7 script (https://github.com/kebarr/biocompatibility_study).
References
Perucca, P., Dubeau, F. & Gotman, J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain 137, 183–196 (2014).
Modur, P. N. High frequency oscillations and infraslow activity in epilepsy. Ann. Indian Acad. Neurol. 17, S99–S106 (2014).
Revankar, G. S. et al. in Seizures in Critical Care: A Guide to Diagnosis and Therapeutics (eds Varelas, P. N. and Claassen, J.) 77–90 (Humana Press, 2017); https://doi.org/10.1007/978-3-319-49557-6_5
Dreier, J. P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 17, 439–447 (2011).
Staba, R. J., Stead, M. & Worrell, G. A. Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11, 334–346 (2014).
Dell, K. L., Cook, M. J. & Maturana, M. I. Deep brain stimulation for epilepsy: biomarkers for optimization. Curr. Treat. Options Neurol. 21, 47 (2019).
Kuhlmann, L., Lehnertz, K., Richardson, M. P., Schelter, B. & Zaveri, H. P. Seizure prediction—ready for a new era. Nat. Rev. Neurol. 14, 618–630 (2018).
Chari, A., Thornton, R. C., Tisdall, M. M. & Scott, R. C. Microelectrode recordings in human epilepsy: a case for clinical translation. Brain Commun. 2, fcaa082 (2020).
Lee, S. et al. DC shifts, high frequency oscillations, ripples and fast ripples in relation to the seizure onset zone. Seizure 77, 52–58 (2020).
Li, C. et al. Evaluation of microelectrode materials for direct-current electrocorticography. J. Neural Eng. 13, 16008 (2015).
Hartings, J. A. How slow can you go? Nat. Mater. 18, 194–196 (2019).
Major, S., Gajovic-Eichelmann, N., Woitzik, J. & Dreier, J. P. Oxygen-induced and pH-induced direct current artifacts on invasive platinum/iridium electrodes for electrocorticography. Neurocrit. Care 35, 146–159 (2021).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Kostarelos, K., Vincent, M., Hebert, C. & Garrido, J. A. Graphene in the design and engineering of next-generation neural interfaces. Adv. Mater. 29, 1700909 (2017).
Blaschke, B. M. et al. Mapping brain activity with flexible graphene micro-transistors. 2D Mater. 4, 025040 (2017).
Hébert, C. et al. Flexible graphene solution-gated field-effect transistors: efficient transducers for micro-electrocorticography. Adv. Funct. Mater. 28, 1703976 (2017).
Masvidal-Codina, E. et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 18, 280–288 (2019).
Weltman, A., Yoo, J. & Meng, E. Flexible, penetrating brain probes enabled by advances in polymer microfabrication. Micromachines 7, 180 (2016).
Tien, L. W. et al. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes. Adv. Funct. Mater. 23, 3185–3193 (2013).
Coenen, A. M. L. & Van Luijtelaar, E. L. J. M. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav. Genet. 33, 635–655 (2003).
Terlau, J. et al. Spike-wave discharges in absence epilepsy: segregation of electrographic components reveals distinct pathways of seizure activity. J. Physiol. https://doi.org/10.1113/JP279483 (2020).
Zijlmans, M. et al. High-frequency oscillations as a new biomarker in epilepsy. Ann. Neurol. 71, 169–178 (2012).
Jacobs, J. et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog. Neurobiol. 98, 302–315 (2012).
Ikeda, A. et al. Focal ictal direct current shifts in human epilepsy as studied by subdural and scalp recording. Brain 122, 827–838 (1999).
Wu, S. et al. Role of ictal baseline shifts and ictal high-frequency oscillations in stereo-electroencephalography analysis of mesial temporal lobe seizures. Epilepsia 55, 690–698 (2014).
Vanhatalo, S. et al. Very slow EEG responses lateralize temporal lobe seizures: an evaluation of non-invasive DC-EEG. Neurology 60, 1098–1104 (2003).
Duan, X. et al. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 7, 174–179 (2012).
Hess, L. H. Graphene transistors for biosensing and bioelectronics. Proc. IEEE 101, 1780–1792 (2013).
Hess, L. H. et al. High-transconductance graphene solution-gated field effect transistors. Appl. Phys. Lett. 99, 033503 (2011).
Garcia-Cortadella, R. et al. Distortion-free sensing of neural activity using graphene transistors. Small 16, 1906640 (2020).
Lecomte, A. et al. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 25, 125003 (2015).
Fueta, Y. & Avoli, M. Effects of antiepileptic drugs on 4-aminopyridine-induced epileptiform activity in young and adult rat hippocampus. Epilepsy Res. 12, 207–215 (1992).
Padmanabhan, K. & Urban, N. N. Disrupting information coding via block of 4-AP-sensitive potassium channels. J. Neurophysiol. 112, 1054–1066 (2014).
Zakharov, A., Chernova, K., Burkhanova, G., Holmes, G. L. & Khazipov, R. Segregation of seizures and spreading depolarization across cortical layers. Epilepsia 60, 2386–2397 (2019).
Hartings, J. A. et al. Direct current electrocorticography for clinical neuromonitoring of spreading depolarizations. J. Cereb. Blood Flow Metab. 37, 1857–1870 (2017).
Harriott, A. M., Takizawa, T., Chung, D. Y. & Chen, S. P. Spreading depression as a preclinical model of migraine. J. Headache Pain 20, 45 (2019).
Buzsáki, G. & Lopes da Silva, F. L High frequency oscillations in the intact brain. Prog. Neurobiol. 98, 241–249 (2012).
Ikeda, A. et al. Active direct current (DC) shifts an “Red slow”: two new concepts for seizure mechanisms and identification of the epileptogenic zone. Neurosci. Res. 156, 95–101 (2020).
Kamarajan, C., Pandey, A. K., Chorlian, D. B. & Porjesz, B. The use of current source density as electrophysiological correlates in neuropsychiatric disorders: a review of human studies. Int. J. Psychophysiol. 97, 310–322 (2015).
Flynn, S. P., Barrier, S., Scott, R. C., Lenck-Santini, P. P. & Holmes, G. L. Status epilepticus induced spontaneous dentate gyrus spikes: in vivo current source density analysis. PLoS ONE 10, e0132630 (2015).
Coenen, A. M. L. & Van Luijtelaar, E. L. J. M. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1, 297–301 (1987).
Orlowska-Feuer, P. et al. Infra-slow modulation of fast beta/gamma oscillations in the mouse visual system. J. Physiol. 599, 1631–1650 (2021).
Garcia-Cortadella, R. et al. Graphene active sensor arrays for long-term and wireless mapping of wide frequency band epicortical brain activity. Nat. Commun. 12, 211 (2021).
Bahari, F. et al. Seizure-associated spreading depression is a major feature of ictal events in two animal models of chronic epilepsy. Preprint at bioRxiv https://doi.org/10.1101/455519 (2020).
Dreier, J. P. et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 135, 259–275 (2012).
Dreier, J. P. et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J. Cereb. Blood Flow Metab. 37, 1595–1625 (2017).
De Tisi, J. et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378, 1388–1395 (2011).
Kanazawa, K. et al. Intracranially recorded ictal direct current shifts may precede high frequency oscillations in human epilepsy. Clin. Neurophysiol. 126, 47–59 (2015).
Lauritzen, M. et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J. Cereb. Blood Flow Metab. 31, 17–35 (2011).
Schaefer, N. et al. Improved metal-graphene contacts for low-noise, high-density microtransistor arrays for neural sensing. Carbon 161, 647–655 (2020).
Jin, H. J. et al. Water-stable silk films with reduced β-sheet content. Adv. Funct. Mater. 15, 1241–1247 (2005).
Rockwood, D. N. et al. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6, 1612–1631 (2011).
Vepari, C. & Kaplan, D. L. Silk as a biomaterial. Prog. Polym. Sci. 32, 991–1007 (2007).
Cao, Y. & Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 10, 1514–1524 (2009).
Gobin, A. S., Froude, V. E. & Mathur, A. B. Structural and mechanical characteristics of silk fibroin and chitosan blend scaffolds for tissue regeneration. J. Biomed. Mater. Res. A 74, 465–473 (2005).
Russo, E. et al. Upholding WAG/Rij rats as a model of absence epileptogenesis: hidden mechanisms and a new theory on seizure development. Neurosci. Biobehav. Rev. 71, 388–408 (2016).
van Luijtelaar, G. & van Oijen, G. Establishing drug effects on electrocorticographic activity in a genetic absence epilepsy model: advances and pitfalls. Front. Pharmacol. 11, 395 (2020).
Acknowledgements
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 881603 (GrapheneCore3). ICN2 is supported by the Severo Ochoa Centres of Excellence programme, funded by the Spanish Research Agency (AEI, grant no. SEV-2017–0706), and by the CERCA Programme/Generalitat de Catalunya. A.B.C. is supported by the International PhD Programme La Caixa-Severo Ochoa (Programa Internacional de Becas ‘la Caixa’-Severo Ochoa). This work has made use of the Spanish ICTS Network MICRONANOFABS, partially supported by MICINN and the ICTS ‘NANBIOSIS’, more specifically by the Micro-NanoTechnology Unit of the CIBER in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN) at the IMB-CNM. We also acknowledge funding from the Generalitat de Catalunya (2017 SGR 1426), and the 2DTecBio project (FIS2017-85787-R) funded by the Ministerio de Ciencia, Innovación y Universidades of Spain, the Agencia Estatal de Investigación (AEI) and the Fondo Europeo de Desarrollo Regional (FEDER/UE). Part of this work was co-funded by the European Regional Development Funds (ERDF) allocated to the Programa operatiu FEDER de Catalunya 2014–2020, with the support of the Secretaria d’Universitats i Recerca of the Departament d’Empresa i Coneixement of the Generalitat de Catalunya for emerging technology clusters devoted to the valorization and transfer of research results (GraphCAT 001-P-001702). A.B.C. acknowledges that this work has been carried out within the framework of a PhD in Electrical and Telecommunication Engineering at the Universitat Autònoma de Barcelona. R.C.W. is funded by a Senior Research Fellowship awarded by the Worshipful Company of Pewterers. D.R. is a Biotechnology and Biological Sciences Research Council (BBSRC) LIDo sponsored PhD student. We thank M. Walker and L. Lemieux (UCL Queen Square Institute of Neurology) for their comments on the manuscript.
Author information
Authors and Affiliations
Contributions
A.B.C. carried out most of the fabrication and characterization of the gDNPs, contributed to the design and performance of the in vivo experiments, analysed the data and wrote the manuscript. E.M.-C. contributed to the design and planning of the in vivo experiments, to the data analysis and particularly to the DC-shift and SD analysis of the in vivo data. R.C.W. and T.M.S. performed the in vivo experiments. D.R. contributed to the in vivo experiments and DC-coupled recordings with the glass micropipette. N.S., E.R.-L., X.I. and J.M.D.l.C. contributed to the fabrication and characterization of the gDNPs. E.D.C., J.B. and C.H. contributed to the growth, transfer and characterization of the CVD graphene used in the gDNPs. E.P.-A., A.H. and E.R.-L. contributed to the optimization of the SF stiffening protocol of the gDNPs. J.M.-A. contributed to the fabrication of the custom electronic instrumentation and development of a Python-based user interface. D.V. contributed to the Python scripts and technical discussions. J.R.S. reviewed the manuscript. J.F. and J.S. contributed to the mechanical assessment of the SF and the SF back-coated gDNPs. M.D. performed all surgeries for the biocompatibility study. A.D. and K.B. contributed to the capture of histological images and image processing and analysis. S.S. and K.B. contributed to the preparation and review of the manuscript. A.G.-B. contributed to the design and fabrication of the custom electronic instrumentation, the development of a custom gSGFET Python library and analysis of the data. R.V., K.K., R.C.W., A.G.-B. and J.A.G. participated in the design of all experiments and thoroughly reviewed the manuscript. All authors read and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.G. is the owner of g.tec medical engineering and Guger Technologies. J.A.G, K.K and A.G.-B declare financial interest in INBRAIN Neuroelectronics. All other authors have no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Matthew Nelson, Bozhi Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–23 and Table 1.
Rights and permissions
About this article
Cite this article
Bonaccini Calia, A., Masvidal-Codina, E., Smith, T.M. et al. Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 17, 301–309 (2022). https://doi.org/10.1038/s41565-021-01041-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-01041-9
This article is cited by
-
Clinical translation of graphene-based medical technology
Nature Reviews Electrical Engineering (2024)
-
High-density transparent graphene arrays for predicting cellular calcium activity at depth from surface potential recordings
Nature Nanotechnology (2024)
-
Control of polymers’ amorphous-crystalline transition enables miniaturization and multifunctional integration for hydrogel bioelectronics
Nature Communications (2024)
-
Graphene-integrated mesh electronics with converged multifunctionality for tracking multimodal excitation-contraction dynamics in cardiac microtissues
Nature Communications (2024)
-
The ultra-thin, minimally invasive surface electrode array NeuroWeb for probing neural activity
Nature Communications (2023)