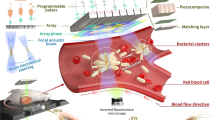
Abstract
Recent advances in molecular engineering and synthetic biology provide biomolecular and cell-based therapies with a high degree of molecular specificity, but limited spatiotemporal control. Here we show that biomolecules and cells can be engineered to deliver potent mechanical effects at specific locations inside the body through ultrasound-induced inertial cavitation. This capability is enabled by gas vesicles, a unique class of genetically encodable air-filled protein nanostructures. We show that low-frequency ultrasound can convert these biomolecules into micrometre-scale cavitating bubbles, unleashing strong local mechanical effects. This enables engineered gas vesicles to serve as remotely actuated cell-killing and tissue-disrupting agents, and allows genetically engineered cells to lyse, release molecular payloads and produce local mechanical damage on command. We demonstrate the capabilities of biomolecular inertial cavitation in vitro, in cellulo and in vivo, including in a mouse model of tumour-homing probiotic therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Plasmids sequences are given in Supplementary Table 3, and will be made available through Addgene. All the raw data related to the plots and graphs are available at https://github.com/shapiro-lab/GV_cavitation.git. All the other materials and data are available from the corresponding author upon reasonable request.
Code availability
MATLAB codes are available from the corresponding author upon reasonable request.
References
Milenic, D. E., Brady, E. D. & Brechbiel, M. W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 3, 488–498 (2004).
Din, M. O. et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85 (2016).
Danino, T. et al. Programmable probiotics for non-invasive urinary detection of cancer. Sci. Transl. Med. 7, 289ra84 (2015).
Claesen, J. & Fischbach, M. A. Synthetic microbes as drug delivery systems. ACS Synth. Biol. 4, 358–364 (2015).
Daniel, C., Roussel, Y., Kleerebezem, M. & Pot, B. Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol. 29, 499–508 (2011).
Steidler, L. et al. Treatment of murine colitis by Lactococcus lactus secreting interleukin-10. Adv. Sci. 289, 1352–1355 (2011).
Davili, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Scheller, L. & Fussenegger, M. From synthetic biology to human therapy: engineered mammalian cells. Curr. Opin. Biotechnol. 58, 108–116 (2019).
Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 16, 214–225 (2018).
Walsby, A. E. Gas vesicles. Annu. Rev. Plant Physiol. 26, 427–439 (1975).
Pfeifer, F. Distribution, formation and regulation of gas vesicles. Nat. Rev. Microbiol. 10, 705–715 (2012).
Dutka, P. et al. Measuring gas vesicle dimensions by electron microscopy. Protein Sci. 30, 1081–1086 (2021).
Shapiro, M. G. et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol. 9, 311–316 (2014).
Lu, G. J. et al. Imaging of gas-filled protein nanostructures. Nat. Mater. 17, 456–463 (2018).
Shapiro, M. G. et al. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat. Chem. 6, 629–634 (2014).
Lakshmanan, A. et al. Molecular engineering of acoustic protein nanostructures. ACS Nano 10, 7314–7322 (2016).
Kunth, M., Lu, G. J., Witte, C., Shapiro, M. G. & Schro, L. Protein nanostructures produce self-adjusting hyperpolarized magnetic resonance imaging contrast through physical gas partitioning. ACS Nano 11, 10939–10948 (2018).
Maresca, D., Sawyer, D. P., Renaud, G., Lee-Gosselin, A. & Shapiro, M. G. Nonlinear X-wave ultrasound imaging of acoustic biomolecules. Phys. Rev. X 8, 41002 (2018).
Maresca, D. et al. Nonlinear ultrasound imaging of nanoscale acoustic biomolecules. Appl. Phys. Lett. 110, 0073704 (2017).
Bourdeau, R. W. et al. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts. Nature 553, 86–90 (2018).
Farhadi, A., Ho, G. H., Sawyer, D. P., Bourdeau, R. W. & Shapiro, M. G. Ultrasound imaging of gene expression in mammalian cells. Science 365, 1469–1475 (2019).
Airan, R. D. et al. Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 17, 652–659 (2017).
Kwan, J. J. et al. Ultrasound-propelled nanocups for drug delivery. Small 11, 5305–5314 (2015).
Kwan, J. J. et al. Ultrasound-induced inertial cavitation from gas-stabilizing nanoparticles. Phys. Rev. E 92, 023019 (2015).
Ferrara, K., Pollard, R. & Borden, M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 9, 415–447 (2007).
Kooiman, K., Vos, H. J., Versluis, M. & De Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 72, 28–48 (2014).
Kotopoulis, S., Dimcevski, G., Gilja, O. H., Hoem, D. & Postema, M. Treatment of human pancreatic cancer using combined ultrasound, microbubbles, and gemcitabine: a clinical case study. Med. Phys. 40, 072902 (2013).
Sheeran, P. S. & Dayton, P. A. Phase-change contrast agents for imaging and therapy. Curr. Pharm. Des. 18, 2152–2165 (2012).
Kripfgans, O. D., Fowlkes, J. B., Miller, D. L., Eldevik, O. P. & Carson, P. L. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med. Biol. Biol. 26, 1177–1189 (2000).
Köse, G., Darguzyte, M. & Kiessling, F. Molecular ultrasound imaging. Nanomaterials 10, 1935 (2020).
Woudstra, L. et al. Development of a new therapeutic technique to direct stem cells to the infarcted heart using targeted microbubbles: StemBells. Stem Cell Res. 17, 6–15 (2016).
Ashokkumar, M., Lee, J., Kentish, S. & Grieser, F. Bubbles in an acoustic field: an overview. Ultrason. Sonochem. 14, 470–475 (2007).
Coussios, C. C. & Roy, R. A. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annu. Rev Fluid Mech. 40, 395–420 (2008).
Szabo, T. L. Diagnostic Ultrasound Imaging: Inside Out (Academic, 2004).
Tran, B. C., Seo, J., Hall, T. L., Fowlkes, J. B. & Cain, C. A. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 50, 1296–1304 (2003).
Church, C. C. Frequency, pulse length, and the mechanical index. Acoust. Res. Lett. 6, 162 (2007).
Holland, C. K. & Apfel, R. E. An improved theory for the prediction of microcavitation thresholds. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 36, 204–208 (1989).
Cherin, E. et al. Acoustic behavior of Halobacterium salinarum gas vesicles in the high-frequency range: experiments and modeling. Ultrasound Med. Biol. 43, 1016–1030 (2017).
Walsby, A. E. The pressure relationships of gas vacuoles. Proc. R. Soc. Lond. B 178, 301–326 (1971).
Lakshmanan, A. et al. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nat. Protocols 12, 2050–2080 (2017).
Le Floc’h, J. et al. In vivo biodistribution of radiolabeled acoustic protein nanostructures. Mol. Imaging Biol. 20, 230–239 (2018).
Duong, M. T. Q., Qin, Y., You, S. H. & Min, J. J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 51, 1–15 (2019).
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W. & Vogelstein, B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl Acad. Sci. USA 98, 15155–15160 (2001).
Leschner, S. et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-α. PLoS ONE 4, e6692 (2009).
Kang, S.-R. et al. Imaging of tumor colonization by Escherichia coli using 18F-FDS PET. Theranostics 10, 4958–4966 (2020).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12, eaax0876 (2020).
Jiang, S.-N. et al. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol. Ther. 18, 635–642 (2010).
Ryan, R. M. et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 16, 329–339 (2009).
Groot, A. J. et al. Functional antibodies produced by oncolytic clostridia. Biochem. Biophys. Res. Commun. 364, 985–989 (2007).
Leventhal, D. S. et al. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 11, 2739 (2020).
Hurt, R. C. et al. Genomically mined acoustic reporter genes enable on-demand in vivo monitoring of tumor-homing bacteria. Preprint at bioRxiv https://doi.org/10.1101/2021.04.26.441537 (2021).
Errico, C. et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502 (2015).
Eranki, A. et al. High-intensity focused ultrasound (hIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin. Cancer Res. 26, 1152–1161 (2020).
Qu, S. et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 8, 1–12 (2020).
Chavez, M. et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics 8, 3611–3628 (2018).
Häcker, G., Redecke, V. & Häcker, H. Activation of the immune system by bacterial CpG-DNA. Immunology 105, 245–251 (2002).
Forbes, N. S., Munn, L. L., Fukumura, D. & Jain, R. K. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 63, 5188–5193 (2003).
Ling, B. et al. Biomolecular ultrasound imaging of phagolysosomal function. ACS Nano 14, 12210–12221 (2020).
Jang, M. J. & Nam, Y. NeuroCa: integrated framework for systematic analysis of spatiotemporal neuronal activity patterns from large-scale optical recording data. Neurophotonics 2, 035003 (2015).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Bar-Zion, A., Yin, M., Adam, D. & Foster, F. S. Functional flow patterns and static blood pooling in tumors revealed by combined contrast-enhanced ultrasound and photoacoustic imaging. Cancer Res. 76, 4320–4331 (2016).
Acknowledgements
The authors thank D. Piraner, A. Lakshmanan, A. Farhadi and P. Ramesh for helpful discussions. In addition, we thank A. Farhadi for his help with the GvpC-RGD variant and H. Davis for his inputs on the optical design of the high-speed set-up. We thank M. Harel (www.maayanillustration.com) for the illustrations in this paper. We also thank A. McDowall for help with electron microscopy and C. Rabut for help with the animal experiments. This project was supported by the David and Lucile Packard Fellowship for Science and Engineering (M.G.S.) and the Heritage Medical Research Institute (M.G.S.). In addition, this project received funding from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement no. 792866 (A.B.-Z.). A.B.-Z. was also supported by the Lester Deutsch Fellowship. A.N. was supported by the Amgen scholars programme. S.S. is supported by the NSF Graduate Research Fellowship. M.H.A. is supported by the NSF Graduate Research Fellowship and the P.D. Soros Fellowship. M.T.B. is supported by the NSF Graduate Research Fellowship. D. Maresca is supported by the Human Frontiers Science Program Cross-Disciplinary Fellowship.
Author information
Authors and Affiliations
Contributions
A.B.-Z. and M.G.S. conceived the study. A.B.-Z., A.N., D. Maresca, D.R.M., S.Y. and S.S. designed, planned and conducted the in vitro experiments. A.B.-Z., A.N., M.T.B., R.C.H., A.L.-G. and M.B.S. designed, planned and conducted in vivo experiments. A.B.-Z. edited the gene circuits with the guidance of M.H.A. A.B.-Z., A.N., D.R.M., S.Y. and D. Maresca analysed the data. D. Malounda prepared the purified GVs. All the authors discussed the results. A.B.-Z., A.N. and M.G.S wrote the manuscript with input from all the authors. All the authors have given their approval for the final version of the manuscript. M.G.S. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The California Institute of Technology has filed a patent application related to this manuscript. The authors have no other competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Mark Borden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 GVs attenuate ultrasound at high concentrations.
(a) Illustration of the sample chamber and setup as seen in the images. (b) B-mode images of purified Ana GVs in different concentrations showing acoustic shadowing at high concentrations. Scale bar, 3 mm. (c) Average broadband emissions measured as a function of GV concentration. GVs were insonated with a single 30-cycle pulse with PNP = 1.0 MPa (n = 5).
Extended Data Fig. 2 GV-seeded cavitation at 3 MHz requires higher pressure levels.
(a) Broadband signals recorded from GVs (0.3 nM) and BSA (matched in mg/mL to GVs concentration) insonated at 3 MHz. Broadband signal increased with pressure and was significantly higher for GV samples at PNP≥0.5 MPa (p < 0.05 for PNP < 1.33 MPa, and p < 0.001 at higher pressure levels, n = 8 independent samples). (b) Comparison between broadband signals from GVs insonated with 670 kHz and 3 MHz pulses (n = 16 and n = 8 independent samples, respectively). Error bars, mean ± s.e.m (a-b).
Extended Data Fig. 3 High frame rate optical imaging of GV collapse and bubble cavitation.
High-speed camera frames (left to right then top to bottom) of GV collapse and cavitation (200 ns between each frame, 31×31 μm field of view), focusing on a single bubble. Initial black spots, which correspond to intact GVs, first disappear due to collapse, liberating gas that coalesces into a cavitating bubble in this region. GVs were insonated with a single 30-cycle pulse with PNP of 1.4 MPa and a central frequency of 670 kHz.
Extended Data Fig. 4 Simulation of free bubble dissolution.
The kinetics of gas bubble dissolution were calculated based on the modified-EP (Epstein and Plesset) equation, following the analysis in [25]: \(- \frac{{{{{\mathbf{dr}}}}}}{{{{{\mathbf{dt}}}}}} = \frac{{{{\mathbf{L}}}}}{{{{{\mathbf{r}}}}/{{{\mathbf{D}}}}_{{{\mathbf{w}}}}}}\left( {\frac{{1 + 2\sigma /{{{\mathbf{P}}}}_a{{{\mathbf{r}}}} - {{{\mathbf{f}}}}}}{{1 + 3\sigma /4{{{\mathbf{P}}}}_a{{{\mathbf{r}}}}}}} \right)\), where Pa = 101.3 kPa is the hydrostatic pressure outside the bubble and r is the bubble radius. Here, L = 0.02 is Ostwald’s coefficient, Dw = 2 × 10−5 cm2 s−1 is the gas diffusivity in water, σ = 72 mNm−1 is the surface tension, and f = 1 is the ratio between the gas concentration in the medium versus that at saturation. This model assumes a perfectly spherical geometry and neglects the potentially stabilizing effects of the nearby collapsed GV shell. However, it provides useful simulations that illustrate the time constants relevant to the process of GV cavitation. (a) Radius-time curves of free air-filled bubbles of different initial sizes. The gas liberated from a collapsed GV occupied the volume of a sphere with a radius of 89 nm under atmospheric conditions and no surface tension, and is expected to have an initial radius slightly larger than 20 nm when surface tension between air and water is assumed across its surface. The actual initial radius is expected to be somewhere between these two values, depending on the degree of stabilization by collapsed GV shells or other solution components. (b) Time before 50% volume reduction for free air-filled bubbles of different sizes. These time constants support the ability of nanobubble to survive the half-cycle between GV collapse (peak pressure) and peak rarefaction. In addition, they can guide the selection of the pulse repetition interval after the initial bubble growth.
Extended Data Fig. 5 High frame rate optical imaging of GVs attached to tumour cells.
(a) GVs attached to U87 cells (0.4 µs) are collapsed by the ultrasound wave (0.8 µs). (b) Differential map comparing pre- and post-collapse images (c) Only after the collapse of the GVs are cavitation events seen (3.4 µs and 9.2 µs). The samples were insonated with a single 30-cycle pulse with PNP = 1.4 MPa a central frequency of 670 kHz. The representative result in panels a-c belongs to one of the 8 repeats presented in Fig. 3g. Scale bars represent 20 µm (a-c).
Extended Data Fig. 6 Targeted and expressed GVs are frequently grouped in close proximity.
The close proximity between expressed or targeted GVs could play an important role in GV cavitation (a) SEM image of Ana GVs attached to U87 cell, forming large patches. (b) TEM image of GVs expressed in S. typhimurium showing a large cluster. Scale bar is 5 μm (a), 1 μm (b). SEM scans of GVs attached to cells and TEM scans of GV expressing S. typhimurium were repeated more than 10 times, all with similar results showing patches or clusters of GVs.
Extended Data Fig. 7 Ultrasound images comparing GV expression in bacteria and mammalian cells.
(a) Ultrasound images of agarose phantoms containing S. typhimurium cells expressing GVs . The initial frame shows the echo from collapsing GVs (left, Peak US,), and the second one presents the residual signal from the cells after bubble dissolution (middle, Collapsed). The GV-specific signal, calculated as the difference between these two images, reveals high GV content in bacteria (right, Difference). (b) Ultrasound images of agarose phantoms containing GV-expressing HEK293 cells. The bacteria and mammalian cell samples were loaded into agarose phantoms at the same concentration as used in cavitation experiments. The volume of each well was 25 µl and it contained either 2 × 105 trypsinized mARG-HEK cells or OD600 = 1 (about 2 × 106) GV-expressing S. typhimurium cells. The combined volume of the mARG-HEK cells greatly exceeds the combined volume of the bacterial cells. However, the partial volume occupied by GVs in mammalian cell is much lower than in bacteria, resulting in lower GV-specific signal.
Extended Data Fig. 8 Color deconvolution of H&E stains reveals effects of GV cavitation on surrounding tissue.
Histologic stains of liver samples were collected after systemic saline injection followed by FUS exposure (negative control, a-d) or GV injection followed by FUS exposure (e-h). Color deconvolution was applied to H&E stains of liver sections (a, e) to obtain separate images of red blood cells (b, f) and tissue (c, g). The residual unmixed images are presented in (d, h). Necrotic regions in the H&E images (e, zoom-in in i) were found around hemorrhagic foci (f, zoom-in in j) in the livers of mice injected with GVs following FUS exposure. Scale bar is 2 mm (d, h), 200 μm (j). The representative results in this figure belong to one of the 5 repeats presented in Fig. 5g, h.
Extended Data Fig. 9 Flow Cytometry Quantification.
Gating strategy for quantifying cell death in mArg-HEK cells, including the SSC-FSC gating of one sample from each population and its Zombie NIR fluorescence histogram. Cell death was quantified by gating the fraction of cells that emitted Zombie NIR fluorescence. The cutoff was the same for all samples.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
41565_2021_971_MOESM3_ESM.avi
Supplementary Video 1 Representative high frame rate movie of GV attached to a Mylar plate. A series of 256 images showing cavitation nucleated by GVs attached to a Mylar plate were collected over 51.2 µs at 5 million frames per second (fps) The movie is displayed at 5 fps, 1 million times slower than the real time.
41565_2021_971_MOESM4_ESM.avi
Supplementary Video 2 Representative high frame rate movie of GVs attached to tumour cells. A series of 256 images showing cavitation nucleated by GVs attached to U87 tumour cells were collected over 51.2 µs at 5 million frames per second (fps) The movie is displayed at 5 fps, 1 million times slower than the real time. The swaying background is a result of the movement of the Mylar membrane at the bottom of the dish during of the ultrasound pulse.
Rights and permissions
About this article
Cite this article
Bar-Zion, A., Nourmahnad, A., Mittelstein, D.R. et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nat. Nanotechnol. 16, 1403–1412 (2021). https://doi.org/10.1038/s41565-021-00971-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00971-8
This article is cited by
-
Phase transition of GvpU regulates gas vesicle clustering in bacteria
Nature Microbiology (2024)
-
Exploiting sound for emerging applications of extracellular vesicles
Nano Research (2024)
-
Genomically mined acoustic reporter genes for real-time in vivo monitoring of tumors and tumor-homing bacteria
Nature Biotechnology (2023)
-
In-vivo programmable acoustic manipulation of genetically engineered bacteria
Nature Communications (2023)
-
Remote control of cellular immunotherapy
Nature Reviews Bioengineering (2023)