Abstract
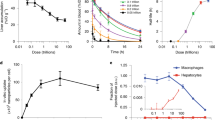
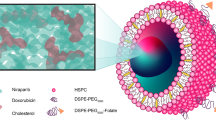
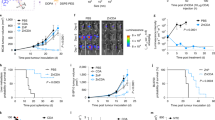
Nanoparticulate albumin bound paclitaxel (nab-paclitaxel, nab-PTX) is among the most widely prescribed nanomedicines in clinical use, yet it remains unclear how nanoformulation affects nab-PTX behaviour in the tumour microenvironment. Here, we quantified the biodistribution of the albumin carrier and its chemotherapeutic payload in optically cleared tumours of genetically engineered mouse models, and compared the behaviour of nab-PTX with other clinically relevant nanoparticles. We found that nab-PTX uptake is profoundly and distinctly affected by cancer-cell autonomous RAS signalling, and RAS/RAF/MEK/ERK inhibition blocked its selective delivery and efficacy. In contrast, a targeted screen revealed that IGF1R kinase inhibitors enhance uptake and efficacy of nab-PTX by mimicking glucose deprivation and promoting macropinocytosis via AMPK, a nutrient sensor in cells. This study thus shows how nanoparticulate albumin bound drug efficacy can be therapeutically improved by reprogramming nutrient signalling and enhancing macropinocytosis in cancer cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available from corresponding authors upon request. RNA-seq data used to calculate the MAPK/AMPK activity score for each cell line were obtained from the Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/ccle).
Code availability
This study did not generate new custom code or mathematical algorithms.
References
Davidson, S. M. et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241 (2017).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Lee, S. W. et al. EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell 50, 381–392.e5 (2019).
Yao, W. et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature 568, 410–414 (2019).
Yardley, D. A. nab-Paclitaxel mechanisms of action and delivery. J. Control. Release 170, 365–372 (2013).
Hoogenboezem, E. N. & Duvall, C. L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 130, 73–89 (2018).
Barkat, M. A., Beg, S., Pottoo, F. H. & Ahmad, F. J. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomed. 14, 1323–1341 (2019).
Havel, H. A. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 18, 1351–1353 (2016).
Socinski, M. A. et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J. Clin. Oncol. 30, 2055–2062 (2012).
Von Hoff, D. D. et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Waters, A. M. & Der, C. J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb Perspect. Med. 8, a031435 (2018).
Tempero, M. A. et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J. Clin. Oncol. 37:15, 4000 (2019).
Desai, N., Trieu, V., Damascelli, B. & Soon-Shiong, P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2, 59–64 (2009).
Hidalgo, M. et al. SPARC expression did not predict efficacy of nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT trial. Clin. Cancer Res. 21, 4811–4818 (2015).
Neesse, A. et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut 63, 974–983 (2014).
Cullis, J. et al. Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol. Res. 5, 182–190 (2017).
Lukinavičius, G. et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 11, 731–733 (2014).
DuPage, M., Dooley, A. L. & Jacks, T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat. Protoc. 4, 1064–1072 (2009).
Cuccarese, M. F. et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 8, 14293 (2017).
Sparreboom, A. et al. Cremophor EL-mediated alteration of paclitaxel distribution in human blood. Cancer Res. 59, 1454–1457 (1999).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Regot, S., Hughey, J. J., Bajar, B. T., Carrasco, S. & Covert, M. W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014).
Kim, H. Y. et al. Quantitative imaging of tumor-associated macrophages and their response to therapy using 64Cu-labeled macrin. ACS Nano 12, 12015–12029 (2018).
Redelman-Sidi, G. et al. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 78, 4658–4670 (2018).
Langer, C. J. et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 32, 2059–2066 (2014).
Ajona, D. et al. Short-term starvation reduces IGF-1 levels to sensitize lung tumors to PD-1 immune checkpoint blockade. Nat. Cancer 1, 75–85 (2020).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Kim, S. M. et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Disco. 8, 866–883 (2018).
Ning, J., Xi, G. & Clemmons, D. R. Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology 152, 3143–3154 (2011).
Tosca, L., Chabrolle, C., Crochet, S., Tesseraud, S. & Dupont, J. IGF-1 receptor signaling pathways and effects of AMPK activation on IGF-1-induced progesterone secretion in hen granulosa cells. Domest. Anim. Endocrinol. 34, 204–216 (2008).
Wagle, M. C. et al. A transcriptional MAPK Pathway Activity Score (MPAS) is a clinically relevant biomarker in multiple cancer types. NPJ Precis Oncol. 2, 7 (2018).
Wan, L. et al. Phosphorylation of EZH2 by AMPK suppresses PRC2 methyltransferase activity and oncogenic function. Mol. Cell 69, 279–291.e5 (2018).
Cui, M. et al. Multifunctional albumin nanoparticles as combination drug carriers for intra-tumoral chemotherapy. Adv. Health. Mater. 2, 1236–1245 (2013).
Zaro, J. L. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J. 17, 83–92 (2015).
Bush, M. A. et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes. Metab. 11, 498–505 (2009).
Suo, Z. et al. Investigation on the interaction of dabrafenib with human serum albumin using combined experiment and molecular dynamics simulation: exploring the binding mechanism, esterase-like activity, and antioxidant activity. Mol. Pharm. 15, 5637–5645 (2018).
Scaltriti, M. & Baselga, J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin. Cancer Res. 12, 5268–5272 (2006).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Dinulescu, D. M. et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 11, 63–70 (2005).
McAuliffe, S. M. et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc. Natl Acad. Sci. USA 109, E2939–E2948 (2012).
McFadden, D. G. et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc. Natl Acad. Sci. USA 111, E1600–E1609 (2014).
Vanden Borre, P. et al. Combined BRAF(V600E)- and SRC-inhibition induces apoptosis, evokes an immune response and reduces tumor growth in an immunocompetent orthotopic mouse model of anaplastic thyroid cancer. Oncotarget 5, 3996–4010 (2014).
Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2, 578–588 (2018).
Vanden Borre, P. et al. The next generation of orthotopic thyroid cancer models: immunocompetent orthotopic mouse models of BRAF V600E-positive papillary and anaplastic thyroid carcinoma. Thyroid 24, 705–714 (2014).
Girnita, A. et al. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 64, 236–242 (2004).
Mulvihill, M. J. et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 1, 1153–1171 (2009).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Pineda, J. J. et al. Site occupancy calibration of taxane pharmacology in live cells and tissues. Proc. Natl Acad. Sci. USA 115, E11406–E11414 (2018).
Devaraj, N. K., Keliher, E. J., Thurber, G. M., Nahrendorf, M. & Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug Chem. 20, 397–401 (2009).
Josephson, L., Tung, C. H., Moore, A. & Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 10, 186–191 (1999).
Langer, K. et al. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 257, 169–180 (2003).
Langer, K. et al. Human serum albumin (HSA) nanoparticles: reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 347, 109–117 (2008).
Tsubaki, M. et al. Trametinib suppresses chemotherapy-induced cold and mechanical allodynia via inhibition of extracellular-regulated protein kinase 1/2 activation. Am. J. Cancer Res. 8, 1239–1248 (2018).
Menu, E. et al. Inhibiting the IGF-1 receptor tyrosine kinase with the cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse model. Blood 107, 655–660 (2006).
Xu, W., Tamura, T. & Takatsu, K. CpG ODN mediated prevention from ovalbumin-induced anaphylaxis in mouse through B cell pathway. Int. Immunopharmacol. 8, 351–361 (2008).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Ng, T. S. C. et al. Detecting immune response to therapies targeting PDL1 and BRAF using ferumoxytol MRI and Macrin in anaplastic thyroid cancer. Radiology 298, 123–132 (2020).
Miller, M. A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314ra183 (2015).
Miller, M. A. et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med. 9, eaal0225 (2017).
Acknowledgements
We acknowledge S. Schmidt and G. Wojtkiewicz for assistance with imaging and biodistribution through the MGH-CSB MIP program. Part of this work was supported by NIH/NCI grant nos. R00CA207744 (M.A.M), DP2CA259675 (M.A.M), U01CA206997 (R.W.), R01HL131495 (R.W.), R01CA206890 (R.W.), T32CA079443 (R.L.), R01GM069668 (D.A.L.), R01CA96504 (D.A.L.), U54CA112967 (D.A.L.), U54CA217377 (D.A.L.), the NSF Graduate Research Fellowship Program (S.J.W.), the American Cancer Society-Ellison Foundation Postdoctoral Fellowship PF-20-106-01-LIB (R.L.), MGH FMD Fellowship (R.L.) and an American Thyroid Association/Thyroid Cancer Survivors’ Association Research Grant (T.S.C.N.).
Author information
Authors and Affiliations
Contributions
R.L., R.W. and M.A.M. developed the concept. R.L., T.S.C.N., S.J.W., C.B.R., H.M., N.B., R.W. and M.A.M designed the experiments. R.L., T.S.C.N., S.J.W, M.P., C.B.R, H.M., R.H.K. and M.A.G. performed the experiments. R.L., R.W. and M.A.M. wrote the paper. R.L., T.S.C.N., S.J.W., M.P., C.B.R., H.M., R.H.K., M.A.G., D.A.L., S.P., D.M.D., N.B., R.W. and M.A.M. analysed the results and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
R.W. is a cofounder of T2Biosystems and Lumicell, serves as a scientific adviser for ModeRNA Therapeutics, Tarveda Therapeutics and Alivio Therapeutics. None of these activities are related to the paper. The other authors declare that they have no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Twan Lammers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5, Figs. 1–23 and reference.
Rights and permissions
About this article
Cite this article
Li, R., Ng, T.S.C., Wang, S.J. et al. Therapeutically reprogrammed nutrient signalling enhances nanoparticulate albumin bound drug uptake and efficacy in KRAS-mutant cancer. Nat. Nanotechnol. 16, 830–839 (2021). https://doi.org/10.1038/s41565-021-00897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-021-00897-1
This article is cited by
-
Progesterone receptor potentiates macropinocytosis through CDC42 in pancreatic ductal adenocarcinoma
Oncogenesis (2024)
-
NIR-dye bridged human serum albumin reassemblies for effective photothermal therapy of tumor
Nature Communications (2023)
-
Drug delivery systems for RNA therapeutics
Nature Reviews Genetics (2022)
-
Nano-biotechnology, an applicable approach for sustainable future
3 Biotech (2022)