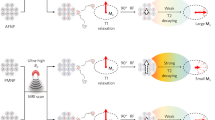
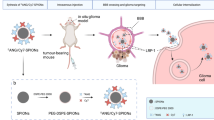
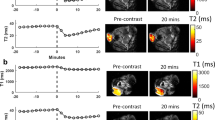
Abstract
Distance-dependent magnetic resonance tuning (MRET) technology enables the sensing and quantitative imaging of biological targets in vivo, with the advantage of deep tissue penetration and fewer interactions with the surroundings as compared with those of fluorescence-based Förster resonance energy transfer. However, applications of MRET technology in vivo are currently limited by the moderate contrast enhancement and stability of T1-based MRET probes. Here we report a new two-way magnetic resonance tuning (TMRET) nanoprobe with dually activatable T1 and T2 magnetic resonance signals that is coupled with dual-contrast enhanced subtraction imaging. This integrated platform achieves a substantially improved contrast enhancement with minimal background signal and can be used to quantitatively image molecular targets in tumours and to sensitively detect very small intracranial brain tumours in patient-derived xenograft models. The high tumour-to-normal tissue ratio offered by TMRET in combination with dual-contrast enhanced subtraction imaging provides new opportunities for molecular diagnostics and image-guided biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Code availability
The subtraction imaging was obtained in MATLAB R2013b software by using the following commands:
A = imread (‘D:T1WI’); B = imread (‘D: T2WI’); C = imsubtract (A, B); J = imcomplement c;
J, subtraction images of the T1- and T2-weighted imaging.
The last step involved the antiphase processing of subtraction images using MATLAB R2013b software.
References
Liu, G. L. et al. A nanoplasmonic molecular ruler for measuring nuclease activity and DNA footprinting. Nat. Nanotechnol. 1, 47–52 (2006).
Nguyen, A. W. & Daugherty, P. S. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 23, 355–360 (2005).
Sönnichsen, C., Reinhard, B. M., Liphardt, J. & Alivisatos, A. P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 23, 741–745 (2005).
Ma, Y. et al. A FRET sensor enables quantitative measurements of membrane charges in live cells. Nat. Biotechnol. 35, 363–370 (2017).
Schuler, B., Lipman, E. A. & Eaton, W. A. Probing the free-energy surface for protein folding with single-molecule fluorescence spectroscopy. Nature 419, 743–747 (2002).
Rizzo, M. A., Springer, G. H., Granada, B. & Piston, D. W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449 (2004).
Biskup, C., Zimmer, T. & Benndorf, K. FRET between cardiac Na+ channel subunits measured with a confocal microscope and a streak camera. Nat. Biotechnol. 22, 220–224 (2004).
Jares-Erijman, E. A. & Jovin, T. M. FRET imaging. Nat. Biotechnol. 21, 1387–1395 (2003).
Choi, J. S. et al. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 16, 537–542 (2017).
Mizukami, S. et al. Paramagnetic relaxation-based 19F MRI probe to detect protease activity. J. Am. Chem. Soc. 130, 794–795 (2008).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Hong, R. et al. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 128, 1078–1079 (2006).
Tam, N. C. M. et al. Porphyrin–lipid stabilized gold nanoparticles for surface enhanced Raman scattering based imaging. Bioconj. Chem. 23, 1726–1730 (2012).
Lovell, J. F. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10, 324–332 (2011).
Xue, X. et al. Trojan horse nanotheranostics with dual transformability and multifunctionality for highly effective cancer treatment. Nat. Commun. 9, 3653 (2018).
Zhou, Z. et al. T1–T2 dual-modal magnetic resonance imaging: from molecular basis to contrast agents. ACS Nano 11, 5227–5232 (2017).
Santra, S. et al. Gadolinium-encapsulating iron oxide nanoprobe as activatable NMR/MRI contrast agent. ACS Nano 6, 7281–7294 (2012).
Mørup, S., Hansen, M. F. & Frandsen, C. Magnetic interactions between nanoparticles. Beilstein J. Nanotechnol. 1, 182–190 (2010).
Köseoğlu, Y., Yıldız, F., Kim, D. K., Muhammed, M. & Aktaş, B. EPR studies on Na-oleate coated Fe3O4 nanoparticles. Phys. Status Solidi C 1, 3511–3515 (2004).
Kuch, W. et al. Three-dimensional noncollinear antiferromagnetic order in single-crystalline FeMn ultrathin films. Phys. Rev. Lett. 92, 017201 (2004).
Webb, M. R., Ash, D. E., Leyh, T. S., Trentham, D. R. & Reed, G. H. Electron paramagnetic resonance studies of Mn(ii) complexes with myosin subfragment 1 and oxygen 17-labeled ligands. J. Biol. Chem. 257, 3068–3072 (1982).
Bulte, J. W., Brooks, R. A., Moskowitz, B. M., Bryant, L. H. Jr. & Frank, J. A. Relaxometry and magnetometry of the MR contrast agent MION-46L. Magn. Reson. Med. 42, 379–384 (1999).
Li, Y. et al. Probing of the assembly structure and dynamics within nanoparticles during interaction with blood proteins. ACS Nano 6, 9485–9495 (2012).
Li, Y. et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials 32, 6633–6645 (2011).
Li, Y., Xiao, K., Zhu, W., Deng, W. & Lam, K. S. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv. Drug Deliv. Rev. 66, 58–73 (2014).
Maeda, H. et al. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and l-buthionine-sulfoximine. Cell Death Differ. 11, 737–746 (2004).
Pileblad, E., Magnusson, T. & Fornstedt, B. Reduction of brain glutathione by l‐buthionine sulfoximine potentiates the dopamine‐depleting action of 6‐hydroxydopamine in rat striatum. J. Neurochem. 52, 978–980 (1989).
Zheng, X. et al. Successively activatable ultrasensitive probe for imaging tumour acidity and hypoxia. Nat. Biomed. Eng. 1, 0057 (2017).
Hori, S. S., Tummers, W. S. & Gambhir, S. S. Cancer diagnostics: on-target probes for early detection. Nat. Biomed. Eng. 1, 0062 (2017).
Li, Y. et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 5, 4712 (2014).
Kato, J. et al. Disulfide cross-linked micelles for the targeted delivery of vincristine to B-cell lymphoma. Mol. Pharm. 9, 1727–1735 (2012).
Xiao, K. et al. Disulfide cross-linked micelles of novel HDAC inhibitor thailandepsin A for the treatment of breast cancer. Biomaterials 67, 183–193 (2015).
Wang, Z. et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 127, 25–35 (2017).
Zhang, Z. et al. Facile synthesis of folic acid-modified iron oxide nanoparticles for targeted MR imaging in pulmonary tumor xenografts. Mol. Imaging Biol. 18, 569–578 (2016).
Acknowledgements
We thank financial support from the National Institutes of Health (NIH)/National Cancer Institute (R01CA199668), NIH/Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD086195), UC Davis Comprehensive Cancer Center Support Grant (CCSG) awarded by the National Cancer Institute (NCI P30CA093373) and National Science Foundation (NSF) (ECCS-1611424 and ECCS-1933527). The acquisition of a magnetic property measurements system (MPMS3) at Georgetown University was supported by the NSF (DMR-1828420).
Author information
Authors and Affiliations
Contributions
Y.L., X.X. and Z.W. conceived the idea and designed the TMRET nanoprobe. Z.W. conducted most of the experiments, X.X. assisted with some of the experiments. X.X. and Z.W. analysed the data. X.X. led the revisions of the manuscript. H.L. worked on the POP materials and animal experiments. Y.H. assisted with the animal studies. Z.W. and Z.L. conducted the DESI process. Z.C., L.Q., N.C., D.A.G., X.X. and K.L. performed the magnetic characterization and assisted with the explanation of T2 quench mechanism. Y.Y. assisted with the MRI studies. N.T. assisted with the MRI data analysis. T.L. assisted with the design and data analysis of biological experiments. K.S.L., A.Y.L. and K.W.F. provided valuable suggestions on the project methodology. X.X., Y.L. and Z.W. wrote the paper and all the authors commented on the manuscript. Y.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
Y.L., X.X. and Z.W. are the co-inventors on the pending patent application filed by the Regents of the University of California on the TMRET nanotechnology and DESI.
Additional information
Peer review information Nature Nanotechnology thanks Jeff Bulte, John Waterton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26 and Tables 1–4.
Rights and permissions
About this article
Cite this article
Wang, Z., Xue, X., Lu, H. et al. Two-way magnetic resonance tuning and enhanced subtraction imaging for non-invasive and quantitative biological imaging. Nat. Nanotechnol. 15, 482–490 (2020). https://doi.org/10.1038/s41565-020-0678-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0678-5
This article is cited by
-
In vivo ultrasound-induced luminescence molecular imaging
Nature Photonics (2024)
-
Doping engineering of iron oxide nanoparticles towards high performance and biocompatible T1-weighted MRI contrast agents
Rare Metals (2024)
-
Dual-ratiometric magnetic resonance tunable nanoprobe with acidic-microenvironment-responsive property to enhance the visualization of early tumor pathological changes
Nano Research (2023)
-
Molecular imaging: design mechanism and bioapplications
Science China Chemistry (2023)
-
Advances in magnetic nanoparticle-based magnetic resonance imaging contrast agents
Nano Research (2023)