Abstract
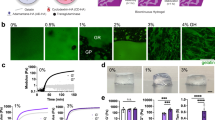
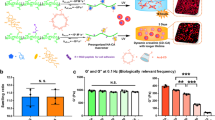
Cells release extracellular vesicles (EVs) to communicate over long distances, which requires EVs to traverse the extracellular matrix (ECM). However, given that the size of EVs is usually larger than the mesh size of the ECM, it is not clear how they can travel through the dense ECM. Here we show that, in contrast to synthetic nanoparticles, EVs readily transport through nanoporous ECM. Using engineered hydrogels, we demonstrate that the mechanical properties of the matrix regulate anomalous EV transport under confinement. Matrix stress relaxation allows EVs to overcome the confinement, and a higher crosslinking density facilitates a fluctuating transport motion through the polymer mesh, which leads to free diffusion and fast transport. Furthermore, water permeation through aquaporin-1 mediates the EV deformability, which further supports EV transport in hydrogels and a decellularized matrix. Our results provide evidence for the nature of EV transport within confined environments and demonstrate an unexpected dependence on matrix mechanics and water permeation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Code availability
The codes used to analyse the data in this study are available from the corresponding author upon reasonable request.
References
Huleihel, L. et al. Matrix-bound nanovesicles within ECM bioscaffold. Sci. Adv. 6, e1600502 (2015).
Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 28, R435–R444 (2018).
Tomlins, P., Grant, P., Mikhalovsky, S., James, S. & Mikhalovska, L. Measurement of pore size and porosity of tissue scaffolds. J. ASTM Int. 1, 1–8 (2004).
Luker, K. E. et al. Comparative study reveals better far-red fluorescent protein for whole body imaging. Sci. Rep. 5, 10332 (2015).
Rakian, R. et al. Native extracellular matrix preserves mesenchymal stem cell ‘stemness’ and differentiation potential under serum-free culture conditions. Stem Cell Res. Ther. 6, 235 (2015).
Vining, K. H., Stafford, A. & Mooney, D. J. Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 188, 187–197 (2019).
Lee, K. Y. & Mooney, D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Grant, G. T., Morris, E. R., Rees, D. A., Smith, P. J. C. & Thom, D. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett. 32, 195–198 (1973).
Armstrong, J. K., Wenby, R. B., Meiselman, H. J. & Fisher, T. C. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys. J. 87, 4259–4270 (2004).
Skotland, T., Hessvik, N. P., Sandvig, K. & Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 60, 9–18 (2019).
Branco da Cunha, C. et al. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35, 8927–8936 (2014).
Metzler, R., Jeon, J. H., Cherstvy, A. G. & Barkai, E. Anomalous diffusion models and their properties: non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 16, 24128–24164 (2014).
Etoc, F. et al. Non-specific interactions govern cytosolic diffusion of nanosized objects in mammalian cells. Nat. Mater. 17, 740–746 (2018).
Schirmache, W., Ruocco, G. & Mazzone, V. Heterogeneous viscoelasticity: a combined theory of dynamic and elastic heterogeneity. Phys. Rev. Lett. 115, 015901 (2015).
Lieleg, O., Vladescu, I. & Ribbeck, K. Characterization of particle translocation through mucin hydrogels. Biophys. J. 98, 1782–1789 (2010).
Goiko, M., De Bruyn, J. R. & Heit, B. Short-lived cages restrict protein diffusion in the plasma membrane. Sci. Rep. 6, 34987 (2016).
Weigel, A. V., Simon, B., Tamkun, M. M. & Krapf, D. Ergodic and nonergodic processes coexist in the plasma membrane as observed by single-molecule tracking. Proc. Natl Acad. Sci. USA 108, 6438–6443 (2011).
Manzo, C. et al. Weak ergodicity breaking of receptor motion in living cells stemming from random diffusivity. Phys. Rev. X 5, 011021- (2015).
Parry, B. R. et al. The bacterial cytoplasm has glass-like properties and is fluidized by metabolic activity. Cell 156, 183–194 (2014).
Kusuma, G. D. et al. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front. Pharmacol. 9, 01199 (2018).
Frank, J. et al. Extracellular vesicles protect glucuronidase model enzymes during freeze-drying. Sci. Rep. 8, 12377 (2018).
Stroka, K. M. et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014).
Blanc, L. et al. The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood 114, 3928–3934 (2009).
Cai, L. H., Panyukov, S. & Rubinstein, M. Hopping diffusion of nanoparticles in polymer matrices. Macromolecules 48, 847–862 (2015).
Wong, I. Y. et al. Anomalous diffusion probes microstructure dynamics of entangled F-actin networks. Phys. Rev. Lett. 92, 178101 (2004).
Ritchie, K. et al. Detection of non-Brownian diffusion in the cell membrane in single molecule tracking. Biophys. J. 88, 2266–2277 (2005).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Manno, S., Takakuwa, Y. & Mohandas, N. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl Acad. Sci. USA 99, 1943–1948 (2002).
Guo, P. et al. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 9, 130 (2018).
McGinley, L. et al. Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res. Ther. 2, 12 (2011).
Lobb, R. J. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasm. J. Extracell. Vesicles 4, 27031 (2015).
Bonenfant, N. R. et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 34, 3231–3245 (2013).
Pena, A. M. et al. Three-dimensional investigation and scoring of extracellular matrix remodeling during lung fibrosis using multiphoton microscopy. Microsc. Res. Tech. 70, 162–170 (2007).
Jos, RANDBLOCK (10584) (MATLAB Central File Exchange, accessed 28 May 2019); https://www.mathworks.com/matlabcentral/fileexchange/17981-randblock
Desai, R. M., Koshy, S. T., Hilderbrand, S. A., Mooney, D. J. & Joshi, N. S. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials 50, 30–37 (2015).
Chaudhuri, O. et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6365 (2015).
Hosford, W. F. Mechanical Behavior of Materials (Cambridge Univ. Press, 2005).
Carr, D. A. & Peppas, N. A. Molecular structure of physiologically-responsive hydrogels controls diffusive behavior. Macromol. Biosci. 9, 497–505 (2009).
Berger, J. et al. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 57, 19–34 (2004).
Boontheekul, T., Kong, H. J. & Mooney, D. J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26, 2455–2465 (2005).
Iza, M., Woerly, S., Danumah, C., Kaliaguine, S. & Bousmina, M. Determination of pore size distribution for mesoporous materials and polymeric gels by means of DSC measurements: thermoporometry. Polymer 41, 5885–5893 (2000).
Backlund, M. P., Joyner, R. & Moerner, W. E. Chromosomal locus tracking with proper accounting of static and dynamic errors. Phys. Rev. E. 91, 062716 (2015).
Vorselen, D. et al. The fluid membrane determines mechanics of erythrocyte extracellular vesicles and is softened in hereditary spherocytosis. Nat. Commun. 9, 4960 (2018).
Segur, J. B. & Oderstar, H. E. Viscosity of glycerol and its aqueous solutions. Ind. Eng. Chem. 43, 2117–2120 (1951).
Calò, A. et al. Force measurements on natural membrane nanovesicles reveal a composition-independent, high Young’s modulus. Nanoscale 6, 2275–2285 (2014).
Acknowledgements
We thank B. Hoffman (Duke University) and L. Cai (University of Virginia) for critical reading of the manuscript and invaluable comments. We acknowledge P. Toth and the Core Imaging Facility at UIC, J. Li at the Department of Pharmacology at UIC, T. Foroozan at the UIC Nanotechnology Core Facility, T. Teng and J. Lee at the Department of Bioengineering at UIC, A. Song at UIC and the ANTEC facility at Northwestern University for their technical help and support. This work made use of instruments in the Fluorescence Imaging Core (Research Resources Center, UIC). This work was supported by National Institutes of Health Grant R01-HL141255 (J.-W.S.), R00-HL125884 (J.-W.S.), T32 HL07829 (S.L.) and American Heart Association Grant 19PRE34380087 (S.L.).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.L. and J-W.S.; data curation, S.L.; formal analysis, S.L.; funding acquisition, S.L. and J-W.S.; investigation, S.L., R.B. and G.C.; methodology, S.L. and J-W.S.; project administration, S.L. and J-W.S.; resources, J-W.S.; software, S.L.; supervision, J-W.S.; validation, S.L., R.B., G.C. and J-W.S.; visualization, S.L.; writing original draft, S.L. and J-W.S; writing the revision, S.L. and J-W.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Gregor Fuhrmann, Neill Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Table 1 and captions for Supplementary Videos 1–6.
Supplementary Video 1
Tracking data overlaid with imaging data for representative transport of a single EV in stiff stress relaxing matrix shown in Fig. 3A. The length scale is micrometers and the time scale is seconds.
Supplementary Video 2
Tracking data overlaid with imaging data for representative transport of a single EV in soft stress relaxing matrix shown in Fig. 3A. The length scale is micrometers and the time scale is seconds.
Supplementary Video 3
Tracking data overlaid with imaging data for representative transport of a single EV in stiff elastic matrix shown in Fig. 3A. The length scale is micrometers and the time scale is seconds.
Supplementary Video 4
Tracking data overlaid with imaging data for representative transport of multiple EVs in stiff stress relaxing matrix. The length scale is micrometers and the time scale is seconds.
Supplementary Video 5
Tracking data overlaid with imaging data for representative transport of multiple EVs in soft stress relaxing matrix. The length scale is micrometers and the time scale is seconds.
Supplementary Video 6
Tracking data overlaid with imaging data for representative transport of multiple EVs in stiff elastic matrix. The length scale is micrometers and the time scale is seconds.
Rights and permissions
About this article
Cite this article
Lenzini, S., Bargi, R., Chung, G. et al. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 15, 217–223 (2020). https://doi.org/10.1038/s41565-020-0636-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-0636-2