Abstract
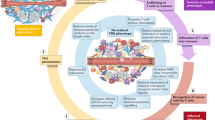
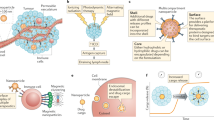
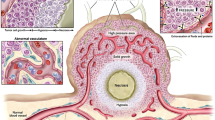
Immunotherapy is known to be clinically beneficial for cancer patients and in many cases represents the new standard of care. Because of this success, the interest in integrating nanomedicine with cancer immunotherapy to further improve clinical response and toxicity profiles has grown. However, unlike conventional systemic therapies, which are directly cytotoxic to tumour cells, cancer immunotherapy relies on the host’s immune system to generate tumouricidal effects. As such, proper design of cancer immune nanomedicine requires scrutiny of tumours’ intrinsic and extrinsic factors that may impact host antitumour immunity. Here, we highlight key parameters that differentiate cancer immunotherapy from conventional cytotoxic agents, and we discuss their implications for designing preclinical cancer immune nanomedicine studies. We emphasize that these factors, including intratumoural genomic heterogeneity, commensal diversity, sexual dimorphism and biological ageing, which were largely ignored in traditional cancer nanomedicine experiments, should be carefully considered and incorporated into cancer immune nanomedicine investigations given their critical involvement in shaping the body’s antitumour immune responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Nam, J. et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414 (2019).
Weiss, S. A., Wolchok, J. D. & Sznol, M. Immunotherapy of melanoma: facts and hopes. Clin. Cancer Res. 25, 5191–5201 (2019).
Peters, S., Reck, M., Smit, E. F., Mok, T. & Hellmann, M. D. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann. Oncol. 30, 884–896 (2019).
Haslam, A. & Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2, e192535 (2019).
Jiang, W. et al. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng. 1, 0029 (2017).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Lizotte, P. H. et al. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 11, 295–303 (2016).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2, 578–588 (2018).
Fadel, T. R. et al. A carbon nanotube-polymer composite for T-cell therapy. Nat. Nanotechnol. 9, 639–647 (2014).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Faria, M. et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 13, 777–785 (2018).
Ioannidis, J. P. A., Kim, B. Y. S. & Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2, 797–809 (2018).
Leong, H. S. et al. On the issue of transparency and reproducibility in nanomedicine. Nat Nanotechnol 14, 629–635 (2019).
Bergthaler, A. & Menche, J. The immune system as a social network. Nat. Immunol. 18, 481–482 (2017).
Slagel, D. E., Feola, J., Houchens, D. P. & Ovejera, A. A. Combined modality treatment using radiation and/or chemotherapy in an athymic nude mouse-human medulloblastoma and glioblastoma xenograft model. Cancer. Res. 42, 812–816 (1982).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714 (2015).
Liu, J. et al. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat. Oncol. 12, 90 (2017).
Ioannidis, J. P. A., Kim, B. Y. S. & Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2, 797–809 (2018).
O’Neil, N. J., Bailey, M. L. & Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 18, 613–623 (2017).
Brown, J. S., O’Carrigan, B., Jackson, S. P. & Yap, T. A. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 7, 20–37 (2017).
An, X. et al. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk. Res. 34, 1255–1268 (2010).
Hughes, T. P. et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 349, 1423–1432 (2003).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Havel, J. J., Chowell, D. & Chan, T. A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer. 19, 133–150 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377, 2500–2501 (2017).
Chowell, D. et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587 (2018). This study showed that increased diversity in the major histocompatibility complex (MHC) was associated with better survival in patients treated with immune checkpoint blockades, showing that the germline genetic diversity contributes to heterogeneous immunotherapy response.
Luksza, M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Andor, N. et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat. Med. 22, 105–113 (2016).
Rodig, S. J. et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 10, eaar3342 (2018).
Want, M. Y. et al. Neoantigens retention in patient derived xenograft models mediates autologous T cells activation in ovarian cancer. Oncoimmunology 8, e1586042 (2019).
Kievit, F. M. et al. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano 6, 2591–2601h (2012).
Chung, W. J. et al. Kras mutant genetically engineered mouse models of human cancers are genomically heterogeneous. Proc. Natl Acad. Sci. USA 114, E10947–E10955 (2017).
Chauhan, V. P. & Jain, R. K. Strategies for advancing cancer nanomedicine. Nat. Mater. 12, 958–962 (2013).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). This study showed that the patients who responded to anti-PD-1 therapy had a different gut microbiome composition compared with non-responders, and that faecal transplants from responding patients can enhance antitumour immunity in germ-free mice.
Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Taur, Y. et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182 (2014).
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Gharaibeh, R. Z. & Jobin, C. Microbiota and cancer immunotherapy: in search of microbial signals. Gut 68, 385–388 (2018).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019).
Liu, H. X. et al. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 142, 769–778 (2018).
Ramirez-Labrada, A. G. et al. the influence of lung microbiota on lung carcinogenesis, immunity, and immunotherapy. Trends Cancer 6, 86–97 (2020).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Chaput, N. et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 28, 1368–1379 (2017).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20, e77–e91 (2019).
Song, W. et al. Trapping of lipopolysaccharide to promote immunotherapy against colorectal cancer and attenuate liver metastasis. Adv. Mater. 30, e1805007 (2018).
Lu, Z., Rong, K., Li, J., Yang, H. & Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 24, 1465–1471 (2013).
Poh, T. Y. et al. Inhaled nanomaterials and the respiratory microbiome: clinical, immunological and toxicological perspectives. Part. Fibre Toxicol. 15, 46 (2018).
Lee, Y. et al. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 19, 118–126 (2019).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Wilson, D. S. et al. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 9, 923–928 (2010).
Javurek, A. B. et al. Gut dysbiosis and neurobehavioral alterations in rats exposed to silver nanoparticles. Sci Rep 7, 2822 (2017).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Edgren, G., Liang, L., Adami, H. O. & Chang, E. T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 27, 187–196 (2012).
Cook, M. B., McGlynn, K. A., Devesa, S. S., Freedman, N. D. & Anderson, W. F. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomarkers Prev. 20, 1629–1637 (2011).
Yang, W. et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 11 (2019).
Conforti, F. et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 19, 737–746 (2018). This meta-analysis of twenty randomized immunotherapy trials with over 11,000 patients found that immune checkpoint inhibitors were more effective in male than in female patients.
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Schneider-Hohendorf, T. et al. Sex bias in MHC I-associated shaping of the adaptive immune system. Proc. Natl Acad. Sci. USA 115, 2168–2173 (2018).
Serpooshan, V. et al. Effect of cell sex on uptake of nanoparticles: the overlooked factor at the nanobio interface. ACS Nano 12, 2253–2266 (2018). This study showed male and female human amniotic stem cells had differences in nanoparticle uptake, suggesting the effect of sex should be considered as a factor at the nano–bio interface.
La-Beck, N. M. et al. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother. Pharmacol. 69, 43–50 (2012).
Lora-Cruz, C. et al. Gold nanoparticle uptake is enhanced by estradiol in MCF-7 breast cancer cells. Int. J. Nanomedicine 14, 2705–2718 (2019).
Schmiedel, B. J. et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 175, 1701–1715 (2018).
Clayton, J. A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 187, 2–5 (2018).
De La Rochere, P. et al. Humanized mice for the study of immuno-oncology. Trends Immunol. 39, 748–763 (2018).
Nikolich-Zugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Kaur, A. et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532, 250–254 (2016).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Sceneay, J. et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discov. 9, 1208–1227 (2019). This study showed that aged mice had a deficiency in innate immune response that causes resistance to immune checkpoint blockade.
Saitoh, T. et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA 106, 20842–20846 (2009).
Mitzel, D. N., Lowry, V., Shirali, A. C., Liu, Y. & Stout-Delgado, H. W. Age-enhanced endoplasmic reticulum stress contributes to increased Atg9A inhibition of STING-mediated IFN-beta production during Streptococcus pneumoniae infection. J. Immunol. 192, 4273–4283 (2014).
Kugel, C. H. 3rd et al. age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin. Cancer Res. 24, 5347–5356 (2018).
Lichtenstein, M. R. L. et al. Impact of age on outcomes with immunotherapy in patients with non-small cell lung cancer. J. Thorac. Oncol. 14, 547–552 (2019).
Zamboni, W. C. et al. Bidirectional pharmacodynamic interaction between pegylated liposomal CKD-602 (S-CKD602) and monocytes in patients with refractory solid tumors. J. Liposome Res. 21, 158–165 (2011).
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Diaz Brinton, R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 153, 3571–3578 (2012).
Scharf, A., Piechulek, A. & von Mikecz, A. Effect of nanoparticles on the biochemical and behavioral aging phenotype of the nematode Caenorhabditis elegans. ACS Nano 7, 10695–10703 (2013).
Kurz, C. L. & Tan, M. W. Regulation of aging and innate immunity in C. elegans. Aging Cell 3, 185–193 (2004).
Garcia-Gonzalez, A. P. et al. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell 169, 431–441 (2017).
Brandt, J. P. & Ringstad, N. Toll-like receptor signaling promotes development and function of sensory neurons required for a C. elegans pathogen-avoidance behavior. Curr. Biol. 25, 2228–2237 (2015).
Mitchell, S. J., Scheibye-Knudsen, M., Longo, D. L. & de Cabo, R. Animal models of aging research: implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 3, 283–303 (2015).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
Ferrara, R. & Matos, I. Atypical patterns of response and progression in the era of immunotherapy combinations. Future Oncol. 16, 1707–1713 (2020).
Ferrara, R., Caramella, C., Besse, B. & Champiat, S. pseudoprogression in non-small cell lung cancer upon immunotherapy: few drops in the ocean? J. Thorac. Oncol. 14, 328–331 (2019).
Wang, Q., Gao, J. & Wu, X. Pseudoprogression and hyperprogression after checkpoint blockade. Int. Immunopharmacol. 58, 125–135 (2018).
Tetzlaff, M. T. et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol 29, 1861–1868 (2018).
June, C. H., Warshauer, J. T. & Bluestone, J. A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 23, 540–547 (2017).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Malysheva, A., Lombi, E. & Voelcker, N. H. Bridging the divide between human and environmental nanotoxicology. Nat. Nanotechnol. 10, 835–844 (2015).
Dobrovolskaia, M. A., Germolec, D. R. & Weaver, J. L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 4, 411–414 (2009).
Hauck, T. S., Anderson, R. E., Fischer, H. C., Newbigging, S. & Chan, W. C. In vivo quantum-dot toxicity assessment. Small 6, 138–144 (2010).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Amos, S. M. et al. Autoimmunity associated with immunotherapy of cancer. Blood 118, 499–509 (2011).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019). This is a comprehensive review of immune-related adverse events associated with different immune checkpoint blockades in different organs.
Weber, J. S., Kahler, K. C. & Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012).
Byun, D. J., Wolchok, J. D., Rosenberg, L. M. & Girotra, M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 13, 195–207 (2017).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Nishino, M., Sholl, L. M., Hodi, F. S., Hatabu, H. & Ramaiya, N. H. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N. Engl. J. Med. 373, 288–290 (2015).
Liu, J., Blake, S. J., Smyth, M. J. & Teng, M. W. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin. Transl. Immunology 3, e22, https://doi.org/10.1038/cti.2014.18 (2014).
Gu, L. et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer 19, 559, https://doi.org/10.1186/s12885-019-5785-z (2019).
Chiang, C. S. et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 13, 746–754 (2018).
Chen, Q. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14, 89–97 (2019).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Luo, M. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017).
Yuan, H. et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Nanotechnol. 12, 763–769 (2017).
Chen, Q. et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Acknowledgements
This work is supported by the Cancer Prevention and Research Institute of Texas (grant RR180017) (W.J.), the National Cancer Institute (grant K08CA241070) (W.J.), the Susan G. Komen Foundation (grant CCR19605871) (W.J.), the American Brain Tumor Association (grant DG1900021) (W.J.), the National Institute of Neurological Disorders and Stroke (grant R01 NS104315) (B.Y.S.K.) and the Department of Defense (grant W81XWH-19-1-0325) (B.Y.S.K.). The authors would like to thank Dr Damiana Chiavolini from the Department of Radiation Oncology at UT Southwestern Medical Center for editorial help with the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks James Moon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, W., Wang, Y., Wargo, J.A. et al. Considerations for designing preclinical cancer immune nanomedicine studies. Nat. Nanotechnol. 16, 6–15 (2021). https://doi.org/10.1038/s41565-020-00817-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-020-00817-9
This article is cited by
-
Age-associated disparity in phagocytic clearance affects the efficacy of cancer nanotherapeutics
Nature Nanotechnology (2024)
-
Tumor-derived systems as novel biomedical tools—turning the enemy into an ally
Biomaterials Research (2023)
-
Cu-doped TiO2 nanoparticles improve local antitumor immune activation and optimize dendritic cell vaccine strategies
Journal of Nanobiotechnology (2023)
-
Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy
Nature Communications (2023)
-
Small molecule–engineered nanoassembly for lipid peroxidation–amplified photodynamic therapy
Drug Delivery and Translational Research (2023)