Abstract
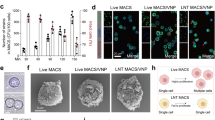
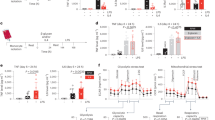
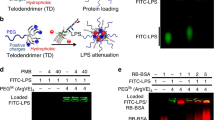
Sepsis, a condition caused by severe infections, affects more than 30 million people worldwide every year and remains the leading cause of death in hospitals1,2. Moreover, antimicrobial resistance has become an additional challenge in the treatment of sepsis3, and thus, alternative therapeutic approaches are urgently needed2,3. Here, we show that adoptive transfer of macrophages containing antimicrobial peptides linked to cathepsin B in the lysosomes (MACs) can be applied for the treatment of multidrug-resistant bacteria-induced sepsis in mice with immunosuppression. The MACs are constructed by transfection of vitamin C lipid nanoparticles that deliver antimicrobial peptide and cathepsin B (AMP-CatB) mRNA. The vitamin C lipid nanoparticles allow the specific accumulation of AMP-CatB in macrophage lysosomes, which is the key location for bactericidal activities. Our results demonstrate that adoptive MAC transfer leads to the elimination of multidrug-resistant bacteria, including Staphylococcus aureus and Escherichia coli, leading to the complete recovery of immunocompromised septic mice. Our work provides an alternative strategy for overcoming multidrug-resistant bacteria-induced sepsis and opens up possibilities for the development of nanoparticle-enabled cell therapy for infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Change history
28 April 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41565-020-0675-8
References
Reinhart, K. et al. Recognizing sepsis as a global health priority—a WHO resolution. N. Engl. J. Med. 377, 414–417 (2017).
van der Poll, T. Immunotherapy of sepsis. Lancet Infect. Dis. 1, 165–174 (2001).
Huttunen, R. & Aittoniemi, J. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J. Infect. 63, 407–419 (2011).
Hotchkiss, R. S. & Karl, I. E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 (2003).
Otto, G. P. et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 15, R183 (2011).
Hotchkiss, R. S., Monneret, G. & Payen, D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268 (2013).
Czermak, B. J. et al. Protective effects of C5a blockade in sepsis. Nat. Med. 5, 788–792 (1999).
Ward, P. A. & Fattahi, F. New strategies for treatment of infectious sepsis. J. Leukoc. Biol. 106, 187–192 (2019).
Huang, X. et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl Acad. Sci. USA 106, 6303–6308 (2009).
Docke, W. D. et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3, 678–681 (1997).
Presneill, J. J., Harris, T., Stewart, A. G., Cade, J. F. & Wilson, J. W. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am. J. Respir. Crit. Care Med. 166, 138–143 (2002).
Galbraith, N., Walker, S., Galandiuk, S., Gardner, S. & Polk, H. C. Jr The significance and challenges of monocyte impairment: for the ill patient and the surgeon. Surg. Infect. 17, 303–312 (2016).
Bo, L., Wang, F., Zhu, J., Li, J. & Deng, X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit. Care 15, R58 (2011).
Foster, T. J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3, 948–958 (2005).
Garzoni, C. & Kelley, W. L. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 17, 59–65 (2009).
Lewis, A. J., Richards, A. C. & Mulvey, M. A. Invasion of host cells and tissues by uropathogenic bacteria. Microbiol. Spectr. 4, UTI-0026-2016 (2016).
Pauwels, A. M., Trost, M., Beyaert, R. & Hoffmann, E. Patterns, receptors, and signals: regulation of phagosome maturation. Trends Immunol. 38, 407–422 (2017).
Giles, F. J., Redman, R., Yazji, S. & Bellm, L. Iseganan HCl: a novel antimicrobial agent. Expert Opin. Investig. Drugs 11, 1161–1170 (2002).
Linke, M., Herzog, V. & Brix, K. Trafficking of lysosomal cathepsin B-green fluorescent protein to the surface of thyroid epithelial cells involves the endosomal/lysosomal compartment. J. Cell Sci. 115, 4877–4889 (2002).
Vasey, P. A. et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents–drug-polymer conjugates. Clin. Cancer Res. 5, 83–94 (1999).
Frimodt-Møller, N., Knudsen, J. & Espersen, F. in Handbook of Animal Models of Infection 127–136 (1999).
McVicker, G. et al. Clonal expansion during Staphylococcus aureus infection dynamics reveals the effect of antibiotic intervention. PLoS Pathog. 10, e1003959 (2014).
Crow, D. Could iPSCs enable “off-the-shelf” cell therapy? Cell 177, 1667–1669 (2019).
Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H. & Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J. Vis. Exp. 76, e50323 (2013).
Zhang, C. et al. Chemotherapy drugs derived nanoparticles encapsulating mRNA encoding tumor suppressor proteins to treat triple-negative breast cancer. Nano Res. 12, 855–861 (2019).
Li, B. et al. An orthogonal array optimization of lipid-like nanoparticles for mRNA delivery in vivo. Nano Lett. 15, 8099–8107 (2015).
Su, X., Fricke, J., Kavanagh, D. G. & Irvine, D. J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol. Pharm. 8, 774–787 (2011).
Zhang, L. et al. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc. Natl Acad. Sci. USA 104, 4606–4611 (2007).
Acknowledgements
We acknowledge the use of the core facility provided by the Campus Microscopy & Imaging Facility at Ohio State University. X.Z. acknowledges support from Fundamental Research Funds for the Central Universities (No. DUT18RC(3)027). Y.D. acknowledges support from National Institutes of Health (NIH) through the Maximizing Investigators’ Research Award R35GM119679 of the National Institute of General Medical Sciences as well as the start-up fund from the College of Pharmacy at Ohio State University.
Author information
Authors and Affiliations
Contributions
X.H. and X.Z. conceived and designed the experiments. X.H. and X.Z. performed the experiments and wrote the paper. W.Z. contributed to animal and live-cell imaging. C. Zeng prepared the mRNA. B.D. and D.W.M. contributed to the Cryo-TEM imaging. S.D. and W.L. contributed to the animal experiments. C. Zhang contributed to flow cytometry assays. Y.D. conceived and supervised the project and wrote the paper. The final manuscript was edited and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Timothy Foster, Anthony Gordon and Liangfang Zhang for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figures 1–10 and RNA sequences.
Rights and permissions
About this article
Cite this article
Hou, X., Zhang, X., Zhao, W. et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15, 41–46 (2020). https://doi.org/10.1038/s41565-019-0600-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0600-1
This article is cited by
-
Applications of peptides in nanosystems for diagnosing and managing bacterial sepsis
Journal of Biomedical Science (2024)
-
Nanotechnology’s frontier in combatting infectious and inflammatory diseases: prevention and treatment
Signal Transduction and Targeted Therapy (2024)
-
Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections
Nature Nanotechnology (2024)
-
Close the cancer–immunity cycle by integrating lipid nanoparticle–mRNA formulations and dendritic cell therapy
Nature Nanotechnology (2023)
-
In vivo bioluminescence imaging of natural bacteria within deep tissues via ATP-binding cassette sugar transporter
Nature Communications (2023)