Abstract
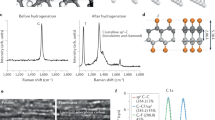
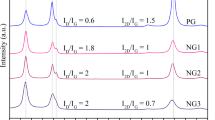
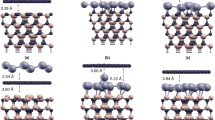
Notwithstanding the numerous density functional studies on the chemically induced transformation of multilayer graphene into a diamond-like film carried out to date, a comprehensive convincing experimental proof of such a conversion is still lacking. We show that the fluorination of graphene sheets in Bernal (AB)-stacked bilayer graphene grown by chemical vapour deposition on a single-crystal CuNi(111) surface triggers the formation of interlayer carbon–carbon bonds, resulting in a fluorinated diamond monolayer (‘F-diamane’). Induced by fluorine chemisorption, the phase transition from (AB)-stacked bilayer graphene to single-layer diamond was studied and verified by X-ray photoelectron, UV photoelectron, Raman, UV-Vis and electron energy loss spectroscopies, transmission electron microscopy and density functional theory calculations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Chernozatonskii, L. A., Sorokin, P. B., Kvashnin, A. G. & Kvashnin, D. G. Diamond-like C2H nanolayer, diamane: simulation of the structure and properties. JETP Lett 90, 134–138 (2009).
Ribas, M. A., Singh, A. K., Sorokin, P. B. & Yakobson, B. I. Patterning nanoroads and quantum dots on fluorinated graphene. Nano. Res 4, 143–152 (2011).
Barboza, A. P. et al. Room-temperature compression-induced diamondization of few-layer graphene. Adv. Mater. 23, 3014–3017 (2011).
Odkhuu, D., Shin, D., Ruoff, R. S. & Park, N. Conversion of multilayer graphene into continuous ultrathin sp3-bonded carbon films on metal surfaces. Sci. Rep 3, 3276 (2013).
Kvashnin, A. G., Chernozatonskii, L. A., Yakobson, B. I. & Sorokin, P. B. Phase diagram of quasi-two-dimensional carbon, from graphene to diamond. Nano Lett. 14, 676–681 (2014).
Martins, L. G. P. et al. Raman evidence for pressure-induced formation of diamondene. Nat. Commun. 8, 96 (2017).
Gao, Y. et al. Ultrahard carbon film from epitaxial two-layer graphene. Nat. Nanotechnol. 13, 133–138 (2018).
Rajasekaran, S., Abild-Pedersen, F., Ogasawara, H., Nilsson, A. & Kaya, S. Interlayer carbon bond formation induced by hydrogen adsorption in few-layer supported graphene. Phys. Rev. Lett. 111, 085503 (2013).
Rajasekaran, S. et al. Reversible graphene-metal contact through hydrogenation. Phys. Rev. B 86, 075417 (2012).
Pumera, M. & Wong, C. Graphane and hydrogenated graphene. Chem. Soc. Rev. 42, 5987–5995 (2013).
Piazza, F. et al. Low temperature, pressureless sp2 to sp3 transformation of ultrathin, crystalline carbon films. Carbon 145, 10–20 (2019).
Smith, D. et al. Hydrogenation of graphene by reaction at high pressure and high temperature. ACS Nano 9, 8279–8283 (2015).
Elias, D. C. et al. Control of graphene’s properties by reversible hydrogenation: evidence of graphane. Science 323, 610–613 (2009).
Burgess, J. S. et al. Tuning the electronic properties of graphene by hydrogenation in a plasma enhanced chemical vapor deposition reactor. Carbon 49, 4420–4426 (2011).
Nair, R. R. et al. Fluorographene: a two-dimensional counterpart of teflon. Small 6, 2877–2884 (2010).
Robinson, J. T. et al. Properties of fluorinated graphene films. Nano Lett. 10, 3001–3005 (2010).
Jeon, K.-J. et al. Fluorographene: a wide bandgap semiconductor with ultraviolet luminescence. ACS Nano 5, 1042–1046 (2011).
Fleisch, T. H. & Mains, G. J. Reduction of copper oxides by UV radiation and atomic hydrogen studied by XPS. Appl. Surf. Sci. 10, 51–62 (1982).
Grosvenor, A. P., Biesinger, M. C., Smart, R. St. C. & McIntyre, N. S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci 600, 1771–1779 (2006).
Hoffman, S. in Practical Surface Analysis, Vol. 2 (eds Briggs, D. & Seah, M. P.) Ch. 4 (Wiley, 1990).
Watanabe, N. Characteristics and applications of graphite fluoride. Physica B & C 105, 17–21 (1981).
Asanov, I. P., Paasonen, V. M., Mazalov, L. N. & Nazarov, A. S. X-ray photoelectron study of fluorinated graphite intercalation compounds. J. Struct. Chem 39, 1127–1133 (1998).
An, K. H. et al. X-ray photoemission spectroscopy study of fluorinated single-walled carbon nanotubes. Appl. Phys. Lett. 80, 4235–4237 (2002).
Sato, Y., Itoh, K., Hagiwara, R., Fukunaga, T. & Ito, Y. On the so-called “semi-ionic” C-F bond character in fluorine-GIC. Carbon 42, 3243–3249 (2004).
Okotrub, A. V., Yudanov, N. F., Asanov, I. P., Vyalikh, D. V. & Bulusheva, L. G. Anisotropy of chemical bonding in semifluorinated graphite C2F revealed with angle-resolved X-ray absorption spectroscopy. ACS Nano 7, 65–74 (2013).
Grayfer, E. D. et al. Synthesis, properties, and dispersion of few-layer graphene fluoride. Chem.-Asian J. 8, 2015–2022 (2013).
Claves, D. Spectroscopic study of fluorinated carbon nanostructures. New J. Chem. 35, 2477–2482 (2011).
Yun, S.-M. et al. An XPS study of oxyfluorinated multiwalled carbon nano tubes. Carbon Lett 8, 292–298 (2007).
Wu, Y. et al. Growth mechanism and controlled synthesis of AB-stacked bilayer graphene on CuNi alloy foils. ACS Nano 6, 77311–77738 (2012).
Liu, L. et al. High-yield chemical vapor deposition growth of high-quality large-area AB-stacked bilayer graphene. ACS Nano 6, 8241–8249 (2012).
Luo, Z. et al. Large-scale synthesis of Bi-layer graphene in strongly coupled stacking order. Adv. Funct. Mater. 21, 911–917 (2011).
Bianconi, A., Hagström, S. B. M. & Bachrach, R. Z. Photoemission studies of graphite high-energy conduction-band and valence-band states using soft X-ray synchrotron radiation excitation. Phys. Rev. B 16, 5543–5548 (1977).
Ono, M. et al. UPS study of VUV-photodegradation of polytetrafluoroethylene (PTFE) ultrathin film by using synchrotron radiation. Nucl. Instrum. Meth. B 236, 377–382 (2005).
Pate, B. B. The diamond surface: atomic and electronic structure. Surf. Sci 165, 83–142 (1986).
Rietwyk, K. J. et al. Work function and electron affinity of the fluorine-terminated (100) diamond surface. Appl. Phys. Lett. 102, 091604 (2013).
Kravets, V. G. et al. Spectroscopic ellipsometry of graphene and an exciton-shifted van Hove peak in absorption. Phys. Rev. B 81, 155413 (2010).
Warner, J. H. et al. Structural transformations in graphene studied with high spatial and temporal resolution. Nat. Nanotechnol 4, 500–504 (2009).
Withers, F., Bointon, T. H., Dubois, M., Russo, S. & Craciun, M. F. Nanopatterning of fluorinated graphene by electron beam irradiation. Nano Lett. 11, 3912–3916 (2011).
Martins, S. E., Withers, F., Dubois, M., Craciun, M. F. & Russo, S. Tuning the transport gap of functionalized graphene via electron beam irradiation. New J. Phys. 15, 033024 (2013).
Hayashi, T. et al. NanoTeflons: structure and EELS characterization of fluorinated carbon nanotubes and nanofibers. Nano Lett. 2, 491–496 (2002).
Acknowledgements
We acknowledge support from the Institute for Basic Science (IBS-R019-D1).
Author information
Authors and Affiliations
Contributions
R.S.R. and P.V.B. conceived of the experiment. P.V.B. wrote the manuscript; designed and constructed the experimental setup; and performed experiments, characterizations and data analyses. P.V.B. and R.S.R. revised the manuscript. M.H. prepared CuNi(111) alloys by electroplating and annealing synthesized and characterized graphene films on CuNi(111) alloys and performed the graphene transfer onto TEM grids and SiO2/Si wafers; M.B. participated in making the metal alloy foils and synthesizing the graphene films. M.S. conducted the experiments and characterizations (transfer of the samples, assisting with building experimental setups and assisting with XPS and Raman characterizations). S.J. and Y.K. converted polycrystalline commercial Cu foils into single-crystal Cu(111). S.W.L. and Z.L. performed the TEM/STEM/EELS characterizations and TEM image simulations. S.H.J., S.O.P. and S.K.K. performed the DFT optimization and calculated formation energies as well as electronic and optical band gaps for various C2F configurations (Supplementary Figs. 4, 13 and 18). J.D. and F.D. performed the DFT calculations to simulate the transformation of BLG into F-diamane (Supplementary Fig. 7), as well as performing EELS simulations. D.C.M. performed DFT calculations, electronic band structure calculations and the TEM/diffraction pattern image simulations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Nanotechnology thanks Rolf Erni and Anirudha Sumant for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs. 1–22, Tables 1 and 2, DFT calculations and XPS quantitative analysis.
Rights and permissions
About this article
Cite this article
Bakharev, P.V., Huang, M., Saxena, M. et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 15, 59–66 (2020). https://doi.org/10.1038/s41565-019-0582-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-019-0582-z
This article is cited by
-
Strain effects of stability, transport, and electro-optical properties of novel Ga2TeS monolayer
Journal of Materials Science (2024)
-
2D materials: increscent quantum flatland with immense potential for applications
Nano Convergence (2022)
-
Two-dimensional diamonds from sp2-to-sp3 phase transitions
Nature Reviews Materials (2022)
-
Synthesis of a monolayer fullerene network
Nature (2022)
-
Ultra-low lattice thermal conductivity and promising thermoelectric figure of merit in borophene via chlorination
Nano Research (2022)