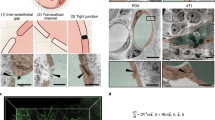
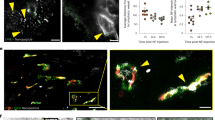
Abstract
While most cancer nanomedicine is designed to eliminate cancer, the nanomaterial per se can lead to the formation of micrometre-sized gaps in the blood vessel endothelial walls. Nanomaterials-induced endothelial leakiness (NanoEL) might favour intravasation of surviving cancer cells into the surrounding vasculature and subsequently extravasation, accelerating metastasis. Here, we show that nanoparticles induce endothelial leakiness through disruption of the VE-cadherin–VE-cadherin homophilic interactions at the adherens junction. We show that intravenously injected titanium dioxide, silica and gold nanoparticles significantly accelerate both intravasation and extravasation of breast cancer cells in animal models, increasing the extent of existing metastasis and promoting the appearance of new metastatic sites. Our results add to the understanding of the behaviour of nanoparticles in complex biological systems. The potential for NanoEL needs to be taken into consideration when designing future nanomedicines, especially nanomedicine to treat cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Steeg, P. S. Tumour metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904 (2006).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012).
Schroeder, A. et al. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 12, 39–50 (2012).
Gupta, G. P. & Massagué, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Reymond, N., d‘Água, B. B. & Ridley, A. J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 13, 858–870 (2013).
Weis, S., Cui, J., Barnes, L. & Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 167, 223–229 (2004).
Anderberg, C. et al. Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. J. Exp. Med. 210, 563–579 (2013).
Zhou, F. et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-β signalling. Nat. Commun. 5, 3388 (2014).
Parodi, A. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotech. 8, 61–68 (2012).
Tay, C. Y. et al. Reality check for nanomaterials-mediated therapy with 3D biomimetic culture systems. Adv. Funct. Mater. 26, 4046–4065 (2016).
Shi, X., von dem Bussche, A., Hurt, R. H., Kane, A. B. & Gao, H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol. 6, 714–719 (2011).
Molinaro, R. et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15, 1037–1046 (2016).
Matsumoto, Y. et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 11, 533–538 (2016).
Setyawati, M. I. et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 4, 1673 (2013).
Setyawati, M. I., Tay, C. Y., Bay, B. H. & Leong, D. T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 11, 5020–5030 (2017).
Tay, C. Y., Setyawati, M. I. & Leong, D. T. Nanoparticle density: a critical biophysical regulator of endothelial permeability. ACS Nano 11, 2764–2772 (2017).
Wang, J., Zhang, L., Peng, F., Shi, X. & Leong, D. T. Targeting endothelial cell junctions with negatively charged gold nanoparticles. Chem. Mater. 30, 3759–3767 (2018).
Qiu, Y. et al. Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions. Nat. Commun. 8, 15594 (2017).
Ding, X. et al. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun. 10, 41 (2019).
Li, L. et al. Actively targeted deep-tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded X-ray-responsive bismuth sulfide@mesoporous silica core-shell nanoparticles. Adv. Funct. Mater. 28, 1704623 (2018).
Peng, F. et al. Silicon-nanowire-based nanocarriers with ultrahigh drug-loading capacity for in vitro and in vivo cancer therapy. Angew. Chem. Int. Ed. 52, 1457–1461 (2013).
Setyawati, M. I., Mochalin, V. M. & Leong, D. T. Tuning endothelial permeability with functionalized nanodiamonds. ACS Nano 10, 1170–1181 (2016).
Yamashita, K. et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 6, 321–328 (2011).
DeLoid, G. et al. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat. Commun. 5, 3514 (2014).
Hirai, T. et al. Metal nanoparticles in the presence of lipopolysaccharides trigger the onset of metal allergy in mice. Nat. Nanotechnol. 11, 808–816 (2016).
Dejana, E. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5, 261–270 (2004).
Xie, L. et al. Regulation of thrombin-induced lung endothelial cell barrier disruption by protein kinase C delta. PLoS One 11, e0158865 (2016).
Tay, C. Y. et al. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 14, 83–88 (2014).
Setyawati, M. I. et al. Nano-TiO2 drives epithelial–mesenchymal transition in intestinal epithelial cancer cells. Small 14, 1800922 (2018).
Bettini, S. et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 7, 40373 (2017).
Chen, Q. et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016).
Chao, Y. et al. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat. Biomed. Eng. 2, 611–621 (2018).
Elgrabli, D. et al. Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS One 10, e0124490 (2015).
Rodriguez-Vita, J. & Morales-Ruiz, M. Down the liver sinusoidal endothelial cell (LSEC) hole. Is there a role for lipid rafts in LSEC fenestration? Hepatology 57, 1272–1274 (2013).
Setyawati, M. I., Tay, C. Y., Docter, D., Stauber, R. H. & Leong, D. T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 44, 8174–8199 (2015).
Bastús, N. G., Comenge, J. & Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: size focusing versus Ostwald ripening. Langmuir 27, 11098–11105 (2011).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Acknowledgements
This work was supported by Ministry of Education Academic Research Grants (R-279–000–418–112, R-279-000-498-114 and R148–000–217–112).
Author information
Authors and Affiliations
Contributions
D.T.L. and H.K.H. conceived the hypotheses, designed the experiments, performed the analysis and interpretations, and wrote the paper. F.P., M.I.S. and J.K.T. designed and performed the experiments, analysed the results and wrote the paper. X.D. and J.P.W. performed the experiments. M.E.N. analysed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figure 1–25 and Supplementary Table 1
Rights and permissions
About this article
Cite this article
Peng, F., Setyawati, M.I., Tee, J.K. et al. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 14, 279–286 (2019). https://doi.org/10.1038/s41565-018-0356-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0356-z
This article is cited by
-
Forefronts and hotspots evolution of the nanomaterial application in anti-tumor immunotherapy: a scientometric analysis
Journal of Nanobiotechnology (2024)
-
Microfluidic high-throughput 3D cell culture
Nature Reviews Bioengineering (2024)
-
Microplastics dampen the self-renewal of hematopoietic stem cells by disrupting the gut microbiota-hypoxanthine-Wnt axis
Cell Discovery (2024)
-
Endothelial leakiness elicited by amyloid protein aggregation
Nature Communications (2024)
-
Anti-lymphangiogenesis for boosting drug accumulation in tumors
Signal Transduction and Targeted Therapy (2024)